Attention Bias Modification Treatment for Pediatric Anxiety Disorders: A Randomized Controlled Trial
Abstract
Objective:
While attention bias modification (ABM) is a promising novel treatment for anxiety disorders, clinical trial data remain restricted to adults. The authors examined whether ABM induces greater reductions in pediatric anxiety symptoms and symptom severity than multiple control training interventions.
Method:
From a target sample of 186 treatment-seeking children at a hospital-based child anxiety clinic, 40 patients with an ongoing anxiety disorder who met all inclusion criteria were enrolled in the study. Children were randomly assigned to one of three conditions: ABM designed to shift attention away from threat; placebo attention training using stimuli identical to those in the ABM condition; and placebo attention training using only neutral stimuli. All participants completed four weekly 480-trial sessions (1,920 total trials). Before and after the attention training sessions, children's clinical status was determined via semistructured interviews and questionnaires. Reduction in the number of anxiety symptoms and their severity was compared across the three groups.
Results:
Change in the number of anxiety symptoms and their severity differed across the three conditions. This reflected significant reductions in the number of anxiety symptoms and symptom severity in the ABM condition but not in the placebo attention training or placebo-neutral condition.
Conclusions:
ABM, compared with two control conditions, reduces pediatric anxiety symptoms and severity. Further study of efficacy and underlying mechanisms is warranted.
The attentional system in anxious individuals is biased toward threat (1, 2). This has led researchers to study a novel anxiety therapy, referred to as attention bias modification (ABM), in randomized controlled trials (3–5). This therapy involves implicit cognitive retraining strategies to alter biases in attention, thereby extending observations suggesting that attention biases act to cause or maintain clinical anxiety (6, 7). For example, in an ABM protocol intended to induce attentional bias away from threat, response targets would appear more frequently at the location of neutral stimuli rather than threat stimuli. This is assumed to induce an implicitly learned bias away from threat following extensive repetitions of such trials (7). ABM in clinical populations has been restricted to adult generalized anxiety disorder (3) or social phobia (4, 5). These studies used the dot-probe task to manipulate attention away from threat (for further detail, see references 7, 8). The treatment effect sizes of these adult ABM randomized controlled trials are comparable to those observed for standard cognitive-behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs) (8). The present study, to our knowledge, is the first randomized controlled trial examining ABM in pediatric anxiety disorders.
Extending ABM to pediatric anxiety disorders is important. First, since most adult anxiety disorders begin during childhood (9), extending ABM to children may affect anxiety symptoms and severity across the lifespan. Second, as reviewed elsewhere (7, 10), aspects of ABM may be particularly well suited for children, given concerns with medication exposure in this age group. Third, the remission rates for first-line treatments for pediatric anxiety disorders (CBT, SSRIs) are up to approximately 70% (11–13). Thus, it is imperative to continue the search for additional efficacious therapies. Fourth, ABM is an extension of neuroscience research on attention-related plasticity suggesting that threat-attention interactions unfold in a developmental context (14). Consequently, it could be beneficial to influence attention early in life, since attention biases have been shown to moderate the development of anxiety.
Two previous studies laid the groundwork for the present randomized controlled trial. First, Eldar et al. (15) found that nonanxious children's responses were similar to those of nonanxious adults (16) in attention training. Second, Bar-Haim et al. (17) found that a form of ABM reduced anxiety symptoms in nondiagnosed highly anxious children. However, neither study examined children with anxiety disorders. In the present study, we tested the hypothesis that ABM produces greater symptom reductions and decreased symptom severity than attention control treatments for pediatric anxiety disorders. As a secondary hypothesis, we examined whether change in attention bias resulting from different training conditions mediates or moderates change in anxiety symptoms from pretreatment to posttreatment. This analysis may clarify the mechanism by which ABM might reduce anxiety.
We used the same active and control conditions used in prior ABM studies of adults as well as a second control condition. The second condition was added because some reductions in anxiety were noted during placebo training in adult ABM studies (3–5). Since such reductions may reflect desensitization to repeatedly presented threat stimuli, our second control condition exposed participants only to neutral stimuli.
Finally, only individuals manifesting attentional threat bias in a prestudy measurement were enrolled. This restriction enabled us to avoid training clinically anxious children to attend away from threat if they did not manifest at least some level of attention bias before training. This decision was derived from findings suggesting that while threat-related attention bias is reliably observed in anxious individuals, as a group-mean effect (1), approximately one-half of clinically anxious individuals do not show an attention bias toward threat. Training such children to avoid threat could carry some risk, given evidence that threat avoidance can contribute to poor outcomes in anxiety.
Method
Sample
Children seeking treatment at the Child Anxiety Clinic at Schneider Children's Medical Center of Israel (Petach-Tikva, Israel) were recruited (mean age: 9.84 years [SD=1.86], range: 8–14 years). All children were assessed using the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions (18, 19). Children meeting criteria for an anxiety disorder were then placed on a waiting list for treatment for approximately 2 months. During this waiting period, children with separation anxiety disorder, social phobia, specific phobia, or generalized anxiety disorder were invited to participate in the study. As in prior randomized controlled trials on pediatric anxiety, the presence of any one diagnosis was considered sufficient for enrollment. Exclusion criteria were 1) posttraumatic stress disorder, obsessive-compulsive disorder, or major depression; 2) current treatment with psychotropic medication; 3) multiple chronic difficulties associated with learning and/or conduct problems; 4) any concurrent psychotherapy; and 5) a baseline attention bias toward threat of less than 8 msec.
An 8-msec cutoff excluded children who showed minimal and possibly meaningless threat bias, thereby minimizing the risk of inducing threat avoidance through ABM in anxious children with no pretreatment threat bias. Typically, bias in the 5- to 10-msec range has been the lower bound in prior studies of anxious children.
Of 186 screened children, 91 met the first four exclusion criteria, and 24 declined to participate in the study. From the remaining 71 children, 31 met the fifth exclusion criterion, leaving 40 eligible participants. Among those children who were eligible, 55% had a primary diagnosis of separation anxiety disorder, 22.5% had generalized anxiety disorder, 20% had specific phobia, and 2.5% had social phobia. Seventy-five percent were also diagnosed with a second anxiety disorder.
Children were randomly assigned to the ABM (N=15), placebo (N=15), or neutral-neutral (N=10) condition. Random assignment to the neutral-neutral group started 4 months after assignment to the other two groups, yielding fewer participants in this group. All children completed the trials. The sessions occurred at the Child Anxiety Clinic from July 2007 to October 2010. The study was approved by the ethics committee of the Schneider Children's Medical Center of Israel, by the Israeli Administration of Health, and by the local ethics committee at Tel Aviv University. Parents provided written consent, and children provided assent.
Outcome Measures
Clinical interviews and questionnaires were used to assess pre- and posttreatment anxiety symptoms and symptom severity. The primary outcome measures were total anxiety symptom counts as reported by parents and children and clinician severity ratings on the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions (range: 0–8). Secondary outcomes were clinical diagnostic status, child- and parent-reported anxiety, and child-reported depression.
Anxiety Disorders Interview Schedule for DSM-IV.
The Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions is a semistructured interview with excellent psychometric properties (19) that has been translated into Hebrew in collaboration with the original authors. Interviewers for this study were two clinical graduate students supervised by two senior clinicians (D.L. and Y.B.). Both interviewers and participants were blind to treatment assignment.
Questionnaires.
Two questionnaires were used: the revised Screen for Child Anxiety Related Emotional Disorders (20), a 66-item parent and child anxiety questionnaire; and the Child Depression Inventory (21), a 27-item child-report depression questionnaire. Both measures have excellent psychometric properties.
Dot-Probe Task (Stimuli and Sequence of Events in a Trial)
The fixation display was a gray cross (2 cm × 2 cm) located in the center of a black screen. The face stimuli were achromatic photographs (55 mm × 80 mm) of 12 actors (six of them men), with each actor contributing two photographs, one with an angry expression and one with a neutral expression (22). Faces of the same actor were presented in angry-neutral or neutral-neutral pairs equidistant from a central fixation cross (16.5 cm center to center). The face pairs were divided into two subsets, each of which consisted of six actors (three of them men). For any given participant, one subset was used at pretreatment, training, and posttreatment (old faces), while the other was used only at posttreatment (new faces). This was done to discern whether any effect of training generalizes to new faces. All experimental factors were randomized and counterbalanced across conditions and participants. Targets were two dots (5 mm center to center; each 2 mm in diameter) oriented either horizontally (..) or vertically (:) and presented at the location of the center of either the left or right photograph. Each trial began with a 500-msec fixation display followed by a 500-msec faces display, which was immediately replaced by the target display until the response, with 1,300-msec intertrial intervals. Participants had to determine the orientation of the dots by pressing one of two buttons.
Pre- and Posttreatment Assessments
Participants completed a dot-probe task using angry-neutral face pairs, with the angry faces equally likely to appear in both hemifields and targets equally likely to appear either horizontally or vertically in the angry or neutral face locations. This generated a fully counterbalanced, randomly mixed design. In the pre-assessment, participants viewed 96 trials (two blocks of 48 trials each). In the postassessment, participants viewed 192 trials (four blocks of 48 trials each), encompassing two blocks of old faces and two blocks of new faces.
ABM and Control Protocols
All children received four training sessions over a 4-week period, with 480 dot-probe trials for each session. Children received training once a week on a prespecified day. When the date of a training appointment had to be changed, the session occurred on another day of the same week of the initial appointment. In the ABM condition, participants viewed angry-neutral stimulus pairs, with targets always appearing at the location of the neutral face. In the placebo condition, participants were presented with the same angry-neutral stimuli but with the targets appearing with equal probability in the angry and neutral locations. In the neutral-neutral condition, participants viewed neutral-neutral face pairs, with targets appearing with equal probability in each hemifield.
Trials with reaction times exceeding two standard deviations of the child's mean reaction time (calculated for each trial type), trials with incorrect response, and trials with reaction times <200 msec were rejected (12.15% of trials). The three groups did not differ in the percentage of eliminated trials. Attention bias scores were calculated by subtracting the mean reaction time in response to targets at the angry face location from the mean reaction time in response to targets at the neutral face location. Positive bias values reflected an attention bias toward angry faces (23).
General Procedure
A CONSORT diagram illustrating the flow of participants through each stage of the trial is shown in Figure 1. Procedures started with the study intake (18), followed by explanation of the study, invitation to participate in the study, consent, and pretreatment assessments.
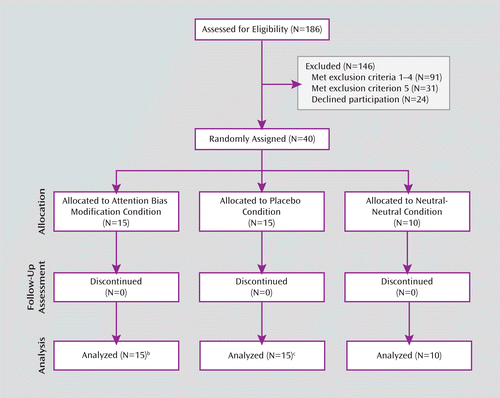
FIGURE 1. CONSORT Diagram Illustrating the Flow of Children Through the Studya
a Children were seeking treatment for symptoms of anxiety at the Child Anxiety Clinic at Schneider Children's Medical Center of Israel (Petach-Tikva, Israel).
b Posttreatment data for the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions were not provided for one child.
c One child was excluded from reaction time analysis (posttreatment bias >3 standard deviations from group mean).
Children were randomly assigned to one of three conditions (ABM, placebo, neutral-neutral). Both families and clinical staff remained blind to group assignment. In sessions 2–5, children trained according to their specific condition. Each session consisted of 480 training trials, presented in 10 blocks of 48 trials each. A short break was allowed at the end of each block. In the sixth session, the child as well as the parent interviewed during the intake session were assessed again with the same instruments. At posttraining, the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions was administered by an interviewer different from the clinician who conducted the intake. Finally, the child was presented with 192 dot-probe measurement trials.
Data Analyses
To test whether randomization generated expectable group characteristics, we conducted chi-square tests for categorical variables and one-way analyses of variance (ANOVAs) for continuous variables. To test the effect of attention training, attention bias scores derived from reaction times in response to the face stimuli used during training (old faces) were submitted to an ANOVA, with time (pretreatment, posttreatment) as a within-subject factor and training condition (ABM, placebo, neutral-neutral) as a between-subjects factor. To test for attention training generalization with regard to new faces, a one-way ANOVA was conducted for attention bias scores, with training condition as a between-subjects factor.
To examine clinical efficacy, the average number of anxiety symptom counts reported by parents and children on the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions and clinician-rated severity scale scores on the same measure were submitted to two separate ANOVAs, with time as a within-subject factor and training condition as a between-subjects factor. As a secondary efficacy test, we used chi-square tests to compare the percentage of children in each condition meeting criteria for their primary anxiety disorder. Questionnaire data were submitted to ANOVAs, with time as a within-subject factor and training condition as a between-subjects factor.
Finally, to assess the interrelations between training and outcome measures, we applied a moderated mediation model analysis (24). This analysis allowed us to simultaneously examine a mediated path linking training condition to change in anxiety symptoms and their severity as a function of the magnitude of attention bias change and a moderation path reflected in an interaction between training condition and change in attention bias. The model examined 1) the relation between training condition and change in attention bias (parameter a); 2) the relation between change in attention bias and change in symptom counts or symptom severity (parameter b); 3) the relation between training condition and change in symptom counts or symptom severity (parameter c); and 4) the residualized effect between training condition and change in symptom counts or symptom severity (parameter c′). Separate models were run for the two outcomes (change in symptom counts and symptom severity).
Results
Between-Group Differences at Preassessment
Comparison of the three conditions at preassessment (Table 1) revealed no group differences beyond a nearly significant difference in gender distribution (χ2=5.52, df=2, 40, p=0.06). We therefore covaried gender in all analyses.
| Training Condition | ||||||
|---|---|---|---|---|---|---|
| Attention Bias Modification (N=15) | Placebo (N=15) | Neutral-Neutral (N=15) | ||||
| Characteristic | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 9.5 | 1.5 | 9.8 | 2.0 | 10.5 | 2.0 |
| Number of siblings | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 |
| Dot-probe task performanceb | ||||||
| Preassessment | ||||||
| Reaction time to threat stimuli (msec) | 637 | 98 | 648 | 117 | 655 | 76 |
| Reaction time to neutral stimuli (msec) | 665 | 101 | 671 | 126 | 675 | 77 |
| Attention bias score | 29 | 19 | 23 | 19 | 20 | 14 |
| Postassessment old faces | ||||||
| Reaction time to threat stimuli (msec) | 572 | 76 | 567 | 81 | 548 | 75 |
| Reaction time to neutral stimuli (msec) | 563 | 73 | 579 | 82 | 551 | 58 |
| Attention bias score | –9 | 19 | 12 | 17 | 3 | 30 |
| Postassessment new faces | ||||||
| Reaction time to threat stimuli (msec) | 601 | 121 | 578 | 83 | 562 | 74 |
| Reaction time to neutral stimuli (msec) | 591 | 112 | 579 | 89 | 556 | 68 |
| Attention bias score | –10 | 20 | 1 | 13 | –6 | 18 |
TABLE 1. Demographic and Clinical Characteristics of Children With Anxiety Disorders Randomly Assigned to Attention Bias Modification, Placebo, and Neutral-Neutral Training Conditionsa
Changes in Attention Bias
The means and standard deviations for accuracy, reaction times, and attention bias scores by training condition are shown in Table 1. Based on the inclusion criteria, at preassessment all participants showed an attention bias toward threat that was significantly different from 0 (ABM: t=6.03, df=14, p<0.001; placebo: t=4.64, df=14, p<0.01; neutral-neutral: t=4.62, df=9, p<0.001), with no difference between the groups.
The ANOVA with the old faces stimuli revealed a time-by-training condition interaction (F=3.35, df=2, 35, p<0.05) but no other main effects or interactions. Follow-up contrasts revealed a reduction in bias scores at postassessment only in the ABM group (t=4.81, df=14, p<0.01, Cohen's d=2.57). Reduction in bias was not significant in the placebo condition or in the neutral-neutral condition.
The one-way ANOVA with the novel faces stimuli, shown only at posttraining, revealed a nonsignificant effect of training condition, suggesting that there was no generalization of training from old to novel faces. However, inspection of the data suggested that the small number of children in the neutral-neutral group might have obscured an effect. An exploratory follow-up contrast between the ABM and placebo conditions revealed stronger bias away from threat in the ABM condition than in the placebo condition (t=2.13, df=28, p<0.05, Cohen's d=0.81).
Changes in Anxiety Symptom Counts and Severity
The primary outcome measures were the average number of anxiety symptoms and symptom severity ratings as derived from clinician interviews. The ANOVA for symptom counts revealed a statistically significant time-by-training condition interaction (F=3.43, df=2, 34, p<0.05 [Figure 2]). Follow-up contrasts revealed a reduction in anxiety symptoms in the ABM condition (preassessment: mean=7.71 [SD=3.36]; postassessment: mean=4.81 [SD=3.92]; t=3.79, df=13, p=0.002, Cohen's d=2.10) but not in the placebo or neutral-neutral condition. The ANOVA for severity scores also revealed a statistically significant time-by-training condition interaction (F=4.22, df=2, 34, p<0.05) (Figure 2). Follow-up contrasts showed a reduction in symptom severity in the ABM condition (preassessment: mean=6.78 [SD=1.09]; postassessment: mean=5.14 [SD=1.76], t=4.06, df=13, p=0.001, Cohen's d=2.25). Neither the placebo nor neutral-neutral condition produced significant reductions.
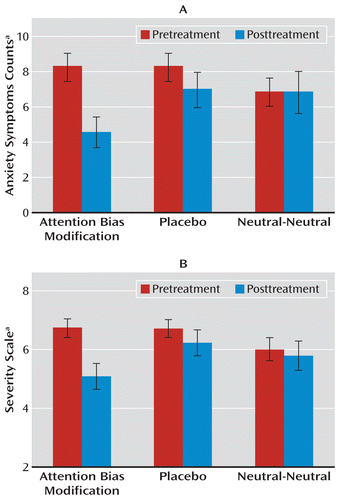
FIGURE 2. Anxiety Symptom Counts and Severity Among Children Randomly Assigned to Attention Bias Modification, Placebo, and Neutral-Neutral Training Conditions
a Per the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions.
Secondary outcomes were diagnostic status and child- and parent-reported symptom ratings. At postassessment, 33.3% of the children in the ABM condition and 13.3% of those in the placebo condition no longer met diagnostic criteria for anxiety disorder, whereas none of the children in the neutral-neutral condition remitted. The ABM group differed from the neutral-neutral group (χ2=4.16, df=1, 25, p<0.05), and the placebo group did not differ from either the ABM or the neutral-neutral group.
For anxiety symptoms (measured using the revised Screen for Child Anxiety Related Emotional Disorders), analyses revealed main effects of time for both child (F=7.13, df=1, 36, p<0.05, Cohen's d=0.89) and parent (F=11.30, df=1, 36, p<0.01, Cohen's d=1.11) reports. Time-by-training interactions were not statistically significant. Depression scores did not change over time as a function of training condition. No significant main or interaction effects were found.
Moderated Mediator Analysis
Reflecting the ANOVA results, there was a strong main effect of training condition for both anxiety symptoms and symptom severity (Table 2). Neither the mediation nor the moderation path was statistically significant, which was likely a result of the small sample size, as can be seen in the nearly significant link between training group and magnitude of attention bias change.
| Parameterb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C′ | AB | |||||||||
| Outcome | β | SE | t | β | SE | t | β | SE | t | β | SE | |
| Symptom counts | –11.46 | 6.68 | –1.72 | –0.09 | 0.05 | –1.72 | 4.08* | 1.11 | 3.67 | 0.04 | 0.02 | 1.60 |
| Symptom severity | –11.46 | 6.68 | –1.72 | –0.02 | 0.02 | –1.06 | 1.14* | 0.37 | 3.11 | 0.01 | 0.01 | 1.42 |
TABLE 2. Prediction of Change in Anxiety Symptom Counts and Severity After Attention Bias Modification, Placebo, or Neutral-Neutral Traininga
Discussion
To our knowledge, this is the first randomized controlled trial of ABM in the treatment of pediatric anxiety disorders. Our study also extends prior research in adult anxiety disorders by including a neutral-neutral control condition, providing assessment beyond the placebo condition in studies of adults. The results suggest that ABM, but neither control condition, reduces pediatric anxiety symptoms and clinician severity ratings, thereby extending findings from the adult ABM literature (8).
The anxiety-reducing effects of attention training seen in other studies could arise from increases in general attentional control regardless of emotional valence (7, 25). For instance, attention training may increase attentional control via enhancement of top-down cognitive capacities that in turn inhibit threat processing (26, 27). The present findings do not support this suggestion, since repeated performance on the dot-probe task did not enhance attention control, nor did it reduce anxiety. Specifically, in the nonaffective training (neutral-neutral condition), none of the participants responded clinically. Thus, our findings, despite the study's reliance on an admittedly small number of children in the neutral-neutral condition, suggest that greater clinical effects follow from training in specific attention-related contingencies than from nonaffective training. Future ABM studies applying training contingencies to nonaffective stimuli (e.g., geometric forms) in considerably larger samples could more definitively address this open issue.
Although the behavioral findings indicate numeric reductions in threat bias in the training condition and two control conditions, only the ABM group showed a significant reduction in threat bias. This training effect did not generalize to the novel faces stimuli, despite some suggestive findings in an exploratory post hoc comparison of the ABM group with the placebo group. Larger studies are needed to address the issue of stimulus generalization.
The results should be considered in light of randomized controlled trial data for adults (3–5). Specifically, there are various technical aspects that might have attenuated the full potential of this ABM trial. First, we used a side-by-side presentation of the face stimuli, whereas in adult trials, vertical presentations generated the largest effects on anxiety (8). Second, the adult studies targeted patients with a specific anxiety disorder (generalized anxiety disorder [3] or social phobia [4, 5]). This allowed tailoring of the training stimuli to the specific anxiety disorder being targeted for treatment. In contrast, the present study enrolled participants with any one of various diagnoses, which is the current standard in randomized controlled trials of pediatric anxiety disorders given the high rates of comorbidity in children. Moreover, face stimuli were used for all participants, regardless of diagnosis; studies of adults tend to use face stimuli for social phobia but word stimuli for other disorders. This could have attenuated the effect of ABM on clinical changes in the present study. Future ABM studies of pediatric anxiety disorders may benefit from a more stimulus-specific approach. Third, the studies of adults used eight biweekly training sessions with a small number of training trials per session (N=160), whereas we applied four weekly sessions with a larger number of trials per session (N=480). This difference in protocol may have affected learning efficacy in our study relative to that in the adult studies (28). Where possible, future randomized controlled trials could attempt formats more similar to those in the adult studies (a standard protocol and software scripts for ABM trials are available upon request from Y.B.). Finally, as in the adult randomized controlled trials, some nonsignificant reduction in symptom counts and severity was observed in our placebo condition. The effect sizes of these clinical improvements are quite large (Cohen's d values, 0.95 and 0.94), indicating that studies with greater statistical power should further monitor the clinical effects of the placebo condition.
Two expected results did not materialize in the present study. First, there were no specific ABM effects on the revised Screen for Child Anxiety Related Emotional Disorders measures. Although both child- and parent-reported scores on this questionnaire significantly correlated with the symptom counts on the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions that were derived from the clinical interviews (range: 0.36–0.74, all p values <0.05), participants from all training conditions self-reported significant reductions in anxiety after treatment. It may be the case that the self-reports are more vulnerable to expectancy or other features related to placebo treatment effects, relative to the structured diagnosis of the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions that is determined by trained clinicians. Second, the moderated mediation analysis failed to establish the expected path between change in attention bias as a function of training condition and change in anxiety symptom counts and severity, which is likely a result of a small sample size. The demonstration of mediation effects in ABM treatment studies has proved elusive (e.g., reference 4), perhaps because of low power in the extant randomized controlled trials. Studies with considerably larger numbers of patients are needed to resolve this issue.
Our results reflect the potential efficacy of ABM in reducing anxiety symptoms in children who have a priori bias toward threat. Future studies may examine the effect of ABM in anxious children with other threat bias profiles. Previous studies (29–31) and our own assessment during the intake procedure reveal that only about one-half of clinically anxious children show a bias toward threat at baseline. The present trial began before publication of many recent reports on ABM. At that time—and arguably even now—it seemed safest to enroll only children who manifested a pretreatment threat bias, unless these children were receiving other known effective treatments, such as CBT. However, recent work suggests that ABM could benefit all anxious children, regardless of their initial bias. This issue of variability in baseline attention bias in anxious individuals was not addressed in the adult trials (3–5), which most certainly included participants with an initial bias away from threat. This raises questions about the mechanism that caused reduction in anxiety symptoms in anxious patients. Finally, if attention profiles at intake are to be considered as a criterion for the provision of ABM treatment, further standardization of attention assessment is needed (7).
In conclusion, our randomized controlled trial using ABM in the treatment of pediatric anxiety disorders generated findings worthy of further study. Although the study's small sample size suggests the importance of replication in larger samples, our findings indicate that ABM reduces pediatric anxiety symptoms and symptom severity. ABM may have several advantages for the treatment of anxiety in children, including the possibility of home-based administration of the intervention and a computer-based interface that may bring therapy into the intuitive lifestyle of many children, thereby improving treatment compliance and opening up the possibility of reaching patients who do not have access to standard therapies. Alternatively, ABM could be easily integrated into the extant CBT toolbox. Further research is needed to elucidate the cognitive mechanisms responsible for the anxiolytic effect of ABM.
1. : Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull 2007; 133:1–24Crossref, Medline, Google Scholar
2. : A cognitive-motivational analysis of anxiety. Behav Res Ther 1998; 36:809–848Crossref, Medline, Google Scholar
3. : Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol 2009; 118:28–33Crossref, Medline, Google Scholar
4. : Attention training in individuals with generalized social phobia: a randomized controlled trial. J Consult Clin Psychol 2009; 77:961–973Crossref, Medline, Google Scholar
5. : Attention training for generalized social anxiety disorder. J Abnorm Psychol 2009; 118:5–14Crossref, Medline, Google Scholar
6. : Whither cognitive bias modification research? commentary on the special section articles. J Abnorm Psychol 2009; 118:89–99Crossref, Medline, Google Scholar
7. : Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry 2010; 51:859–870Crossref, Medline, Google Scholar
8. : Attention bias modification treatment: a meta-analysis towards the establishment of novel treatment for anxiety. Biol Psychiatry 2010; 68:982–990Crossref, Medline, Google Scholar
9. : The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64Crossref, Medline, Google Scholar
10. : Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology 2009; 34:213–228Crossref, Medline, Google Scholar
11. : Systematic review of the efficacy of cognitive behaviour therapies for childhood and adolescent anxiety disorders. Br J Clin Psychol 2004; 43:421–436Crossref, Medline, Google Scholar
12. : Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359:2753–2766Crossref, Medline, Google Scholar
13. the
14. : Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry 2007; 48:631–648Crossref, Medline, Google Scholar
15. : Plasticity in attention: implications for stress response in children. Behav Res Ther 2008; 46:450–461Crossref, Medline, Google Scholar
16. : Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol 2002; 111:107–123Crossref, Medline, Google Scholar
17. : Training to disengage attention from threat reduces vulnerability to stress in anxious children. J Child Psychol Psychiatry 2011; 52:861–869Crossref, Medline, Google Scholar
18. : The Anxiety Disorders Interview Schedule for Children for DSM-IV–Child and Parent Versions. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
19. : Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV–Child and Parent versions. J Am Acad Child Adolesc Psychiatry 2001; 40:937–944Crossref, Medline, Google Scholar
20. : The revised version of the Screen for Child Anxiety Related Emotional Disorders (SCARED-R): factor structure in normal children. Person Individ Diff 1999; 26:99–112Crossref, Google Scholar
21. : Rating scales to assess depression in school-aged children. Acta Paedopsychiatr 1981; 46:305–315Medline, Google Scholar
22. : The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168:242–249Crossref, Medline, Google Scholar
23. : Attention training for threatening facial expressions in anxiety: manipulation of stimulus duration. Cogn Emotion 1998; 12:737–753Crossref, Google Scholar
24. : Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multi Behav Res 2007; 42:185–227Crossref, Medline, Google Scholar
25. : Anxiety and cognitive performance: attentional control theory. Emotion 2007; 7:336–353Crossref, Medline, Google Scholar
26. : How do emotion and motivation direct executive control? Trends Cogn Sci 2009; 13:160–166Crossref, Medline, Google Scholar
27. : Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A 2002; 99:11458–11463Crossref, Medline, Google Scholar
28. : A link between perceptual learning, adaptation and sleep. Vision Res 2006; 46:4071–4074Crossref, Medline, Google Scholar
29. : An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol Med 2001; 31:541–547Crossref, Medline, Google Scholar
30. : Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry 2005; 162:291–296Link, Google Scholar
31. : Biases in visual attention in children and adolescents with clinical anxiety and mixed anxiety-depression. J Abnorm Child Psychol 1999; 27:215–223Crossref, Medline, Google Scholar



