Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study
Abstract
Objective:
Two antidepressant medication combinations were compared with selective serotonin reuptake inhibitor monotherapy to determine whether either combination produced a higher remission rate in first-step acute-phase (12 weeks) and long-term (7 months) treatment.
Method:
The single-blind, prospective, randomized trial enrolled 665 outpatients at six primary and nine psychiatric care sites. Participants had at least moderately severe nonpsychotic chronic and/or recurrent major depressive disorder. Escitalopram (up to 20 mg/day) plus placebo, sustained-release bupropion (up to 400 mg/day) plus escitalopram (up to 20 mg/day), or extended-release venlafaxine (up to 300 mg/day) plus mirtazapine (up to 45 mg/day) was delivered (1:1:1 ratio) by using measurement-based care. The primary outcome was remission, defined as ratings of less than 8 and less than 6 on the last two consecutive applications of the 16-item Quick Inventory of Depressive Symptomatology—Self-Report. Secondary outcomes included side effect burden, adverse events, quality of life, functioning, and attrition.
Results:
Remission and response rates and most secondary outcomes were not different among treatment groups at 12 weeks. The remission rates were 38.8% for escitalopram-placebo, 38.9% for bupropion-escitalopram, and 37.7% for venlafaxine-mirtazapine, and the response rates were 51.6%–52.4%. The mean number of worsening adverse events was higher for venlafaxine-mirtazapine (5.7) than for escitalopram-placebo (4.7). At 7 months, remission rates (41.8%–46.6%), response rates (57.4%–59.4%), and most secondary outcomes were not significantly different.
Conclusions:
Neither medication combination outperformed monotherapy. The combination of extended-release venlafaxine plus mirtazapine may have a greater risk of adverse events.
Major depressive disorder is a serious, disabling, life-shortening illness with a high lifetime risk: 7%–12% for men and 20%–25% for women (1). It is often recurrent, episodes frequently last more than 2 years (i.e., are chronic) (2), and interepisode recovery is often incomplete (3). Chronic episodes and recurrent courses are associated with worse prognoses and are more likely to need longer-term treatment (4–7).
Remission is the aim of treatment (8) because patients whose depression has remitted have better functioning and a better prognosis than those without remission (5). Antidepressant medications, when used as monotherapies in placebo-controlled registration trials, typically result in 30%–35% remission rates (8). Lower remission rates (25%–30%) are reported for patients with more chronic depressions (5, 6).
Could remission rates be increased with a combination of two antidepressant medications used together as initial treatment? Other branches of medicine often employ combination treatments at the outset of chronic illness, especially for the more severely ill (9, 10). In depression treatment, when a single antidepressant medication is not effective (7, 11), a second is often added to the first, with some evidence for efficacy (12). It also appears that some antidepressant medications work for some patients but not for others. A combination of two medications might therefore increase the spectrum of patients who could benefit from the combination (13). Furthermore, an unexpected synergy between medications might produce a rapid onset of benefit, so that fewer patients would drop out of treatment, which, in turn, might enhance remission rates. From a pharmacological perspective, a combination might affect a wider range of neurotransmitter or neuromodulator systems, which would enhance efficacy for some patients (14–16). Finally, clinical experience and a few small randomized, short-term trials (13, 17, 18) suggest that some combinations can be more effective than monotherapy. On the other hand, treatment guidelines do not recommend such an approach as a first treatment step, and the risk of serious adverse events or intrusive side effects has not been fully evaluated. Thus, combining antidepressants as a first-step treatment for depression needs proper evaluation.
The Combining Medications to Enhance Depression Outcomes (CO-MED) trial was designed as a proof-of-concept study to determine whether either of two different antidepressant medication combinations would produce a higher remission rate at 12 weeks and, secondarily, after 7 months than monotherapy with a selective serotonin reuptake inhibitor (SSRI) as a first-step treatment in outpatients with chronic or recurrent major depression. We also compared the treatment effects on patient retention, side effect burden, and quality of life.
Method
Study Overview
CO-MED was a 7-month single-blind, randomized, placebo-controlled trial that compared the efficacy of each of two medication combinations with escitalopram plus placebo in a 1:1:1 ratio as first-step treatment, including acute-phase (12 weeks) and long-term continuation (total 7 months) treatment. We planned a study group of 660 outpatients with nonpsychotic major depression from six primary and nine psychiatric care sites to allow detection of roughly a 15% difference in remission rates between each combination and escitalopram-placebo (with an expected remission rate of 35%). This difference was viewed as sufficiently large to affect practice since it approximates the benefit of a single antidepressant medication over placebo in successful antidepressant registration trials (8).
Site Selection
Clinical sites were selected on the basis of our prior experience and their performance in the Sequenced Treatment Alternatives to Relieve Depression trial to ensure 1) adequate patient flow, 2) committed administrative support, 3) adequate minority representation, and 4) adequate representation of both primary and psychiatric care sites.
Recruitment
Potential participants were screened at each clinical site with each site's standard procedure (variable across sites). Most sites used two to nine questions from the Patient Health Questionnaire (19, 20). Patients identified by screening saw their study clinicians and clinical research coordinator to determine study eligibility following written informed consent.
Participants
Broad inclusion and minimal exclusion criteria ensured a reasonably representative participant group. The outpatient enrollees were 18–75 years old and met the DSM-IV-TR (21) criteria for either recurrent or chronic (current episode lasting at least 2 years) major depression according to a clinical interview and confirmed with a DSM-IV-based symptom checklist completed by the clinical research coordinator. Eligible participants had to have an index episode lasting at least 2 months and had to score at least 16 on the 17-Item Hamilton Depression Rating Scale (HAM-D) (22). Those with any psychotic illness or bipolar disorder and those in need of hospitalization were ineligible. (For a complete list of exclusion criteria, see http://clinicaltrials.gov/ct2/show/NCT00590863.)
The study protocol and all consent and study procedures were approved by the institutional review boards at the national coordinating center (University of Texas Southwestern Medical Center at Dallas), the University of Pittsburgh data coordinating center, and each participating regional center and relevant clinical site.
Baseline Data
Sociodemographic and illness features were recorded at baseline. The anxiety subscale of the HAM-D was used to establish the presence of anxious features at baseline (23). This anxiety/somatization factor, derived from a factor analysis of the HAM-D conducted by Cleary and Guy (24), includes six items from the original 17-item version: item 10 (anxiety, psychic), item 11 (anxiety, somatic), item 12 (somatic symptoms, gastrointestinal), item 13 (somatic symptoms, general), item 15 (hypochondriasis), and item 17 (insight). A HAM-D anxiety/somatization factor score of 7 or higher indicated anxiety. The HAM-D administered at baseline by research outcome assessors (not located at any clinical site) was used to define anxious features. The self-report Psychiatric Diagnostic Screening Questionnaire (25) was used to establish the presence of current axis I disorders. The Self-Administered Comorbidity Questionnaire (26) established the presence, severity, and functional impact of a range of common concurrent general medical conditions.
Antidepressant Treatment
A 12-week study period was chosen for the primary analysis to provide sufficient time for maximal dosing (if needed) and to allow most cases of depression that could remit to do so (27). Treatment visits were planned at baseline and weeks 1, 2, 4, 6, 8, 10, 12, 16, 20, 24, and 28. No washout was required, but clinicians could choose a washout period if they thought it to be clinically advisable. Dose adjustments were based on measurement-based care following an operations manual to provide personally tailored but vigorous dosing (28). Dose adjustments were based on the score on the 16-item Quick Inventory of Depressive Symptomatology—Clinician-Rated (QIDS-C) (29), which was extracted from the 30-item Inventory of Depressive Symptomatology—Clinician-Rated (IDS-C) (30), and the score on the Frequency, Intensity, and Burden of Side Effects Rating Scale (31) obtained at each visit.
Treatment was randomly assigned, stratified by clinical site according to a web-based randomization system (32). Random block sizes of three and six were used to minimize the probability of identifying the next treatment assignment. Dosing schedules were based on prior reports (33–35). Doses were increased only in the context of acceptable side effects. Participants could exit the study if unacceptable or intolerable side effects occurred and could not be resolved with dose reduction or medication treatment of the side effects.
Escitalopram plus placebo (monotherapy).
Escitalopram treatment was begun at 10 mg/day (one pill) and could be increased to 20 mg/day (two pills) at 4 weeks if the score on the QIDS-C was higher than 5 (if side effects allowed). Pill placebo was started at week 2, with the option to increase it to two pills at week 4 if the QIDS-C score was higher than 5 (if side effects allowed).
Bupropion plus escitalopram.
The dose of sustained-release bupropion was 150 mg/day initially and was increased to 300 mg/day at the week 1 visit. Escitalopram was begun at 10 mg/day at the week 2 visit. At week 4, the bupropion dose was raised to 400 mg/day (200 mg b.i.d.) and/or the escitalopram dose was raised to 20 mg/day if the score on the QIDS-C was higher than 5 (if side effects allowed). At week 6 and beyond, doses could be increased up to a maximum of 400 mg/day (200 mg b.i.d.) for bupropion and 20 mg/day for escitalopram if the QIDS-C score was above 5 (if side effects allowed).
Venlafaxine plus mirtazapine.
Treatment with extended-release venlafaxine was begun at 37.5 mg/day for 3 days and then raised to 75 mg/day. At week 1, the dose was raised to 150 mg/day. At week 2, if the score on the QIDS-C was above 5, mirtazapine was added at a dose of 15 mg/day. At week 4, if the QIDS-C score was above 5, the venlafaxine dose was raised to 225 mg/day and/or the mirtazapine dose was increased to 30 mg/day. At week 6, if the QIDS-C score was higher than 5, the mirtazapine dose could be raised to 45 mg/day, the maximum dose. At week 8, if the score was above 5, the venlafaxine dose could be raised to 300 mg/day, the maximum allowed.
Medication Blinding
The first medication given in each treatment group was open label (both participant and study personnel were unblinded), while each second medication was given in a single-blind fashion (participant only) to ensure that the participants all took two types of pills. Specifically, in the escitalopram-placebo cell, placebo administration was blinded. For the bupropion-escitalopram combination, escitalopram was blinded. For venlafaxine-mirtazapine, mirtazapine was blinded. The participants remained blinded to the second medication throughout the 7-month study. The research coordinators and physicians were not blinded, to maximize safety and facilitate informed flexible dosing decisions.
Concurrent Treatments
Only protocol antidepressant medications were allowed. Treatments with possible antidepressant effects were proscribed, as were anxiolytics, sedative-hypnotics, and depression-targeted, empirically validated psychotherapies for depression. Other therapies (e.g., supportive, couples, occupational) were allowed, as were medications for any general medical condition. Given the inhibition of the 2D6 isoenzyme by sustained-release bupropion, we alerted clinicians about nonprotocol medications (e.g., type 1C antiarrhythmics, beta-blockers) for which serum or dose adjustments might be needed. Medications to treat antidepressant medication side effects were allowed; administration was based on clinician judgment.
Research Outcomes
Outcome measures were assessed at baseline and all treatment visits. The primary outcome, symptom remission, was based on the score on the 16-item Quick Inventory of Depressive Symptomatology—Self-Report (QIDS-SR) at 12 weeks (29). The designation of remission was based on the last two consecutive measurements during the 12-week acute-phase trial to ensure that a single “good week” was not falsely signaling remission. At least one of these ratings had to be less than 6, while the other had to be less than 8. If participants exited before 12 weeks, their last two consecutive scores were used to determine remission. Those without two postbaseline measures were considered not to have remission.
Physicians were advised that participants could exit the study if they had received a maximally tolerated dose(s) for 4 or more weeks by week 8 without obtaining at least a 30% reduction from the baseline score on the QIDS-C. They could enter continuation treatment (weeks 12–28) if they had received an acceptable benefit (defined as a QIDS-C score of 9 or less by week 12) or if they reached a score of 10–13 and the clinician and participant judged the benefit to be substantial enough to recommend treatment continuation. Thus, virtually all participants entering the continuation phase had at least a 40% reduction in the QIDS-C score. If a participant exited the study at any time, a study exit form was completed. Clinical research coordinators attempted to contact all participants who did not come for a final exit visit.
Secondary outcomes included attrition, anxiety as reflected in the score on the anxiety subscale of the IDS-C (30), functioning as measured by the Work and Social Adjustment Scale (36), quality of life as measured by the Quality of Life Inventory (37), side effect burden as measured by the Frequency, Intensity, and Burden of Side Effects Rating Scale (31), and specific side effects as measured by the Systematic Assessment for Treatment Emergent Events—Systematic Inquiry (38). Manic symptoms were assessed by using the Altman Self-Rating Mania Scale (39), and cognitive and executive dysfunction was assessed by means of the Cognitive and Physical Functioning Questionnaire (40).
Statistical Analyses
Descriptive statistics, including measures of central tendency and dispersion, were computed for continuous data. Frequency distributions were estimated for categorical data.
Outcome analyses were conducted with the full group, on the basis of the intention-to-treat principle. Each combination therapy was compared to the monotherapy. To control for the overall type I error rate, a type I error rate of 0.025 was planned for the comparison of each combination treatment with monotherapy. The analytic approach for the two comparisons was identical. A chi-square test was used to compare the remission rates across the treatment groups. Fisher's exact test was used when the expected cell frequencies were less than 5. For binary outcomes (e.g., remission), bivariate logistic regression models were fit to estimate the effect of treatment on outcome. Multivariable logistic regression models were then fit to control for the effect of regional center and baseline characteristics that were not balanced across treatment groups. A similar approach was used for discrete outcomes with more than two levels, except a polytomous logistic regression model was used. For continuous outcomes, a t test was used to compare the means when distributions were normal, and the nonparametric Kruskal-Wallis test was used when distributions were nonnormal. Linear regression models were used to compare the means after controlling for regional center and baseline characteristics not balanced across treatment groups. A general linear model with a negative binomial distribution and log link was estimated for outcomes with severely nonnormal distributions (the last number of worsening adverse events indicated by the Systematic Assessment for Treatment Emergent Effects and the score on the IDS-C anxiety subscale).
Results
Baseline Characteristics
Figure 1 contains a chart specifying how the study group was formed. From March 2008 through February 2009, the study enrolled 665 participants. They were moderately to severely ill, as indicated by a mean HAM-D score of 23.8 (SD=4.8) and a score on the QIDS-SR of 15.5 (SD=4.3). About half of the participants were unemployed, and two-thirds were female (Table 1). Over three-quarters had recurrent major depression (Table 2). More than one-half of the participants were in a chronic current major depressive episode. About one-third had both recurrent and chronic depression. Almost one in 10 had made a prior suicide attempt, and for over 40% the illness had begun before age 18. Most (75%) had anxious features. Concurrent comorbid axis I and axis III disorders were common (Table 3).
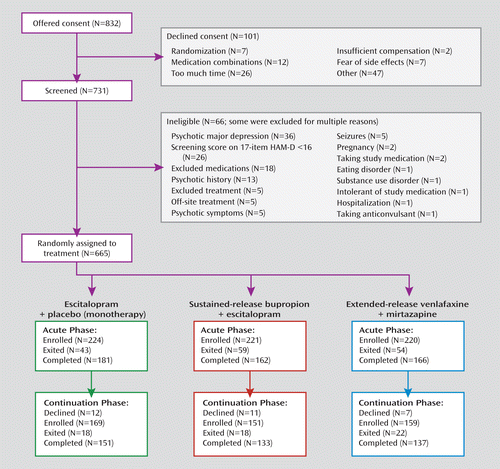
FIGURE 1. Recruitment and Treatment of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | Total (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| Sex | 0.43 | <0.05c | ||||||||
| Male | 213 | 32.0 | 81 | 36.2 | 72 | 32.6 | 60 | 27.3 | ||
| Female | 452 | 68.0 | 143 | 63.8 | 149 | 67.4 | 160 | 72.7 | ||
| Race | 0.90 | 0.84 | ||||||||
| White | 431 | 67.0 | 147 | 67.7 | 142 | 67.0 | 142 | 66.4 | ||
| Black | 174 | 27.1 | 56 | 25.8 | 58 | 27.4 | 60 | 28.0 | ||
| Other | 38 | 5.9 | 14 | 6.5 | 12 | 5.7 | 12 | 5.6 | ||
| Hispanic | 101 | 15.2 | 37 | 16.5 | 36 | 16.3 | 28 | 12.7 | 0.95 | 0.26 |
| Employed | 331 | 49.8 | 99 | 44.2 | 119 | 53.8 | 113 | 51.4 | <0.05c | 0.14 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age (years) | 42.7 | 13.0 | 43.6 | 13.1 | 42.4 | 13.5 | 42.1 | 12.4 | 0.34 | 0.22 |
| Education (years) | 13.8 | 3.0 | 13.8 | 3.2 | 13.8 | 2.6 | 13.7 | 3.1 | 0.85 | 0.82 |
| Monthly household income (dollars) | 2,678 | 5,353 | 2,449 | 3,696 | 2,828 | 5,037 | 2,759 | 6,832 | 0.81 | 0.28 |
TABLE 1. Baseline Sociodemographic Characteristics of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| First episode before age 18 | 296 | 44.6 | 96 | 43.0 | 95 | 43.0 | 105 | 47.9 | 0.99 | 0.31 |
| Recurrent depression | 517 | 78.0 | 171 | 76.7 | 174 | 78.7 | 172 | 78.5 | 0.61 | 0.64 |
| Ever attempted suicide | 59 | 9.2 | 14 | 6.5 | 23 | 10.7 | 22 | 10.3 | 0.13 | 0.16 |
| Abused before age 18 | ||||||||||
| Emotionally | 261 | 39.3 | 94 | 42.2 | 88 | 39.8 | 79 | 35.9 | 0.62 | 0.18 |
| Physically | 131 | 19.7 | 45 | 20.2 | 42 | 19.0 | 44 | 20.0 | 0.76 | 0.97 |
| Sexually | 145 | 21.9 | 43 | 19.3 | 50 | 22.6 | 52 | 23.7 | 0.39 | 0.26 |
| Chronic depression (index episode duration ≥2 years) | 368 | 55.5 | 121 | 54.3 | 121 | 54.8 | 126 | 57.5 | 0.92 | 0.49 |
| Chronic or recurrent depression | 0.81 | 0.53 | ||||||||
| Chronic only | 146 | 22.0 | 52 | 23.3 | 47 | 21.3 | 47 | 21.5 | ||
| Recurrent only | 295 | 44.5 | 102 | 45.7 | 100 | 45.2 | 93 | 42.5 | ||
| Both | 222 | 33.5 | 69 | 30.9 | 74 | 33.5 | 79 | 36.1 | ||
| Anxious features (HAM-D) | 497 | 74.7 | 156 | 69.6 | 177 | 80.1 | 164 | 74.5 | 0.02c | 0.25 |
| Atypical features (IDS-C) | 103 | 15.5 | 33 | 14.7 | 38 | 17.2 | 32 | 14.5 | 0.48 | 0.96 |
| Melancholic features (IDS-C) | 124 | 20.5 | 42 | 20.5 | 36 | 18.0 | 46 | 23.0 | 0.53 | 0.54 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age at first episode (years) | 24.0 | 14.1 | 24.4 | 14.4 | 23.9 | 13.7 | 23.7 | 14.2 | 0.85 | 0.53 |
| Years since first episode | 18.7 | 13.6 | 19.3 | 14.4 | 18.5 | 13.4 | 18.4 | 13.1 | 0.69 | 0.76 |
| Index episode duration (months) | 61.7 | 104.8 | 66.4 | 114.4 | 58.1 | 100.8 | 60.6 | 98.5 | 0.91 | 0.77 |
| Scores on clinical ratings | ||||||||||
| HAM-D | 23.8 | 4.8 | 23.4 | 4.9 | 23.8 | 4.6 | 24.3 | 5.0 | 0.34 | <0.05c |
| IDS-C | 38.0 | 9.1 | 37.0 | 8.8 | 37.8 | 9.2 | 39.3 | 9.3 | 0.39 | 0.02c |
| QIDS-C | 15.8 | 3.4 | 15.6 | 3.4 | 15.7 | 3.5 | 16.1 | 3.5 | 0.72 | 0.13 |
| QIDS-SR | 15.5 | 4.3 | 15.2 | 4.0 | 15.3 | 4.6 | 15.9 | 4.2 | 0.77 | 0.10 |
| Altman Self-Rating Mania Scale (39) | 1.5 | 2.3 | 1.6 | 2.4 | 1.6 | 2.2 | 1.3 | 2.2 | 0.79 | 0.21 |
| Cognitive and Physical Functioning Questionnaire (40) | 27.6 | 5.9 | 27.4 | 5.7 | 27.7 | 6.1 | 27.8 | 5.8 | 0.62 | 0.39 |
| Quality of Life Inventory (37) | –1.2 | 1.9 | –1.2 | 1.9 | –1.1 | 1.9 | –1.3 | 1.9 | 0.85 | 0.41 |
| Work and Social Adjustment Scale (36) | 26.9 | 8.8 | 26.2 | 8.8 | 26.7 | 9.2 | 27.9 | 8.4 | 0.50 | 0.04c |
TABLE 2. Baseline Clinical Characteristics of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Illness Variablea | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| Comorbid axis I disordersc | ||||||||||
| Agoraphobia | 69 | 10.4 | 20 | 8.9 | 28 | 12.7 | 21 | 9.5 | 0.21 | 0.83 |
| Alcohol abuse | 67 | 10.1 | 23 | 10.3 | 24 | 10.9 | 20 | 9.1 | 0.83 | 0.68 |
| Bulimia | 78 | 11.7 | 27 | 12.1 | 22 | 10.0 | 29 | 13.2 | 0.48 | 0.73 |
| Drug abuse | 35 | 5.3 | 15 | 6.7 | 12 | 5.4 | 8 | 3.6 | 0.58 | 0.15 |
| Generalized anxiety | 131 | 19.7 | 39 | 17.4 | 43 | 19.5 | 49 | 22.3 | 0.58 | 0.20 |
| Hypochondriasis | 29 | 4.4 | 9 | 4.0 | 12 | 5.4 | 8 | 3.6 | 0.49 | 0.84 |
| Obsessive-compulsive | 79 | 11.9 | 27 | 12.1 | 25 | 11.3 | 27 | 12.3 | 0.81 | 0.95 |
| Panic | 65 | 9.8 | 16 | 7.1 | 25 | 11.3 | 24 | 10.9 | 0.13 | 0.17 |
| Posttraumatic stress | 81 | 12.2 | 29 | 12.9 | 32 | 14.5 | 20 | 9.1 | 0.64 | 0.20 |
| Social phobia | 178 | 26.8 | 60 | 26.8 | 59 | 26.7 | 59 | 26.8 | 0.99 | 1.00 |
| Somatoform | 21 | 3.2 | 7 | 3.1 | 7 | 3.2 | 7 | 3.2 | 0.98 | 0.98 |
| Number of comorbid axis I disorders | 0.27 | 0.66 | ||||||||
| 0 | 296 | 44.6 | 107 | 47.8 | 85 | 38.6 | 104 | 47.3 | ||
| 1 | 159 | 23.9 | 51 | 22.8 | 67 | 30.5 | 41 | 18.6 | ||
| 2 | 92 | 13.9 | 27 | 12.1 | 29 | 13.2 | 36 | 16.4 | ||
| 3 | 50 | 7.5 | 16 | 7.1 | 19 | 8.6 | 15 | 6.8 | ||
| ≥4 | 67 | 10.1 | 23 | 10.3 | 20 | 9.1 | 24 | 10.9 | ||
| Number of comorbid axis III disordersd | 0.25 | 0.86 | ||||||||
| 0 | 161 | 24.2 | 55 | 24.7 | 59 | 26.7 | 47 | 21.4 | ||
| 1 | 198 | 29.8 | 66 | 29.6 | 67 | 30.3 | 65 | 29.5 | ||
| 2 | 154 | 23.2 | 54 | 24.2 | 43 | 19.5 | 57 | 25.9 | ||
| 3 | 77 | 11.6 | 20 | 9.0 | 32 | 14.5 | 25 | 11.4 | ||
| ≥4 | 74 | 11.1 | 28 | 12.6 | 20 | 9.0 | 26 | 11.8 | ||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Axis III comorbidity scored | 3.4 | 3.5 | 3.5 | 3.7 | 3.0 | 3.1 | 3.6 | 3.8 | 0.41 | 0.56 |
TABLE 3. Baseline Axis I and III Disorders of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
Outcomes at 12 Weeks
During the first 12 weeks, the participants were in treatment for an average of 10 weeks (Table 4). Of the 665 participants, 86.0% (N=572) completed at least 4 weeks of treatment. Overall, 78.3% (N=521) completed week 8 and 72.2% (N=480) completed at least 12 weeks of treatment. For the escitalopram-placebo group, the escitalopram dose was close to the maximum target dose of 20 mg/day. For bupropion-escitalopram, the comparable mean exit escitalopram dose during acute treatment was significantly lower, at 12.5 mg/day (SD=8.3) (χ2=31.15, df=1, p<0.0001). Also of note, while the venlafaxine dose was close to 200 mg/day by 12 weeks, the mean mirtazapine dose was only 20.0 mg/day (SD=15.7) (Table 4).
| Patient Groupb | Comparison With Monotherapyc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| 12 weeks | N | % | N | % | N | % | N | % | p | p |
| Weeks in treatment | ||||||||||
| <4 | 93 | 14.0 | 30 | 13.5 | 33 | 14.9 | 30 | 13.7 | 0.66 | 0.94 |
| <8 | 144 | 21.7 | 43 | 19.3 | 55 | 24.9 | 46 | 21.0 | 0.16 | 0.66 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Weeks in treatment | 9.9 | 3.9 | 10.0 | 3.9 | 9.6 | 4.0 | 10.0 | 3.8 | 0.13 | 0.88 |
| Number of postbaseline visits | 5.3 | 2.2 | 5.4 | 2.1 | 5.1 | 2.2 | 5.3 | 2.2 | 0.21 | 0.88 |
| Maximum open dose (mg/day) | — | — | 17.6 | 4.5 | 324.0 | 80.4 | 207.6 | 69.2 | — | — |
| Last open dose (mg/day) | — | — | 16.8 | 5.3 | 287.7 | 121.2 | 192.3 | 82.2 | — | — |
| Maximum blinded dose (mg/day)d | — | — | 1.4 | 0.7 | 14.0 | 7.2 | 25.3 | 32.0 | — | — |
| Last blinded dose (mg/day)d | — | — | 1.3 | 0.7 | 12.5 | 8.3 | 20.0 | 15.7 | — | — |
| 7 months | N | % | N | % | N | % | N | % | p | p |
| Weeks in treatment <12 | 185 | 27.8 | 56 | 25.1 | 72 | 32.6 | 57 | 26.0 | 0.09 | 0.83 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Weeks in treatment | 19.9 | 10.5 | 20.5 | 10.3 | 19.1 | 10.8 | 20.1 | 10.4 | 0.38 | 0.77 |
| Number of postbaseline visits | 7.7 | 3.7 | 7.9 | 3.6 | 7.4 | 3.8 | 7.7 | 3.7 | 0.26 | 0.81 |
| Maximum open dose (mg/day) | — | — | 17.9 | 4.4 | 328.5 | 81.7 | 217.3 | 73.3 | — | — |
| Last open dose (mg/day) | — | — | 15.6 | 6.9 | 271.0 | 136.8 | 177.6 | 94.0 | — | — |
| Maximum blinded dose (mg/day)d | — | — | 1.5 | 0.7 | 14.2 | 7.3 | 26.7 | 32.2 | — | — |
| Last blinded dose (mg/day)d | — | — | 0.7 | 0.9 | 11.5 | 8.6 | 18.0 | 16.4 | — | — |
TABLE 4. Treatment Characteristics at 12 Weeks and 7 Months of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
As shown in Table 5 and Figure 2, the treatment groups did not differ in either remission or response rates, nor did they differ in the percentage of change in QIDS-SR score (baseline to exit or 12 weeks) or in effects on quality of life. The venlafaxine-mirtazapine combination was associated with more side effect burden than escitalopram-placebo. Patients taking venlafaxine-mirtazapine had more adverse symptoms (ear aches, blurred vision, irritability, etc.) present at baseline that became worse during treatment, as measured by the Systematic Assessment for Treatment Emergent Events (mean number of effects=5.7, SD=5.8), than the monotherapy group (Table 5).
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| 12 weeks | N | % | N | % | N | % | N | % | p | p |
| Early termination | 182 | 27.4 | 55 | 24.6 | 70 | 31.7 | 57 | 25.9 | 0.10 | 0.75 |
| Remissionc | 256 | 38.5 | 87 | 38.8 | 86 | 38.9 | 83 | 37.7 | 0.99 | 0.81 |
| Last QIDS-SR score ≤5 | 242 | 36.6 | 81 | 36.2 | 82 | 37.4 | 79 | 36.2 | 0.78 | 0.99 |
| Reduction in QIDS-SR score ≥50% | 334 | 51.9 | 113 | 51.8 | 111 | 51.6 | 110 | 52.4 | 0.97 | 0.91 |
| Maximum side effect burdend | 0.07 | <0.0001e | ||||||||
| No impairment | 128 | 20.2 | 46 | 21.6 | 44 | 21.0 | 38 | 18.1 | ||
| Minimal/mild | 276 | 43.6 | 110 | 51.6 | 90 | 42.9 | 76 | 36.2 | ||
| Moderate/marked | 167 | 26.4 | 48 | 22.5 | 55 | 26.2 | 64 | 30.5 | ||
| Severe/intolerable | 62 | 9.8 | 9 | 4.2 | 21 | 10.0 | 32 | 15.2 | ||
| Last side effect burdend | 0.66 | 0.64 | ||||||||
| No impairment | 344 | 54.7 | 117 | 55.5 | 118 | 56.5 | 109 | 52.2 | ||
| Minimal/mild | 215 | 34.2 | 74 | 35.1 | 69 | 33.0 | 72 | 34.4 | ||
| Moderate/marked | 53 | 8.4 | 16 | 7.6 | 14 | 6.7 | 23 | 11.0 | ||
| Severe/intolerable | 17 | 2.7 | 4 | 1.9 | 8 | 3.8 | 5 | 2.4 | ||
| At least one serious adverse event | 27 | 4.1 | 8 | 3.6 | 7 | 3.2 | 12 | 5.5 | 0.82 | 0.34 |
| At least one psychiatric serious adverse event | 7 | 1.1 | 1 | 0.4 | 1 | 0.5 | 5 | 2.3 | 1.00 | 0.12 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Last QIDS-SR score | 8.1 | 5.4 | 7.9 | 5.2 | 8.1 | 5.3 | 8.4 | 5.7 | 0.74 | 0.34 |
| Percent change in QIDS-SR score | –45.6 | 35.1 | –46.5 | 35.2 | –44.6 | 34.6 | –45.8 | 35.8 | 0.59 | 0.85 |
| Score on IDS-C anxiety subscale | 2.6 | 2.1 | 2.4 | 2.0 | 2.6 | 2.1 | 2.8 | 2.3 | 0.28 | 0.10 |
| Last Quality of Life Inventory (37) score | 0.2 | 2.3 | 0.1 | 2.4 | 0.3 | 2.1 | 0.1 | 2.4 | 0.57 | 0.92 |
| Last Work and Social Adjustment Scale (36) score | 14.9 | 12.3 | 14.9 | 11.9 | 13.9 | 11.9 | 15.9 | 13.0 | 0.43 | 0.53 |
| Last number of symptom worseningsf | 5.1 | 5.1 | 4.7 | 4.9 | 5.0 | 4.4 | 5.7 | 5.8 | 0.12 | 0.04e |
| 7 months | N | % | N | % | N | % | N | % | p | p |
| Early termination | 244 | 36.7 | 78 | 34.8 | 84 | 38.0 | 82 | 37.3 | 0.49 | 0.60 |
| Remissionc | 298 | 44.8 | 103 | 46.0 | 103 | 46.6 | 92 | 41.8 | 0.90 | 0.38 |
| Last QIDS-SR score ≤5 | 292 | 44.4 | 101 | 45.3 | 101 | 46.3 | 90 | 41.5 | 0.83 | 0.42 |
| Reduction in QIDS-SR score ≥50% | 374 | 58.4 | 129 | 59.4 | 125 | 58.4 | 120 | 57.4 | 0.83 | 0.68 |
| Maximum side effect burdend | 0.14 | <0.0001e | ||||||||
| No impairment | 115 | 18.2 | 43 | 20.2 | 41 | 19.5 | 31 | 14.8 | ||
| Minimal/mild | 269 | 42.5 | 107 | 50.2 | 88 | 41.9 | 74 | 35.2 | ||
| Moderate/marked | 184 | 29.1 | 52 | 24.4 | 60 | 28.6 | 72 | 34.3 | ||
| Severe/intolerable | 65 | 10.3 | 11 | 5.2 | 21 | 10.0 | 33 | 15.7 | ||
| Last side effect burdend | 0.77 | 0.02e | ||||||||
| No impairment | 374 | 59.3 | 135 | 63.7 | 128 | 61.0 | 111 | 53.1 | ||
| Minimal/mild | 184 | 29.2 | 60 | 28.3 | 60 | 28.6 | 64 | 30.6 | ||
| Moderate/marked | 59 | 9.4 | 13 | 6.1 | 15 | 7.1 | 31 | 14.8 | ||
| Severe/intolerable | 14 | 2.2 | 4 | 1.9 | 7 | 3.3 | 3 | 1.4 | ||
| At least one serious adverse event | 46 | 6.9 | 16 | 7.1 | 13 | 5.9 | 17 | 7.7 | 0.60 | 0.82 |
| Last QIDS-SR score | 7.6 | 5.6 | 7.3 | 5.4 | 7.3 | 5.4 | 8.1 | 5.9 | 0.98 | 0.26 |
| Percent change in QIDS-SR score | –49.5 | 36.0 | –50.9 | 34.5 | –49.8 | 37.0 | –47.8 | 36.7 | 0.98 | 0.49 |
| Score on IDS-C anxiety subscale | 2.5 | 2.1 | 2.4 | 2.1 | 2.6 | 2.1 | 2.5 | 2.2 | 0.19 | 0.34 |
| Last Quality of Life Inventory (37) score | 0.5 | 2.4 | 0.4 | 2.6 | 0.6 | 2.1 | 0.4 | 2.4 | 0.27 | 0.87 |
| Last Work and Social Adjustment Scale (36) score | 13.8 | 12.5 | 13.5 | 12.0 | 13.0 | 12.2 | 15.0 | 13.2 | 0.62 | 0.31 |
| Last number of worsening adverse eventsf | 4.9 | 5.3 | 4.7 | 5.4 | 4.8 | 5.2 | 5.4 | 5.3 | 0.47 | <0.05e |
TABLE 5. Outcomes at 12 Weeks and 7 Months of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
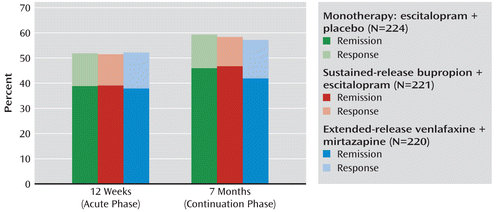
FIGURE 2. Rates of Remission and Response for Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinationsa
a Remission was defined as scores of less than 8 and less than 6 on the 16-item Quick Inventory of Depressive Symptomatology—Self-Report (QIDS-SR) (29) at the last two consecutive assessments. Response was defined as a reduction of at least 50% in QIDS-SR score.
Outcomes at 7 Months
Overall, while 72.2% of the 665 participants (N=480) completed at least 12 weeks of treatment, 65.6% completed 16 weeks or more, 61.4% completed 20 weeks, 55.6% completed 24 weeks, and 58.0% completed 28 weeks. Attrition rates over the 7-month period did not differ among treatment groups. Average drug doses were basically unchanged from 12 weeks to 7 months of treatment, regardless of treatment group (Table 4).
At 7 months (or study exit, if earlier), the three groups were not different in terms of remission rate (range: 41.8%–46.6%), response rate (range: 57.4%–59.4%), or attrition rate. Nor did the groups differ in the percentage of change in QIDS-SR (baseline to exit or 7 months), quality of life, or work and social adjustment.
Table 6 compares the side effect frequency, intensity, and burden in the escitalopram monotherapy group and each of the combination groups. Overall, there were modestly more side effects with escitalopram-bupropion than with escitalopram-placebo in both the 12-week and 7-month comparisons. On the other hand, the venlafaxine-mirtazapine group had greater side effect frequency and intensity at 12 weeks and greater side effect frequency, intensity, and burden at 7 months as compared to escitalopram-placebo.
| Comparison with Monotherapy: Escitalopram Plus Placebo (N=224) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | ||||||||
| Outcomea | Unadjusted | Adjustedb | Unadjusted | Adjustedc | |||||
| 12 weeks | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | |
| Early termination | 1.42 | 0.10 | 1.46 | 0.09 | 1.08 | 0.75 | 1.00 | 1.00 | |
| Side effectsd | |||||||||
| Maximum frequency | 1.51 | 0.02e | 1.42 | 0.06 | 2.12 | <0.0001e | 2.05 | <0.0001e | |
| Maximum intensity | 1.82 | <0.001e | 1.73 | 0.003e | 1.97 | 0.0002e | 1.86 | 0.0008e | |
| Maximum burden | 1.37 | 0.09 | 1.28 | 0.18 | 1.96 | 0.0002e | 1.87 | 0.0008e | |
| Last frequency | 1.25 | 0.23 | 1.12 | 0.57 | 1.70 | <0.004e | 1.62 | <0.02e | |
| Last intensity | 1.31 | 0.15 | 1.19 | 0.36 | 1.68 | <0.005e | 1.58 | <0.02e | |
| Last burden | 0.99 | 0.96 | 0.90 | 0.61 | 1.19 | 0.36 | 1.11 | 0.61 | |
| At least one serious adverse eventf | 0.88 | 0.82 | — | — | 1.56 | 0.35 | — | — | |
| At least one psychiatric serious adverse eventf | 1.01 | 1.00 | — | — | 5.19 | 0.14 | — | — | |
| Last QIDS-SR score ≤5 | 1.06 | 0.78 | 1.08 | 0.72 | 1.00 | 0.99 | 1.14 | 0.54 | |
| Reduction in QIDS-SR score ≥50% | 0.99 | 0.97 | 0.96 | 0.85 | 1.02 | 0.91 | 1.03 | 0.87 | |
| Last Work and Social Adjustment Scale (36) scoreg | 0.93 | 0.67 | 0.98 | 0.93 | 1.15 | 0.43 | 0.95 | 0.80 | |
| β | p | β | p | β | p | β | p | ||
| Maximum number of worsening adverse eventsh | 0.07 | 0.26 | 0.08 | 0.19 | 0.11 | 0.06 | 0.11 | 0.06 | |
| Last number of worsening adverse eventsh | 0.07 | 0.46 | 0.06 | 0.54 | 0.20 | <0.04e | 0.14 | 0.14 | |
| Last QIDS-SR score | 0.17 | 0.74 | 0.13 | 0.80 | 0.50 | 0.34 | 0.02 | 0.97 | |
| Percent change in QIDS-SR score | 1.82 | 0.59 | 2.44 | 0.47 | 0.66 | 0.85 | 0.22 | 0.95 | |
| Score on IDS-C anxiety subscale | 0.10 | 0.25 | 0.06 | 0.44 | 0.16 | 0.06 | 0.09 | 0.24 | |
| Last Quality of Life Inventory (37) score | 0.13 | 0.57 | 0.12 | 0.60 | –0.03 | 0.92 | 0.14 | 0.54 | |
| 7 months | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | |
| Early termination | 1.15 | 0.49 | 1.15 | 0.49 | 1.11 | 0.60 | 1.07 | 0.75 | |
| Side effectsd | |||||||||
| Maximum frequency | 1.53 | <0.02e | 1.44 | <0.05e | 2.31 | <0.0001e | 2.20 | <0.0001e | |
| Maximum intensity | 1.79 | <0.002e | 1.67 | <0.006e | 2.26 | <0.0001e | 2.12 | <0.0001e | |
| Maximum burden | 1.34 | 0.11 | 1.26 | 0.22 | 2.15 | <0.0001e | 2.02 | 0.0002e | |
| Last frequency | 1.40 | 0.08 | 1.32 | 0.16 | 1.80 | <0.002e | 1.76 | <0.004e | |
| Last intensity | 1.53 | <0.03e | 1.48 | <0.05e | 1.94 | 0.0004e | 1.99 | 0.0005e | |
| Last burden | 1.15 | 0.48 | 1.09 | 0.69 | 1.63 | <0.02e | 1.61 | 0.02e | |
| At least one serious adverse event | 0.81 | 0.60 | — | — | 1.09 | 0.82 | — | — | |
| At least one psychiatric serious adverse event | 0.60 | 0.50 | — | — | 1.44 | 0.54 | — | — | |
| Last QIDS-SR score ≤5 | 1.04 | 0.83 | 1.02 | 0.93 | 0.86 | 0.42 | 0.98 | 0.95 | |
| Reduction in QIDS-SR score ≥50% | 0.96 | 0.83 | 0.92 | 0.69 | 0.92 | 0.68 | 0.99 | 0.97 | |
| Last Work and Social Adjustment Scale (36) scoreg | 0.96 | 0.81 | 1.05 | 0.79 | 1.25 | 0.20 | 1.06 | 0.74 | |
| β | p | β | p | β | p | β | p | ||
| Maximum number of worsening adverse eventsh | 0.05 | 0.37 | 0.06 | 0.26 | 0.10 | 0.10 | 0.10 | 0.09 | |
| Last number of worsening adverse eventsh | 0.03 | 0.76 | 0.04 | 0.73 | 0.14 | 0.19 | 0.13 | 0.22 | |
| Last QIDS-SR score | –0.04 | 0.94 | –0.05 | 0.93 | 0.76 | 0.16 | 0.22 | 0.66 | |
| Percent change in QIDS-SR score | 1.14 | 0.74 | 2.09 | 0.54 | 3.17 | 0.36 | 2.12 | 0.53 | |
| Score on IDS-C anxiety subscale | 0.10 | 0.27 | 0.06 | 0.49 | 0.07 | 0.41 | 0.02 | 0.77 | |
| Last Quality of Life Inventory (37) score | 0.26 | 0.27 | 0.24 | 0.28 | –0.04 | 0.87 | 0.11 | 0.64 | |
TABLE 6. Odds Ratios and Beta Coefficients From Regression Models of Outcomes at 12 Weeks and 7 Months of Depressed Patients in a Comparison of Antidepressant Monotherapy With Two Antidepressant Combinations
Discussion
The study has four key findings: 1) remission and response rates were not different at 12 weeks, 2) remission and response rates were not different at 7 months, 3) the effects of the three treatments on quality of life and on work and social adjustment were not different, and 4) extended-release venlafaxine plus mirtazapine was associated with a greater side effect burden at 12 weeks and 7 months than escitalopram plus placebo and a higher number of worsening adverse events than escitalopram plus placebo at 7 months. We found no clinical advantage over escitalopram-placebo from either combination of antidepressant medications in terms of either remission or response rates at either 12 weeks or 7 months. The remission rates approximated those expected on the basis of monotherapy studies of chronic depression (5, 6). Both combination treatments had more side effects (in terms of frequency, intensity, or burden) than escitalopram-placebo in both the acute and continuation phases. Attrition rates, however, were not different across the three treatment groups in either phase of treatment. The venlafaxine-mirtazapine group was at particularly greater risk for side effects. In fact, it had significantly greater worsening of side effects than escitalopram-placebo despite the fact that the mirtazapine dose was not high (about 20 mg/day).
Prior reports have suggested that the response to either medication combination would exceed the effects of monotherapy. An open study of 49 patients (41) given escitalopram (up to 40 mg/day) plus sustained-release bupropion (400–450 mg/day) found a 63% remission rate at week 8. Blier et al. (18) compared mirtazapine, paroxetine, and the combination in a 6-week double-blind, randomized, controlled trial conducted at two research clinics with clinically referred patients and symptomatic volunteers (N=61). Remission rates at 6 weeks were 19% for mirtazapine, 26% for paroxetine, and 43% for the combination. Most of these patients had melancholic symptom features and either had nonrecurrent depression or had an index episode lasting less than 1 year. Drug doses included up to 45 mg/day of mirtazapine and paroxetine amounts that could exceed 30 mg/day (average final or exit doses not reported).
In a recent, larger 6-week double-blind acute randomized, controlled trial, Blier et al. (13) compared fluoxetine (20 mg/day) with mirtazapine (30 mg/day) in combination with fluoxetine (20 mg/day), extended-release venlafaxine (225 mg/day), or sustained-release bupropion (150 mg/day). Each combination had a remission rate (46%–58%) that exceeded that of fluoxetine alone (25%). In the study, 76% of the participants met melancholia criteria, 63% had recurrent major depression, and 61% had an index episode longer than 1 year. It is interesting that the response rates were not significantly different (54% for fluoxetine, 68% for mirtazapine-fluoxetine, 73% for mirtazapine-venlafaxine, 65% for mirtazapine-bupropion). Of note, this 6-week study may have been too brief to allow the full benefit of fluoxetine to be expressed (42).
There are several other possible explanations for why our findings differ from those of Blier et al. (13). The studies differ in terms of length of treatment, primary outcome, and scales used to assess outcomes. Our results are not accounted for by either differential attrition across the three treatments or baseline differences. On the other hand, our participants differed from those studied by Blier et al. Neither participant group was treatment resistant. However, participants in our study were required to have chronic and/or recurrent depression. In fact, there were far more chronically ill participants in our study than in the one by Blier et al. In addition, 62%–85% of their participants had melancholic features (compared to only 20% in this study). Some reports (17, 43) suggest better efficacy, i.e., drug-placebo differences, for inpatients (who are more likely to have melancholic features) with combination medications or dual-action medications. In addition, a meta-analysis by Perry (44) revealed that broader-action agents (e.g., tricyclic antidepressants) have far greater efficacy than SSRIs in melancholic depression.
To evaluate the potential impact of chronicity on outcome, we reanalyzed the data. Chronicity was associated with lower remission rates across all three treatment cells. Specifically, in the bupropion-escitalopram group, 37.2% of the patients with episodes of chronic depression had remissions, whereas the remission rate was 41.0% for nonchronic depression. Analogous rates were 35.5% versus 43.1% for escitalopram-placebo and 34.9% versus 41.9% for venlafaxine-mirtazapine. We conducted a similar analysis to compare patients with and without melancholic features from the current study. Melancholic features were associated with more axis I comorbidity, greater symptom severity, and more suicidal plans and thoughts. However, remission rates ranged from 30.0% to 39.1% for those with melancholic features and from 37.5% to 39.5% for those without. There were no differences across medication groups. Thus, neither the difference in the proportion with chronic illness nor the difference in melancholia seems to account for why our results differ from those of Blier et al. (13). While we enrolled the kinds of patients (i.e., those with chronic or recurrent depression) for whom most clinicians would be likely to consider antidepressant medication combinations (45), we cannot rule out potential impact on outcomes from one or more unknown baseline features.
This group with chronic and/or recurrent depression had high rates of self-reported emotional, sexual, or physical abuse before age 18, and a high proportion had anxious features. While these features could have reduced the overall benefit of any one treatment, they would be unlikely to obscure differences between treatment cells, given their proportional distribution across the cells.
Perhaps the differences between the present study and the results reported by Blier et al. (13) are due to the specific antidepressant medications and doses that we used and to the doses that were administered. There is evidence for the efficacy of venlafaxine plus mirtazapine (13, 35) and bupropion plus escitalopram (16, 34). Carpenter et al. (46) found that mirtazapine, when used as an adjunct to previously ineffective SSRIs alone, was more effective than placebo in treating depression. In fact, Blier et al. (13) used the venlafaxine-mirtazapine combination and Stewart et al. (41) used bupropion plus escitalopram in their trials, although the doses were higher in both of them. The rationale for a higher venlafaxine dose is that the effect on the norepinephrine system (17, 47) is only realized at doses of at least 225 mg/day. Mirtazapine has antagonistic effects that are modest at 15 mg/day and more clearly evident at 30 mg/day. Thus, as suggested by Blier (personal communication), the doses in CO-MED may have been insufficient in a large enough proportion of participants to preclude the benefit otherwise available from the combination. In an attempt to evaluate that notion, we identified the 86 participants who reached 225 mg/day of venlafaxine and 30 mg/day of mirtazapine at any time during treatment. Their remission rate was 33.7% at 12 weeks and 41.9% at 7 months. These results do not suggest that underdosing was the cause of the poor performance of this combination.
It remains an unanswered question whether these larger doses (if they are required to achieve an advantage for antidepressant combinations) are achievable in practice with more representative patients who have chronic and/or recurrent major depressive disorder and more concurrent axis I and III disorders.
This study had several limitations. While larger than many studies, the study group may not be representative of the universe of outpatients with chronic and/or recurrent major depression. As noted, the doses used may not have been sufficient to realize the full potential value of combination antidepressant medications. The results for the continuation treatment phase are limited by the fact that the subjects were not rerandomized or stratified by level of improvement following the acute phase. Finally, the clinicians were not blind to treatment, and a structured interview was not used to establish axis I diagnoses.
In summary, in outpatients with chronic and/or recurrent major depressive disorder, there appears to be no advantage to either medication combination over escitalopram alone as a first-step treatment for nonresistant depression. Some combinations may incur a risk of higher side effect burden. This conclusion is conditioned on the doses employed.
1. : The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105Crossref, Medline, Google Scholar
2.
3. : Results of the DSM-IV mood disorders field trial. Am J Psychiatry 1995; 152:843–849Link, Google Scholar
4. : Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology 2006; 31:1841–1853Crossref, Medline, Google Scholar
5. : The treatment of chronic depression, part 2: a double-blind, randomized trial of sertraline and imipramine. J Clin Psychiatry 1998; 59:598–607Crossref, Medline, Google Scholar
6. : A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000; 342:1462–1470Crossref, Medline, Google Scholar
7. : Strategies for managing depression refractory to selective serotonin reuptake inhibitor treatment: a survey of clinicians. Can J Psychiatry 2000; 45:476–481Crossref, Medline, Google Scholar
8.
9.
10. ,
11. : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
12. : Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006; 354:1231–1242Crossref, Medline, Google Scholar
13. : Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry 2010; 167:281–288Link, Google Scholar
14. : Neurobiological basis of depression: an update. Metabolism 2005; 54(5 suppl 2): 24–27Crossref, Medline, Google Scholar
15. : Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom 2006; 75:139–153Crossref, Medline, Google Scholar
16. : Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354:1243–1252Crossref, Medline, Google Scholar
17. : Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry 2004; 55:296–300Crossref, Medline, Google Scholar
18. : Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol 2009; 19:457–465Crossref, Medline, Google Scholar
19. : The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292Crossref, Medline, Google Scholar
20. : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
21.
22. : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–61Crossref, Medline, Google Scholar
23. : Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 2008; 165:342–351Link, Google Scholar
24. : Factor analysis of the Hamilton Depression Scale. Drugs Exp Clin Res 1977; 1:115–120Google Scholar
25. : Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord 2005; 87:43–55Crossref, Medline, Google Scholar
26. : The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003; 49:156–163Crossref, Medline, Google Scholar
27. ,
28. : Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology 2007; 32:2479–2489Crossref, Medline, Google Scholar
29. : An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression trial report. Biol Psychiatry 2006; 59:493–501Crossref, Medline, Google Scholar
30. : The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26:477–486Crossref, Medline, Google Scholar
31. : Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract 2006; 12:71–79Crossref, Medline, Google Scholar
32. : Web-based communications and management of a multi-center clinical trial: the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) project. Clin Trials 2004; 1:387–398Crossref, Medline, Google Scholar
33. : Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry 2001; 62(suppl 18):4–11Medline, Google Scholar
34. : An open pilot study of the combination of escitalopram and bupropion-SR for outpatients with major depressive disorder. J Psychiatr Pract 2008; 14:271–280Crossref, Medline, Google Scholar
35. ,
36. : The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002; 180:461–464Crossref, Medline, Google Scholar
37. : Predictive and treatment validity of life satisfaction and the Quality of Life Inventory. Assessment 2005; 12:66–78Crossref, Medline, Google Scholar
38. : General versus specific inquiry with SAFTEE(letter). J Clin Psychopharmacol 1992; 12:448Crossref, Medline, Google Scholar
39. : The Altman Self-Rating Mania Scale. Biol Psychiatry 1997; 42:948–955Crossref, Medline, Google Scholar
40. : Reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire. Psychother Psychosom 2009; 78:91–97Crossref, Medline, Google Scholar
41. : Does dual antidepressant therapy as initial treatment hasten and increase remission from depression? J Psychiatr Pract 2009; 15:337–345Crossref, Medline, Google Scholar
42. : When should a trial of fluoxetine for major depression be declared failed? Am J Psychiatry 2003; 160:734–740Link, Google Scholar
43. : Venlafaxine and paroxetine in treatment-resistant depression: double-blind, randomised comparison. Br J Psychiatry 1999; 175:12–16Crossref, Medline, Google Scholar
44. : Pharmacotherapy for major depression with melancholic features: relative efficacy of tricyclic versus selective serotonin reuptake inhibitor antidepressants. J Affect Disord 1996; 39:1–6Crossref, Medline, Google Scholar
45. : Chronicity of depressive episode in relation to antidepressant-placebo response. Neuropsychopharmacology 1991; 4:125–130Medline, Google Scholar
46. : A double-blind, placebo-controlled study of antidepressant augmentation with mirtaza-pine. Biol Psychiatry 2002; 51:183–188Crossref, Medline, Google Scholar
47. : Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin Psychopharmacol 1999; 19:177–182Crossref, Medline, Google Scholar



