Allelic Differences Between Han Chinese and Europeans for Functional Variants in ZNF804A and Their Association With Schizophrenia
Abstract
Objective:
ZNF804A is a schizophrenia risk gene that was recently identified by genome-wide association studies as well as subsequent replications. Although the results are consistent among studies in European populations, there have been conflicting reports in Chinese populations. The authors conducted both association and functional analyses to test whether ZNF804A is a risk gene for schizophrenia in Chinese populations.
Method:
The authors recruited two case-control samples of independent Han Chinese (a total of 2,207 participants) from southwestern China. A total of six single-nucleotide polymorphisms (SNPs), including the key SNP (rs1344706) that showed significant association with schizophrenia in European populations and the other five promoter SNPs of ZNF804A, were tested. Based on the results of the association analysis, the authors performed two functional assays to test the impact of the risk SNP on transcriptional factor binding affinity and promoter activity.
Results:
The SNP rs1344706 was not associated with schizophrenia in either of the two Han Chinese groups, and this result was confirmed by meta-analyses in five Han Chinese samples. However, the authors identified two ZNF804A promoter SNPs that were significantly associated with schizophrenia in both samples, and the significance was strengthened in the combined samples and further supported by haplotype analysis. The functional assays demonstrated that the risk SNP (rs359895) can influence Sp1 binding affinity, resulting in a higher promoter activity of the risk allele.
Conclusions:
Our results suggest that ZNF804A is a common risk gene for schizophrenia in world populations and that the newly identified functional SNP (rs359895) is likely a risk SNP for schizophrenia.
Schizophrenia is a severe and common neuropsychiatric disorder with an estimated lifetime prevalence approaching 1% worldwide (1). The essential characteristics of this disorder include various psychotic symptoms such as delusions and hallucinations, affective response, social withdrawal, apathy, and cognitive impairment (2). Family, twin, and adoption studies have unequivocally shown a strong genetic component in schizophrenia, with heritability estimates of about 80% (3). Despite such high heritability of schizophrenia, the underlying genetic risk factors have yet to be identified. Numerous genetic association studies of schizophrenia have been reported, and numerous candidate genes have been proposed through linkage analyses, candidate gene association studies, and genome-wide association studies (GWAS) (4–7). Many of them, however, await satisfactory replications in different populations and functional verification of the relevant genetic variants.
ZNF804A, a novel schizophrenia susceptibility gene on chromosome 2q32.1, was first identified by GWAS in U.K. populations with large-scale follow-up replications (8). The risk single-nucleotide polymorphism (SNP), rs1344706, achieved genome-wide significance, and it was further confirmed by subsequent independent replication studies as well as meta-analyses in major populations (9–14). Through fine mapping analysis, Williams et al. (10) observed multiple common SNPs within ZNF804A that were strongly associated with schizophrenia. Meanwhile, Steinberg et al. (9) searched for large copy number variations in 5,408 psychiatric patients (including those with schizophrenia, bipolar disorder, anxiety, and depression) and 39,481 healthy comparison subjects and identified three copy number variations covering part of ZNF804A in psychiatric patients but not in comparison subjects (p=0.0016). Thus, ZNF804A is a promising risk gene for schizophrenia, especially in European populations.
Studies of Chinese populations, however, have been inconsistent. O'Donovan et al. (8) and Steinberg et al. (9) failed to replicate the association of rs1344706 with schizophrenia in Han Chinese (p=0.166 and p=0.62, respectively), but Zhang et al. (11) did replicate the association (p=0.00083). This suggests that either ZNF804A may not be a risk gene for schizophrenia in the Chinese population, or there are other risk SNPs. Additionally, it was reported that when considering disease status, the expression of ZNF804A was higher in patients than it was in comparison subjects but was not significantly different (p=0.107). When considering rs1344706 allele status in comparison samples only, expression was significantly higher from the associated allele (p=0.033) (13). We speculate that those SNPs located in the promoter region of ZNF804A may affect ZNF804A expression and eventually contribute to the pathogenesis of schizophrenia.
To test whether ZNF804A is a risk gene for schizophrenia in the Chinese population, we first examined rs1344706 in two independent case-control samples collected from southwestern China. We then tested five ZNF804A promoter SNPs (rs359895, rs10497655, rs13026173, rs11888068, and rs1021042) in these samples, in which we observed two SNPS (rs1021042 and rs359895) that were significantly associated with schizophrenia. Finally, we investigated the functional impact of rs359895 on transcription factor binding affinity and promoter activity.
Method
Participants
We recruited two case-control samples independently from the southwestern Chinese cities of Yuxi (502 patients with a mean age of 38.5 years [SD=10.4]; 694 comparison subjects with a mean age of 37.1 years [SD=6.8]) and Kunming (404 patients with a mean age of 36.3 years [SD=8.7]; 607 comparison subjects with a mean age of 36.6 years [SD=7.0]). The patients were diagnosed with schizophrenia according to ICD-10 (Yuxi) or DSM-IV (Kunming) criteria. We collected detailed information on the course of the clinical disorder, age at onset, symptoms, and family history of psychiatric illnesses. Potential participants with a history of alcoholism, epilepsy, neurological disorders, or drug abuse were excluded from the study. Meanwhile, unrelated healthy volunteers were recruited from the local communities as comparison subjects. These individuals were asked to provide detailed information about their medical and family psychiatric histories. Those who had a history of psychiatric disorders, psychiatric treatment, alcohol dependence, or drug abuse or a family history of psychiatric disorders were excluded.
The patients and comparison subjects were of Han Chinese origin from the Yunnan province of southwestern China, and they were all unrelated. All participants provided written informed consent, and the research protocol was approved by the internal review board of the Kunming Institute of Zoology at the Chinese Academy of Sciences.
SNP Selection
SNP selection was based on previous studies and our HapMap data analysis (8–13). For the initial screening in the Yuxi sample, a total of six SNPs were selected. We first selected rs1344706, which has been identified by GWAS in European populations (8). We then examined the linkage disequilibrium pattern of the ZNF804A promoter region (about 10 kb) in the Chinese population using data from HapMap. We initially selected five tagging SNPs spanning the promoter region of ZNF804A (rs1021042, rs13026173, rs359895, rs10497655, and rs359894) with the use of the tagger procedure implemented in Haploview (15). Since the minor allele frequency of rs359894 is less than 0.05 in Han Chinese (minor allele frequency=0.022, shown in HapMap), we excluded this SNP from further analysis and selected another one (rs11888068) close to rs359894. All the selected SNPs are biallelic with the minor allele frequency greater than 5%. In total, six SNPs were selected for screening the Yuxi sample (see Figure S1 in the data supplement that accompanies the online edition of this article). For the replication analysis in the Kunming sample, three SNPs were tested, including rs1344706 and the two SNPs (rs359895 and rs1021042) that showed significant associations with schizophrenia in the Yuxi sample. The linkage disequilibrium map of the tested SNPs in the Yuxi sample is shown in Figure 1, and the linkage disequilibrium maps of the ZNF804A promoter SNPs downloaded from the HapMap database are shown in Figure S1 and Figure S2 in the online data supplement. The SNP information is shown in Table 1.
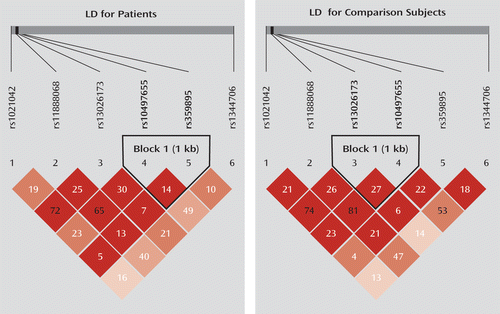
FIGURE 1. Linkage Disequilibrium (LD) Maps of the Six Single Nucleotide Polymorphisms (SNPs) in the Yuxi Samplea
a The linkage disequilibrium of the tested SNPs was calculated using the r2 algorithm by the Haploview program.
| Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | SNP ID | Position | Allele | Patients | Comparison Subjects | p (nominal) | p (corrected) | Odds Ratio | 95% CI |
| Yuxi sample | rs1021042 | 185453158 | G | 0.2814 | 0.2241 | 1.5×10–3 | 7.0×10–3 | 1.36 | 1.12–1.64 |
| rs11888068 | 185460295 | T | 0.4327 | 0.4654 | 0.11 | 0.37 | 0.88 | 0.74–1.03 | |
| rs13026173 | 185460842 | C | 0.2802 | 0.2334 | 0.010 | 0.041 | 1.28 | 1.06–1.54 | |
| rs10497655 | 185462041 | C | 0.4746 | 0.4863 | 0.58 | 0.97 | 0.95 | 0.81–1.12 | |
| rs359895 | 185463185 | A | 0.1573 | 0.2197 | 1.4×10–4 | 7.0×10–4 | 0.66 | 0.54–0.82 | |
| rs1344706 | 185778428 | G | 0.4939 | 0.4971 | 0.88 | 1.0 | 0.99 | 0.84–1.16 | |
| Kunming sample | rs1021042 | 185453158 | G | 0.3284 | 0.2544 | 4.7×10–4 | 9.0×10–4 | 1.43 | 1.17–1.76 |
| rs359895 | 185463185 | A | 0.1725 | 0.2199 | 9.3×10–3 | 0.023 | 0.74 | 0.59–0.93 | |
| rs1344706 | 185778428 | G | 0.5000 | 0.4843 | 0.49 | 0.84 | 1.07 | 0.89–1.27 | |
| Combined sample | rs1021042 | 185453158 | G | 0.3019 | 0.2378 | 2.5×10–6 | 3.0×10–6 | 1.39 | 1.21–1.60 |
| rs359895 | 185463185 | A | 0.1641 | 0.2198 | 5.0×10–6 | 1.0×10–5 | 0.70 | 0.60–0.81 | |
| rs1344706 | 185778428 | G | 0.4966 | 0.4911 | 0.72 | 0.97 | 1.02 | 0.91–1.15 | |
TABLE 1. Allele Frequency and Single Nucleotide Polymorphism (SNP) Association Analysis in Two Chinese Samples
SNP Genotyping
Venous blood was collected from all participants, and genomic DNA was extracted from the blood sample using the standard phenol-chloroform method. DNA samples of the patients and comparison subjects were randomly distributed in the DNA sample plates. All the selected SNPs were genotyped by the SNaPShot method as described in our previous study (16). Details of all primers and assay conditions are available on request. The SNP genotype calls were automatically performed using GeneMapper 4.0 (Applied Biosystems) and verified manually. To make sure of the accuracy of genotyping, we conducted bidirectional sequencing of 100 randomly selected individuals, and no genotyping errors were observed. The genotyping success rate for the six tested SNPs was 98.4%.
Electrophoretic Mobility Shift Assay
For functional prediction of the candidate SNPs, we used the online software program AliBaba ( www.gene-regulation.com/pub/programs.html#alibaba2) to predict the DNA-binding motifs in the ZNF804A promoter region. A 100% match to the matrix yields a maximum score of 1.00, and a matrix similarity score greater than 0.80 is considered a good match.
Electrophoretic mobility shift assay was performed using the gel shift assay system (Pierce Protein Research, Rockford, Ill.) under the guidelines provided. The single-strand oligonucleotides were 3′-end biotin labeled and then annealed to form double strands. The binding reaction contained purified recombinant Sp1 protein (Alexis Biochemicals, San Diego), 10× binding buffer, and, if needed, unlabeled competitors; the labeled probes were added after 20 minutes, and samples were incubated at room temperature for a total of 30 minutes. After incubation, samples were separated on a native 6% polyacrylamide gel and then transferred to a nylon membrane. The positions of biotin end-labeled oligonucleotides were detected by a chemiluminescent reaction with streptavidin-horseradish peroxidase. The nucleotide sequences of the double-stranded oligonucleotides with either A or T allele were:
A allele: 5′-GTATCAGCCCAGTGGCTCCCAGCCATTGGCTCAGTGCAATG-3′
T allele: 5′-GTATCAGCCCAGTGGCTCCCTGCCATTGGCTCAGTGCAATG-3′
Luciferase Reporter Assay
To construct ZNF804A promoter, we amplified fragments encompassing nucleotides from –1089 base pairs (bp) to +65 bp (relative to transcription start site +1) from two individual homozygotes with respect to the corresponding genotypes (TT and AA) for rs359895. Then the amplified fragments were cloned into the pGL3-basic plasmid vectors. We verified all recombinant clones by bidirectional DNA sequencing to make sure no de novo mutation was introduced. These plasmids were all accurately quantified with an Eppendorf (Hamburg) BioPhotometer, and equal amounts of the plasmids were used for transfection. The reporters containing either T allele or A allele were transiently cotransfected into HEK293T and HeLa cells together with pRL-TK plasmid (a standard reporter). After a 36-hour incubation, we collected the cells and measured luciferase activity using the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, Wisc.). All assays were performed in at least three independent experiments with a minimum of five replications.
Statistical Analysis
We tested for Hardy-Weinberg equilibrium using Haploview 4.1 by examining the genotypic distributions of ZNF804A SNPs in each sample. All of the SNPs genotyped in this study were in Hardy-Weinberg equilibrium. Linkage disequilibrium between paired SNPs was estimated by Haploview using the r2 algorithm. Allelic and genotypic associations were accessed with PLINK (17). Since the SNPs may not be totally independent because of linkage disequilibrium, to avoid type II error when applying the Bonferroni correction, we corrected the p values with a max(T) permutation procedure implemented in PLINK, using the “–mperm” option (N=1,000,000), which takes a single parameter (the number of permutations to be performed). This is achieved by comparing each observed test statistic against the maximum of all permuted statistics (i.e., over all SNPs) for each single replicate. We calculated significance for the combined samples (the Yuxi and Kunming samples) using the Cochran-Mantel-Haenszel test and conditioning by site as implemented in PLINK. The 95% confidence intervals (CIs) of odds ratios were calculated with an online tool (http://faculty.vassar.edu/lowry/odds2x2.html). For meta-analysis of rs1344706 in the five Han Chinese samples independently collected from Shanghai, Xi'an, Sichuan, Yuxi, and Kunming (from O'Donovan et al. [8], Steinberg et al. [9], Zhang et al. [11], and the present study), because there is genetic heterogeneity caused by the data from Zhang et al. (11), we used the Mantel-Haenszel method with the random-effects model. For the meta-analysis excluding the sample from Zhang et al. (11), we used the Mantel-Haenszel method with the fixed-effects model, and the analysis was conducted in RevMan, version 4.2 (http://ims.cochrane.org/revman/download/revman-4). Haplotype frequencies and association analysis were estimated using the HaploStats Package in the R environment (18). Correction for multiple haplotypes and global analysis were performed with 20,000 permutations (18). The power analysis was performed using the G*power software program (19). To avoid the false positive association caused by potential population stratification, we used the software program Structure to perform population stratification analysis in our samples (20, 21). For the reporter gene assay data analysis, we used two-tailed t tests to conduct statistical assessment.
Results
Association of ZNF804A With Schizophrenia
The single SNP analysis results of the six SNPs are presented in Table 1 and in Table S1 in the online data supplement. In the Yuxi sample, significant differences (p<0.01) in allele frequencies between patients and comparison subjects were detected for two SNPs after permutation correction (rs1021042, p=7.0×10–3; rs359895, p=7.0×10–4). In the replication Kunming sample, we successfully confirmed the associations (rs1021042, p=9.0×10–4; rs359895, p=0.023, corrected). When the two samples were combined, the significances were strengthened (rs359895, p=1.0×10–5; rs1021042, p=3.0×10–6, corrected). No heterogeneity was detected in the combined samples (rs1021042, χ2=0.15, p=0.69; rs359895, χ2=0.52, p=0.47; rs1344706, χ2=0.38, p=0.54). The analysis of population stratification indicated that there was no obvious population stratification between patients and comparison subjects in both samples (see Figure S3 in the online data supplement). The analysis using genotype data indicated similar results (see Table S1 in the online data supplement).
The association of ZNF804A with schizophrenia was further supported by the results of the haplotype analysis. For the haplotypes incorporating the two SNPs that were significantly associated with schizophrenia (rs1021042 and rs359895), we observed significant associations both in the Yuxi sample (global p<1.0×10–5, corrected) and in the Kunming sample (global p=4.0×10–4, corrected). In the combined sample, we identified a risk haplotype (T-G, p<1.0×10–7, 29.37% in patients and 22.42% in comparison subjects) and a protective haplotype (A-C, p<1.0×10–6, 15.66% in patients and 20.68% in comparison subjects) that was significantly associated with schizophrenia. Global tests also supported the associations of these haplotypes with schizophrenia (global p<1.0×10–8, corrected; Table 2).
| Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Haplotype | Schizophrenia | Comparison Subjects | p (Nominal) | p (Corrected) | Odds Ratio | 95% CI | Global p (Nominal) | Global p (Corrected) |
| Yuxi sample | A-C | 0.1524 | 0.2086 | 2.0×10–4 | <1.0×10–4 | 0.73 | 0.58–0.92 | <1.0×10–5 | <1.0×10–5 |
| A-G | 0.0051 | 0.0112 | 0.28 | 0.30 | 0.49 | 0.11–2.10 | |||
| T-C | 0.5670 | 0.5674 | 0.87 | 0.87 | 1.00 | ||||
| T-G | 0.2756 | 0.2129 | 4.0×10–4 | 4.0×10–4 | 1.32 | 1.07–1.64 | |||
| Kunming sample | A-C | 0.1624 | 0.2040 | 8.0×10–3 | 7.0×10–3 | 0.83 | 0.63–1.07 | 5.0×10–4 | 4.0×10–4 |
| A-G | 0.0111 | 0.0159 | 0.70 | 0.70 | 0.84 | 0.24–2.99 | |||
| T-C | 0.5083 | 0.5427 | 0.22 | 0.23 | 1.00 | ||||
| T-G | 0.3183 | 0.2373 | 9.0×10–5 | <1.0×10–4 | 1.46 | 1.16–1.83 | |||
| Combined sample | A-C | 0.1566 | 0.2068 | <1.0×10–6 | <1.0×10–6 | 0.77 | 0.65–0.91 | <1.0×10–8 | <1.0×10–8 |
| A-G | 0.0080 | 0.0131 | 0.30 | 0.31 | 0.66 | 0.26–1.68 | |||
| T-C | 0.5417 | 0.5560 | 0.52 | 0.52 | 1.00 | ||||
| T-G | 0.2937 | 0.2242 | <1.0×10–7 | <1.0×10–7 | 1.37 | 1.18–1.61 | |||
TABLE 2. The rs359895-rs1021042 Haplotype Association Analysis in the Yuxi and Kunming Samples
In contrast, the reported association of rs1344706 in GWAS (8) was not replicated either in the Yuxi sample (p=1.00, corrected) or in the Kunming sample (p=0.84, corrected). The same results were obtained when the men and women were analyzed separately for rs1344706 (p=0.96 in women and p=1.00 in men, corrected). We then performed a meta-analysis for rs1344706 in the five Han Chinese samples (a total of 2,892 cases and 3,333 comparison subjects after including three previous studies [8, 9, 11] and the present study), and the result remained nonsignificant (p=0.38, odds ratio=1.05, 95% CI=0.94–1.18; see Table S2 in the online data supplement). Since the test of heterogeneity for rs1344706 in the five Chinese samples indicated a marginal significance (p=0.03) introduced by the data from Zhang et al. (11), we performed the meta-analysis that excluded the samples from Zhang et al. (2,326 patients and 2,759 comparison subjects remained), and no heterogeneity was detected (p=0.63). The association was not significant (p=0.87, odds ratio=1.01, 95% CI=0.93–1.09; see Table S3 in the online data supplement).
We also performed a power analysis using the G*power program (19) based on Cohen's method (22). The Yuxi and Kunming sample sizes revealed >93% and >88% power to detect a significant association (α<0.05), respectively, given an effect size index of 0.1 (which corresponds to a weak gene effect). When an effect size index of 0.2 (a weak to moderate gene effect) was assumed, the Yuxi and Kunming sample sizes showed >98% power to detect significance (α<0.05).
rs359895 Alters the Binding Affinity of Transcription Factor Sp1
We conducted a functional prediction analysis of the two significant promoter SNPs and found one SNP (rs359895) that showed potential functional effects. The SNP rs359895 resides in the core promoter region (about –500 bp from the transcription start site) of ZNF804A, and it is located in a putative binding site of the common transcriptional factor Sp1 (Figure 2A). The change from A to T at rs359895 may influence the binding affinity of Sp1 and promoter activity. Thus, rs359895 is likely a functional SNP.

FIGURE 2. rs359895 Allele Variation Affects Sp1 Bindinga
a Panel A shows the oligonucleotides for testing the binding activity of Sp1. The predicted binding sequence is underlined and contains the rs359895 variation site (boxed). Panel B shows lanes 1–9. Lane 1: negative control; lanes 2 and 3: the probes containing the T allele and the A allele can both bind Sp1, but the binding affinity is stronger for the T allele; lanes 4 and 5: the competition assay showing the binding specificity of the probes to Sp1; lanes 6–9: the relative binding strength tested by the competition assay using increasing concentrations of the unlabeled probes containing the opposite alleles.
To verify the functional consequences of rs359895, we performed an electrophoretic mobility shift assay with purified recombinant human Sp1 protein and the labeled double-stranded oligonucleotides containing either the T allele or the A allele. The electrophoretic mobility shift assay analysis showed that the predicted binding sequence containing the T allele of rs359895 had a higher binding affinity than the A allele (Figure 2B, lanes 2–3). The addition of 100× molar excess of unlabeled corresponding probes indicated the specific binding of Sp1 to the predicted binding sequence (Figure 2B, lanes 4–5). A cross-competition experiment further confirmed the observation of higher binding affinity of the probe containing the T allele (Figure 2B, lanes 6–9).
The T Allele of rs359895 Increases the Promoter Activity of ZNF804A
To test whether rs359895 influences the ZNF804A promoter activity, we designed a reporter gene assay. Two fragments containing either the T allele or the A allele were cloned into the pGL3 vector, and the promoter activities were determined in HEK293T and HeLa cell lines. As expected, the transcriptional activity of the ZNF804A promoter containing the T allele of rs359895 was significantly higher than that of the A allele in the HeLa (p=1.0×10–7) and HEK293T (p=2.3×10–5) cell lines (Figure 3). Collectively, these data indicate that rs359895 is likely a functional SNP and that the sequence change at this site can influence the expression of ZNF804A. Since our computer-aided analysis did not reveal any potential function for rs1021042, whether it plays any functional role for ZNF804A is yet to be determined.
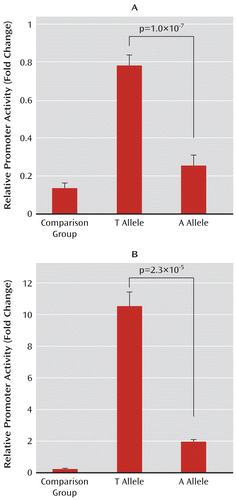
FIGURE 3. Results of the Reporter Gene Assay Testing the Promoter Activitiesa
a Effects of rs359895 allele variation on the ZNF804A promoter activity are shown in the panels for HeLa (panel A) and HEK293T cells (panel B). The values represent fold changes of luciferase activity relative to the control pGL3 vector. The means and standard deviations of at least three independent experiments are shown.
Discussion
In 2008, O'Donovan et al. (8) undertook a GWAS of 479 U.K. schizophrenia patients and 2,937 comparison subjects, with follow-up replications in approximately 17,000 subjects, and identified rs1344706 within ZNF804A as significantly associated with schizophrenia. Subsequently, the association of rs1344706 with schizophrenia was consistently reported by the International Schizophrenia Consortium, the Irish Case-Control Study of Schizophrenia, and the SGENE-plus Consortium (9, 13, 23). Further investigations have indicated that there are allelic differences of rs1344706 in ZNF804A expression and nuclear protein binding affinity (10, 13, 24). Since it is located in the intron region, however, there is still the possibility that rs1344706 is a tagging SNP linked to the causative variant at this locus. To address this, Williams et al. (10) sought to localize the association signal at this locus through a process of genomic resequencing and fine-scale mapping. After detailed association analysis, rs1344706 remained one of the most strongly associated markers in ZNF804A. In addition, a meta-analysis of rs1344706 in 18,945 schizophrenia patients and 38,675 comparison subjects (10) again supported the association between rs1344706 and schizophrenia (p=2.5×10–11). These data have strongly indicated that rs1344706 is a promising risk SNP for schizophrenia, especially in European populations.
In contrast, the association of rs1344706 with schizophrenia in Han Chinese is inconsistent among studies. No significant association between rs1344706 and schizophrenia in Han Chinese was observed in the replication studies by O'Donovan et al. (8) and Steinberg et al. (9), while Zhang et al. (11) reported significant associations (p<0.001). In the two independent samples tested in our study, we failed to detect significance for rs1344706, and this finding was further confirmed by the meta-analyses of Chinese populations (p=0.38). These analyses suggest that rs1344706 is probably not a risk SNP for schizophrenia in Han Chinese.
Notably, the T allele frequency of rs1344706 in our comparison subjects was 0.509, which is similar to the frequencies reported by O'Donovan et al. (frequency=0.514) and Steinberg et al. (frequency=0.546) as well as the frequency in Han Chinese from the HapMap database (frequency=0.524). It is quite different, however, from the frequency reported by Zhang et al. (frequency=0.457), implying that there is either sampling bias in Zhang et al. (11) or potential genetic heterogeneity among regional Han Chinese populations as reflected in the results of heterogeneity tests in the meta-analysis. Moreover, the inconsistent association of rs1344706 with schizophrenia between Chinese and European populations also suggests genetic heterogeneity of ZNF804A sequence variations among continental populations. The population-specific factors (population history, differences in genetic structure, environmental exposure, diet, and culture) may play an important role in the observed inconsistency of replications.
We identified a novel functional SNP (rs359895, minor allele frequency=0.2198) that is strongly associated with schizophrenia in two independent Han Chinese samples (p=1.0×10–5), and the haplotype analysis further supported these associations (global p<1.0×10–8). Our functional assays indicated that rs359895 can influence the affinity of the Sp1 binding sequence and that the T allele has a higher binding affinity to Sp1, leading to a higher promoter activity and higher ZNF804A expression than the A allele. This is consistent with previous findings in which the risk allele of rs1344706 showed significantly higher ZNF804A expression in comparison subjects (p=0.033), and an elevated expression was detected in the prefrontal cortex of schizophrenia patients relative to comparison subjects, although it did not reach significance (13). Additionally, another newly identified SNP (rs1021042) was significantly associated with schizophrenia in our samples (p=3.0×10–6), but it showed no potential function based on the prediction. The SNP rs1021042 is in low linkage disequilibrium with rs359895 in both the patients and comparison subjects as well as in the HapMap Han Chinese in Beijing (CHB) (r2≤0.05), indicating that the association of rs1021042 with schizophrenia is likely independent.
The previous fine-mapping study of ZNF804A by Williams et al. (10) reported many SNPs that are strongly associated with schizophrenia; however, rs359895 and rs1021042 were not tested. Interestingly, according to the HapMap data, there is a large heterogeneity for rs359895 among continental populations. The T (risk) allele of rs359895 is prevalent in East Asian populations (frequency=0.800 in Chinese and frequency=0.920 in Japanese), but much less frequent in Europeans (frequency=0.408) and Africans (frequency=0.217), and the linkage disequilibrium structures of the ZNF804A promoter region also differ among major populations (see Figure S2 in the online data supplement). Therefore, whether rs359895 is also associated with schizophrenia in European populations has yet to be tested.
Despite many genetic association studies, the function of ZNF804A is still unknown. ZNF804A, consisting of four exons and encoding a protein of 1,210 amino acids, is expressed in the brain (25). The amino acid sequence contains a C2H2-type domain characteristic of the classical zinc-finger (ZnF) family of proteins, which may interact with other types of molecules and have many roles in cellular function (25). Interestingly, the mouse homologue of ZNF804A, zfp804a, has recently been reported as a target for HOXC8, suggesting that ZNF804A may be involved in the regulation of early neurodevelopment (26). Additionally, recent investigations have indicated ZNF804A may also act on neural activation and cognitive performance (12, 27–29).
The data we present here are limited, and we are cautious in the interpretation of our results. First, we tested only the ZNF804A promoter SNPs, and there are other ZNF804A risk SNPs in Chinese populations that have yet to be studied, although the importance of promoter regions in identifying risk genetic variants for schizophrenia has been verified (30, 31). Second, although we tested two independent samples (more than 2,000 individuals) in this study, our sample size is still relatively small compared with the current large-scale genetic studies (8–10, 32), and the association of rs359895 needs to be tested in more samples.
1. : Incidence worldwide of schizophrenia. Br J Psychiatry 1987; 151:408–409Crossref, Medline, Google Scholar
2. : Symptoms, signs, and diagnosis of schizophrenia. Lancet 1995; 346:477–481Crossref, Medline, Google Scholar
3. : Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373:234–239Crossref, Medline, Google Scholar
4. : Association of neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry 2007; 12:94–104Crossref, Medline, Google Scholar
5. : A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 2002; 71:1296–1302Crossref, Medline, Google Scholar
6. : Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1). Arch Gen Psychiatry 2004; 61:336–344Crossref, Medline, Google Scholar
7. : Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol Psychiatry 2004; 9:1037–1041Crossref, Medline, Google Scholar
8. : Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 2008; 40:1053–1055Crossref, Medline, Google Scholar
9. : Expanding the range of ZNF804A variants conferring risk of psychosis. Mol Psychiatry 2011; 16:59–66Crossref, Medline, Google Scholar
10. : Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry 2011; 16:429–441Crossref, Medline, Google Scholar
11. : Population-based and family-based association studies of ZNF804A locus and schizophrenia. Mol Psychiatry 2011; 16:360–361Crossref, Medline, Google Scholar
12. : Psychosis susceptibility gene ZNF804A and cognitive performance in schizophrenia. Arch Gen Psychiatry 2010; 67:692–700Crossref, Medline, Google Scholar
13. : Replication of association between schizophrenia and ZNF804A in the Irish case-control study of schizophrenia sample. Mol Psychiatry 2009; 15:29–37Crossref, Medline, Google Scholar
14. : Evidence of sex-modulated association of ZNF804A with schizophrenia. Biol Psychiatry 2011; 69:914–917Crossref, Medline, Google Scholar
15. : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21:263–265Crossref, Medline, Google Scholar
16. : Association of haplotypes spanning PDZ-GEF2, LOC728637 and ACSL6 with schizophrenia in Han Chinese. J Med Genet 2008; 45:818–826Crossref, Medline, Google Scholar
17. : PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575Crossref, Medline, Google Scholar
18. : Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 2002; 70:425–434Crossref, Medline, Google Scholar
19. : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–191Crossref, Medline, Google Scholar
20. : Association mapping in structured populations. Am J Hum Genet 2000; 67:170–181Crossref, Medline, Google Scholar
21. : Inference of population structure using multilocus genotype data. Genetics 2000; 155:945–959Medline, Google Scholar
22. : Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
23. : Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460:748–752Crossref, Medline, Google Scholar
24. : Allelic differences in nuclear protein binding at a genome-wide significant risk variant for schizophrenia in ZNF804A. Mol Psychiatry (Epub ahead of print, March 1, 2011)Crossref, Google Scholar
25. : The psychosis susceptibility gene ZNF804A: associations, functions, and phenotypes. Schizophr Bull 2010; 36:904–909Crossref, Medline, Google Scholar
26. : Mouse homologue of the schizophrenia susceptibility gene ZNF804A as a target of hoxc8. J Biomed Biotechnol (Epub ahead of print, May 25, 2010)Crossref, Google Scholar
27. : Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry 2011; 16:462–470Crossref, Medline, Google Scholar
28. : Neural mechanisms of a genome-wide supported psychosis variant. Science 2009; 324:605Crossref, Medline, Google Scholar
29. : A schizophrenia risk gene, ZNF804A, influences neuroanatomical and neurocognitive phenotypes. Neuropsychopharmacology 2010; 35:2284–2291Crossref, Medline, Google Scholar
30. : A 5′ promoter region SNP in NRG1 is associated with schizophrenia risk and type III isoform expression. Mol Psychiatry 2009; 14:741–743Crossref, Medline, Google Scholar
31. : Functional variants in the promoter region of chitinase 3-like 1 (CHI3L1) and susceptibility to schizophrenia. Am J Hum Genet 2007; 80:12–18Crossref, Medline, Google Scholar
32. : GWA study data mining and independent replication identify cardiomyopathy-associated 5 (CMYA5) as a risk gene for schizophrenia. Mol Psychiatry (Epub ahead of print, Sept 14, 2010)Google Scholar



