Toxoplasma Infection and Later Development of Schizophrenia in Mothers
Abstract
Objective:
Several studies based on clinical samples have found an association between Toxoplasma gondii infection and schizophrenia, and a case-control study among U.S. military personnel with specimens available from both before and after diagnosis found a positive association between T. gondii immunoglobulin G (IgG) antibody level and schizophrenia. These findings have never been replicated in a prospective cohort study. The purpose of this study was to determine whether mothers infected with T. gondii have an elevated risk of schizophrenia or related disorders and whether the risk depends on IgG antibody level.
Method:
In a register-based prospective cohort study of 45,609 women born in Denmark, the level of T. gondii-specific IgG antibodies was measured in connection with childbirth between 1992 and 1995. Women were followed up from the date of delivery until 2008.
Results:
A significant positive association between T. gondii IgG antibody level and schizophrenia spectrum disorders was found. Mothers with the highest IgG level had a relative risk of 1.73 (95% confidence interval [CI]=1.12–2.62) compared with mothers with the lowest IgG level. For schizophrenia, the relative risk was 1.68 (95% CI=0.77–3.46). When the mothers were classified according to IgG level, only those with the highest IgG levels had a significantly higher risk of schizophrenia spectrum disorders.
Conclusions:
Women with high levels of T. gondii-specific IgG antibodies have a significantly elevated risk of developing schizophrenia spectrum disorders.
Toxoplasma gondii is a protozoan parasite. Humans are infected mainly by consumption of undercooked meat containing T. gondii cysts, by ingestion of oocysts from the feces of infected cats (e.g., through contact with a contaminated litter box, ingestion of unwashed vegetables), or by congenital infection if the mother has a primary infection during pregnancy and passes the infection to the fetus (1). After infection, rapidly dividing intracellular T. gondii tachyzoites are disseminated throughout the body. The tachyzoites eventually transform to slowly dividing bradyzoites in tissue cysts, most commonly in brain and muscle. These cysts may remain throughout the life of the host, and unlike tachyzoites, they fail to provoke an inflammatory response. Reactivation of the infection may occur if the host becomes immunosuppressed (2, 3).
The first studies of an association between T. gondii and psychosis were published during the period 1953–1957. Since then, many studies have compared T. gondii status in individuals with schizophrenia and healthy comparison subjects. Yolken et al. (4–6) have provided an overview of previous research and discussion of possible mechanisms by which T. gondii could cause schizophrenia. In a meta-analysis using schizophrenia cases and controls from 23 studies, Torrey et al. (7) reported that the odds ratio for schizophrenia associated with T. gondii was 2.73 (95% confidence interval [CI]=2.10–3.60) and that the odds ratio based on the seven studies with first-episode schizophrenia was 2.54. Subsequent studies also found an association between T. gondii infection and schizophrenia (8–12). One study found significantly more individuals with high-titer T. gondii response in the schizophrenia group compared with the healthy comparison group and no significant difference for low-titer positive response (13). A limitation of these various studies is that antibody levels for individuals with schizophrenia were measured at the time of diagnosis, yet psychiatric symptoms are often present sometime before the first diagnosis of schizophrenia. It is unknown whether such incipient psychiatric illness causes behavioral changes that increase the risk of infection with T. gondii, which then might explain the observed association between T. gondii and schizophrenia. To avoid this problem, there is a need for prospective cohort studies assessing T. gondii in adults prior to onset of psychiatric illness. Only one previous study, among U.S. military personnel, was based on measurements of immunoglobulin G (IgG) level in adults before as well as after diagnosis of schizophrenia (14); in that study, researchers found an association between increasing IgG levels and schizophrenia.
In the present study, we used the women included in a population-based Danish study of neonatal screening for Toxoplasma infection (15) as a prospective cohort to investigate whether T. gondii-infected mothers suffer an elevated risk of schizophrenia and schizophrenia spectrum disorders and whether the risk depends on the level of IgG antibodies.
Method
Study Population
The individuals included in the study population were originally recruited for a study of neonatal screening for T. gondii (15). Pregnant women living in five counties in Denmark from 1992 to 1996 were offered screening of their child for T.gondii shortly after birth. The five counties (encompassing Copenhagen and its suburbs, the northeastern part of Zealand, and the southern part of Jutland) represented one-third of Danish deliveries during that period. Only 0.18% of eligible women declined to take part in the original study; 9.26% of the women had no serum sample from the first trimester and were excluded from the study. A heel-stick blood sample from the child was drawn 5–10 days after birth and stored on filter paper for testing for phenylketonuria and other metabolic abnormalities. For children of mothers included in the study, two 3.2-mm discs from the phenylketonuria card were analyzed by enzyme immunoassay for T. gondii IgG antibodies (16). The level of antibodies was expressed as a percentage of the optical density obtained for World Health Organization international standard serum, and the mean of the two results measured the IgG level. In the present study, mothers of children with an IgG level above 24 were regarded as T. gondii-positive from the time of delivery. The IgG antibodies measured in the blood from the child were maternal in origin, as IgG passes through the placenta and infected newborn children will only begin producing T. gondii-specific IgG at approximately 3 months of age (1). For one-fourth of the women (N=12,693) in the study population, data were also available on IgG level based on the first-trimester serum sample. The IgG levels measured in mother and offspring were highly correlated (Spearman correlation=0.76, p<0.0001).
All people living in Denmark from 1968 onward are registered in the Danish Civil Registration System (17). Among many other variables, the register includes information on personal identification number, gender, date and place of birth, continuously updated information on vital status, and the personal identification numbers of the parents. The personal identification number is used in all national registers, enabling accurate linkage between registers. Our study population included mothers born in Denmark who gave birth between May 15, 1992, and January 15, 1995, who had no diagnosis of a schizophrenia spectrum disorder (defined below) at time of delivery and whose child was screened for T. gondii (N=45,609). For mothers who gave birth more than once during the study period, only the first delivery was included in the study.
Assessment of Schizophrenia and Mental Illness in Cohort Members and Their Parents
The study population and their parents were linked with the Danish Psychiatric Central Register (18) to obtain information on any history of mental illness. The Danish Psychiatric Central Register, which was computerized in 1969, contains data on all admissions to Danish psychiatric inpatient facilities and, from 1995 on, information on outpatient visits to psychiatric departments. From 1969 to 1993, the diagnostic system used was the Danish modification of ICD-8, and from 1994, ICD-10. For cohort members and their parents, data were extracted for diagnoses of schizophrenia (ICD-8 codes 295.x except 295.7; ICD-10 code F20), diagnoses of schizophrenia spectrum disorder (ICD-8 codes 295, 297, 298.39, and 301.83; ICD-10 codes F20–F29), as well as a psychiatric history (any diagnosis) if they had been admitted to a psychiatric hospital or been in outpatient care with a diagnosis of a psychiatric disorder. Date of onset was defined as the first day of the first contact (inpatient or outpatient) with the diagnosis in question.
Assessment of Urbanization
For each cohort member, we compiled information on degree of urbanization at place of residence at time of delivery according to the classification of municipalities in Denmark by Statistics Denmark: capital, capital suburb, provincial city with more than 100,000 inhabitants, provincial town with more than 10,000 inhabitants, or rural areas (19). Provincial cities were not represented in the cohort.
Study Design
Prevalences of T. gondii at the time of delivery (based on serology from the newborns) were estimated using a cross-sectional study design (20). The prevalence and 95% likelihood ratio-based confidence intervals were calculated using the GENMOD procedure in SAS version 9.2 (SAS Institute, Cary, N.C.).
A total of 45,609 mothers were followed from the day they gave birth (May 15, 1992, to January 15, 1995) until the date of onset of schizophrenia or schizophrenia spectrum disorder, date of death, date of emigration from Denmark, or December 31, 2008, whichever came first.
Incidence rate ratios of schizophrenia, referred to here as relative risks, were estimated by the Cox proportional hazards model (Cox regression) (21). Time since delivery was used as the underlying time scale. The proportional hazards assumption was evaluated by comparing estimated log-minus-log survival curves. All relative risks were adjusted for age at delivery. We also investigated the potential confounding effect of a history of mental illness in a parent (19) and place of residence at time of delivery. Three time-dependent variables were used to adjust for history of mental illness in the women's parents: an indicator of a schizophrenia spectrum disorder in a parent and indicators of a psychiatric history (any diagnosis) in each parent separately. All other variables were treated as time-independent variables. T. gondii IgG level was analyzed as both a continuous and a categorical variable. Two categorical models were considered: a dichotomous model (seropositive and seronegative) and a model with seropositive IgG levels divided according to quartiles; for schizophrenia spectrum disorder, the upper quartile was divided further into the 10% highest IgG levels and the 10%–25% highest IgG levels. To compare our findings with those of Niebuhr et al. (14), we also included the IgG level as a continuous variable, scoring the lowest IgG values as 0 and the highest IgG values as 1. However, to avoid bias due to extreme observations, the 2% highest values were truncated to their mean value. Using this scoring, the relative risk estimate measured the effect of the highest IgG level compared with the lowest IgG.
All p values and 95% confidence intervals were based on likelihood ratio tests (21).
The investigators were blind to the identity of individuals in the study, and because the study did not involve any contact with the cohort members, it did not require written informed consent. The study was approved by the Danish Data Protection Agency.
Results
The distribution of the IgG level measured in the newborn child showed a clear bimodal distribution (Figure 1) in which mothers at the lower end of the distribution were T. gondii seronegative and those at the higher end of the distribution were T. gondii seropositive. In accordance with Lebech and Petersen (16) and consistent with the bimodal distribution of IgG levels in Figure 1, an IgG level above 24 was considered seropositive.
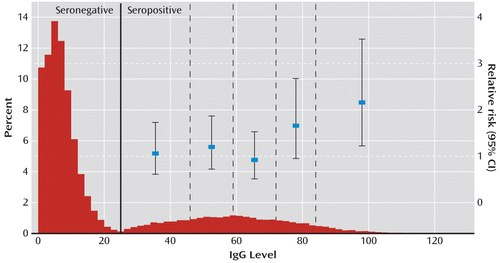
FIGURE 1. Distribution of Toxoplasma gondii-Specific IgG Level and Relative Risk of Schizophrenia Spectrum Disorders in a Cohort of Danish Mothersa
a IgG level is expressed as percentage of the optical density obtained for World Health Organization international standard serum for the 45,609 Danish mothers in the cohort. Points and vertical line segments are relative risks and 95% confidence intervals of schizophrenia spectrum disorder for T. gondii seropositive mothers in five subgroups defined by the dotted lines as compared to seronegative mothers (IgG level below 25).
Prevalence of T. gondii Infection
The general characteristics of the study cohort are summarized in Table 1. Overall, the prevalence of T. gondii was 26.8% at the time of delivery. Higher maternal age at delivery and lower degree of urbanization at the time of delivery were associated with a higher prevalence of T. gondii infection. Women whose mothers had a psychiatric history had a slightly higher prevalence of T. gondii infection than did women whose mothers did not have such a history.
| Variable | N | Prevalence (%) | 95% CI |
|---|---|---|---|
| Total | 45,609 | 26.80 | 26.33–27.28 |
| Age at delivery (years)a | |||
| 14–19 | 749 | 23.23 | 19.95–26.86 |
| 20–24 | 6,544 | 21.29 | 20.19–22.43 |
| 25–29 | 19,255 | 24.74 | 24.04–25.44 |
| 30–34 | 13,726 | 29.29 | 28.39–30.20 |
| ≥35 | 5,335 | 35.15 | 33.58–36.77 |
| Residence at time of delivery | |||
| Capital | 15,949 | 25.70 | 24.92–26.49 |
| Capital suburb | 16,737 | 26.24 | 25.47–27.02 |
| Provincial town | 5,525 | 26.50 | 25.17–27.88 |
| Rural area | 7,398 | 30.69 | 29.44–31.97 |
| Parental psychiatric history, motherb | |||
| Yes | 3,776 | 29.43 | 27.73–31.19 |
| No | 40,629 | 26.19 | 25.70–26.69 |
| Parental psychiatric history, fatherb | |||
| Yes | 3,044 | 27.93 | 26.09–29.85 |
| No | 40,556 | 26.26 | 25.76–26.76 |
TABLE 1. Prevalence of Toxoplasma gondii in a Danish Cohort of Mothers at the Time of Delivery, 1992–1995, by Age Group, Degree of Urbanization at Place of Residence, and Parental Psychiatric History
Relative Risk of Schizophrenia Spectrum Disorder
Among the 45,609 mothers followed from delivery until 2008, 246 developed a schizophrenia spectrum disorder during the 690,048 person-years at risk, corresponding to a crude incidence rate of 3.56 per 10,000 person-years (Table 2). The follow-up was terminated before the end of the study period for 927 (2%) mothers because of death (N=302) or emigration from Denmark (N=625). For mothers with the highest IgG level, the relative risk of schizophrenia spectrum disorder was 1.73 times (95% CI=1.12–2.62) higher than for those with the lowest IgG level. When the IgG level was treated as a dichotomous variable, the relative risk of schizophrenia spectrum disorder for mothers who were T. gondii seropositive was 1.28 (95% CI=0.97–1.67, p=0.076) times higher than for those who were T. gondii seronegative, but the difference fell short of significance. When the seropositive values were further subdivided into 25%, 50%, 75%, and 90% quantiles, the risk of schizophrenia spectrum disorder was increased only for mothers with IgG values in the two upper groups (Figure 1 and Table 2); women with an IgG level between 72 and 83 had a relative risk of 1.66 (95% CI=0.95–2.68) compared to seronegative women, and those with an IgG level above 84 had a relative risk of 2.16 (95% CI=1.22–3.53). Adjustment for place of residence at time of delivery and psychiatric history in the parents had only a limited impact on the results. In analyses with follow-up starting 2 years after delivery, the relative risk in the continuous model was 1.97 (95% CI=1.23–3.07, p=0.005), and in the dichotomous model it was 1.27 (95% CI=0.95–1.70, p=0.111). For women with an IgG level between 72 and 83, the relative risk was 1.97 (95% CI=1.13–3.21) compared with seronegative women, and for those with an IgG level above 83, it was 2.56 (95% CI=1.44–4.22).
| First Adjustmentb | Second Adjustmentc | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Cases (N) | Incidence Ratea | Relative Risk | 95% CI | pd | Relative Risk | 95% CI | pd |
| Total | 246 | 3.56 | ||||||
| Continuouse | 1.80 | 1.16–2.73 | 0.009 | 1.73 | 1.12–2.62 | 0.015 | ||
| Two categories | 0.049 | 0.076 | ||||||
| 0–24f | 166 | 3.29 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| ≥25 | 80 | 4.32 | 1.31 | 1.00–1.71 | 1.28 | 0.97–1.67 | ||
| Six categoriesg | 0.058 | 0.079 | ||||||
| 0–24f | 166 | 3.29 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| 25–45 | 16 | 3.58 | 1.09 | 0.63–1.78 | 1.06 | 0.61–1.73 | ||
| 46–58 | 19 | 4.04 | 1.23 | 0.74–1.92 | 1.20 | 0.72–1.87 | ||
| 59–71 | 14 | 3.13 | 0.95 | 0.53–1.58 | 0.92 | 0.51–1.53 | ||
| 72–83 | 16 | 5.50 | 1.66 | 0.96–2.68 | 1.66 | 0.95–2.68 | ||
| ≥84 | 15 | 7.65 | 2.27 | 1.28–3.72 | 2.16 | 1.22–3.53 | ||
TABLE 2. Adjusted Relative Risk of Schizophrenia Spectrum Disorders Associated With Toxoplasma gondii IgG Antibody Level
To assess the potential bias within the study population, we compared the effect of all selected confounders with the effect of these variables in the total Danish population of women giving birth between 1992 and 1995 (Table 3). The effect of these variables in the study population was not significantly different from that in the total population, and adjustment for these factors had only a limited impact on our estimates (Table 2).
| Study Population | Danish-Born Women Giving Birth in Denmark | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Cases (N) | Incidence Ratea | Relative Riskb | 95% CI | pc | Cases (N) | Incidence Ratea | Relative Riskb | 95% CI | pc |
| Age at delivery (years) | 0.001 | <0.0001 | ||||||||
| 14–19 | 9 | 7.95 | 2.83 | 1.31–5.36 | 37 | 8.18 | 3.15 | 2.19–4.41 | ||
| 20–24 | 52 | 5.23 | 2.01 | 1.40–2.86 | 179 | 4.74 | 1.99 | 1.64–2.43 | ||
| 25–29 | 73 | 2.50 | 1.00 | (Reference) | 226 | 2.30 | 1.00 | (Reference) | ||
| 30–34 | 78 | 3.77 | 1.51 | 1.10–2.08 | 197 | 2.98 | 1.29 | 1.06–1.56 | ||
| ≥35 | 34 | 4.23 | 1.36 | 0.84–2.15 | 79 | 3.27 | 1.16 | 0.85–1.55 | ||
| Residence at time of delivery | 0.013 | 0.002 | ||||||||
| Capital | 108 | 4.49 | 1.20 | 0.85–1.74 | 120 | 4.54 | 1.54 | 1.23–1.91 | ||
| Capital suburb | 69 | 2.72 | 0.73 | 0.50–1.09 | 86 | 2.87 | 0.99 | 0.77–1.26 | ||
| Provincial city | 0 | 76 | 2.69 | 0.95 | 0.73–1.23 | |||||
| Provincial town | 28 | 3.34 | 0.90 | 0.55–1.45 | 201 | 3.11 | 1.06 | 0.88–1.28 | ||
| Rural area | 41 | 3.65 | 1.00 | (Reference) | 235 | 2.89 | 1.00 | (Reference) | ||
| Schizophrenia spectrum disorder in a parentd | 0.001 | <0.0001 | ||||||||
| Yes | 18 | 14.56 | 2.78 | 1.59–4.64 | 49 | 13.77 | 2.58 | 1.85–3.53 | ||
| No | 228 | 3.36 | 1.00 | (Reference) | 669 | 2.94 | 1.00 | (Reference) | ||
| Parental psychiatric history, mother | 0.020 | <0.0001 | ||||||||
| Yes | 46 | 6.36 | 1.54 | 1.07–2.16 | 141 | 6.66 | 1.92 | 1.56–2.34 | ||
| No | 200 | 3.24 | 1.00 | (Reference) | 577 | 2.75 | 1.00 | (Reference) | ||
| Parental psychiatric history, father | 0.003 | <0.0001 | ||||||||
| Yes | 39 | 6.71 | 1.78 | 1.22–2.52 | 99 | 5.80 | 1.61 | 1.28–2.00 | ||
| No | 207 | 3.28 | 1.00 | (Reference) | 619 | 2.90 | 1.00 | (Reference) | ||
| Unknown mother | 0.567 | 0.137 | ||||||||
| Yes | 13 | 7.13 | 1.28 | 0.55–2.97 | 32 | 5.14 | 1.56 | 0.87–2.83 | ||
| No | 233 | 3.47 | 1.00 | (Reference) | 686 | 3.06 | 1.00 | (Reference) | ||
| Unknown father | 0.039 | 0.119 | ||||||||
| Yes | 22 | 7.26 | 2.07 | 1.04–3.73 | 45 | 5.05 | 1.50 | 0.90–2.38 | ||
| No | 224 | 3.40 | 1.00 | (Reference) | 673 | 3.03 | 1.00 | (Reference) | ||
TABLE 3. Adjusted Relative Risk of Schizophrenia Spectrum Disorders Associated With Selected Confounders for the Study Population as Well as the Total Danish Population of Women Giving Birth Between 1992 and 1995
Relative Risk of Schizophrenia
A total of 80 mothers developed schizophrenia during the 691,394 person-years of follow-up, which corresponds to a crude incidence rate of 1.16 per 10,000 person-years at risk (Table 4). The relative risks of schizophrenia associated with T. gondii infection (Table 4) were similar to those of schizophrenia spectrum disorders (Table 2), although they did not reach statistical significance. In analyses with follow-up starting 2 years after delivery, the relative risk in the continuous model was 1.69 (95% CI=0.73–3.63, p=0.214), and in the dichotomous model it was 1.36 (95% CI=0.81–2.22, p=0.237). Mothers with an IgG level above 71 had a relative risk of 1.90 (95% CI=0.87–3.70) compared to seronegative mothers.
| First Adjustmentb | Second Adjustmentc | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Cases (N) | Incidence Ratea | Relative Risk | 95% CI | pd | Relative Risk | 95% CI | pd |
| Total | 80 | 1.16 | ||||||
| Continuouse | 1.74 | 0.80–3.59 | 0.159 | 1.68 | 0.77–3.46 | 0.186 | ||
| Two categories | 0.133 | 0.150 | ||||||
| 0–24f | 53 | 1.05 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| ≥25 | 27 | 1.46 | 1.44 | 0.89–2.27 | 1.42 | 0.88–2.24 | ||
| Five categoriesg | 0.491 | 0.519 | ||||||
| 0–24f | 53 | 1.05 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| 25–45 | 5 | 1.12 | 1.16 | 0.40–2.64 | 1.15 | 0.40–2.64 | ||
| 46–58 | 8 | 1.70 | 1.70 | 0.74–3.38 | 1.68 | 0.74–3.35 | ||
| 59–71 | 5 | 1.12 | 1.10 | 0.38–2.49 | 1.07 | 0.37–2.43 | ||
| ≥72 | 9 | 1.84 | 1.73 | 0.80–3.34 | 1.70 | 0.78–3.28 | ||
TABLE 4. Adjusted Relative Risk of Schizophrenia Associated With Toxoplasma gondii IgG Antibody Level
For both outcomes, we excluded 180 mothers who had a diagnosis of a schizophrenia spectrum disorder at start of follow-up (date of delivery). Mothers who had other psychiatric disorders, such as an affective disorder, before delivery were included (N=917). However, restricting the analysis to mothers without any psychiatric contact before the delivery had no impact on our findings. For both outcomes of interest, further adjustment for maternal place of birth also had no impact on the findings.
Discussion
In a prospective follow-up study of women screened for T. gondii at the time of giving birth, we found a significantly elevated risk of schizophrenia spectrum disorders associated with T. gondii infection. The greater risk was not explained by psychiatric family history, degree of urbanization at place of residence, or age at delivery. When IgG antibody level was analyzed as a categorical variable, only the highest levels had a significant effect.
Because we had information on infection status at time of delivery only, mothers who became infected with T. gondii after delivery were considered seronegative in our analyses, which implies that the effect sizes we report are likely to be conservative. Only one previous study assessed T. gondii antibody levels in adults before as well as after onset of schizophrenia—a nested case-control study by Niebuhr et al. (14) with 15 exposed cases among military personnel. Even though the Niebuhr et al. study contained 83% men and the present study included only women, the size of the association in the two studies was similar.
To potentially interpret observed associations as causal associations, T. gondii antibodies had to be measured prior to the onset of psychiatric symptoms (20). An earlier study of first-episode schizophrenia spectrum disorders in Denmark (22) showed that the duration of untreated psychosis among women was 2 years on average. In a subanalysis, we started follow-up 2 years after delivery, thereby excluding cases with first diagnosis of a schizophrenia spectrum disorder within 2 years after assessment for T. gondii antibodies. Given that the size of the association between T. gondii and schizophrenia and schizophrenia spectrum disorders was unchanged, it is unlikely that the association found was due to reverse causality (onset of psychiatric symptoms before onset of T. gondii infection). Our results were virtually unchanged after adjustment for some important confounders, including family history of mental disorders and degree of urbanization of place of residence at the time of delivery. Although this does not exclude confounding from genetic and environmental risk factors for schizophrenia and related disorders, it does lend further credibility to our results.
To our knowledge, this is the largest study of T. gondii and schizophrenia spectrum disorders. We studied a population-based cohort consisting of women across a large area of Denmark, accounting for about one-third of all deliveries in Denmark between 1992 and 1995, with almost complete follow-up data for up to 16 years. Women were included in the study irrespective of social status, and information on T. gondii IgG level was collected prospectively and independently of the present study. A weakness of the study is that the study population was not a random sample of women giving birth in Denmark. Individuals included in the study population were originally recruited for a study of the maternofetal transmission rate of T. gondii infection (15). Pregnant women from five counties in Denmark were offered screening at delivery for primary T. gondii infection during pregnancy from 1992 to 1996. A first-trimester serum sample from the mother was available for 90.7% of women otherwise eligible for the study. Only 0.18% of eligible women declined to take part in the study. All phenylketonuria cards with blood drawn 5–10 days postpartum from infants of consenting mothers were analyzed for Toxoplasma-specific IgG. When the test result was positive, the mother's first-trimester sample was thawed and analyzed for Toxoplasma-specific IgG, to trace seroconversion (15). For the present study, we were able to retrieve results of IgG measurements for children born only up until January 15, 2005. Apart from this, the only selections made were to restrict the analyses to mothers born in Denmark, to include each mother only once (her first delivery during the study period), and to exclude mothers who had a diagnosis of a schizophrenia spectrum disorder before delivery. We compared the influence of age at delivery, parental history of mental illness, and degree of urbanization of place of residence at the time of delivery on the risk of schizophrenia spectrum disorders in the 45,609 mothers included in the study compared with the 151,950 women who gave birth in Denmark during the study period. We found similar magnitudes and directions for all associations, and thus there was no sign of selection bias with respect to the included confounders. A limitation of the generalizability of the study is the exclusion of nulliparous women, who may have different risk factors for developing schizophrenia. The age distribution of women included in the study was relatively broad, although the largest age group was the 25- to 29-year-olds. Another obvious limitation on the generalizability of the study is that only women were included, although the association between T. gondii and schizophrenia was similar to that reported by Niebuhr et al. (14) in their predominantly male military sample.
As noted earlier, because information on the IgG level in the mothers' first-trimester serum samples was available for only a subset of the cohort, we based our analyses on IgG levels in the newborns. Although IgG levels in the child can be influenced by a number of factors (including placental transport), antibody levels in maternal serum from the first trimester and in the blood sample from the infant were well correlated (Spearman correlation=0.76, p<0.0001). We therefore have confidence in the results based on antibody levels measured in the newborns.
Reliance on routinely acquired clinical information has its limitations, particularly with regard to the validity and reliability of diagnoses. Reassuring results have been obtained from a study that assessed the validity of schizophrenia diagnoses acquired from the Danish Psychiatric Central Register (23). Although research diagnoses would be preferable, we believe our results can be compared with those of other studies.
A number of publications have suggested possible immunological and other mechanisms explaining the association between T. gondii infection and brain disease (24–29). The association with high levels of IgG could have several explanations. High levels could reflect a recently acquired infection or a reactivated infection. One could also speculate that there is a direct effect of the IgG antibodies through molecular mimicry. It has been suggested that IgG antibodies against several infectious agents, including T. gondii, may cross-react with epitopes in neural tissue (30, 31), and it is known that neurological and psychiatric symptoms may be caused by antibodies crossing the blood-brain barrier in autoimmune disorders such as systemic lupus erythematosus (32) as well as in paraneoplastic disorders (33). Anti-NMDA ( N-methyl-d-aspartic acid) receptor antibodies and other specific CNS-related antibodies have been associated with psychosis and other neurological and psychiatric symptoms (34, 35). However, at present all these explanations remain speculative. Further knowledge is needed about the potential differential effects of different strains and stages of T. gondii . One study suggested that psychosis is particularly strongly associated with maternal infection with type I strain (36), but this finding needs to be replicated. Future studies also should elucidate the mechanisms underpinning the association—for example, whether there is a direct effect of T. gondii-specific IgG antibodies on the CNS, whether the effect is mediated through inflammatory mechanisms, and whether there is interaction with gene variants associated with schizophrenia.
1. : Toxoplasma, in Manual of Clinical Microbiology. Edited by Murray PRBaron EJPfaller MATenover FCYolken RH. Washington, DC, American Society for Microbiology, 1999, pp 1374–1382Google Scholar
2. : Biology of toxoplasmosis, in Toxoplasmosis: A Comprehensive Clinical Guide . Edited by Joynson DHMWreghitt TG. Cambridge, UK, Cambridge University Press, 2001, pp 1–42Crossref, Google Scholar
3. : Immunology of Toxoplasma infection, (Ibid), in Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge, UK, Cambridge University Press, 2001, pp 43–57Crossref, Google Scholar
4. : Toxoplasma and schizophrenia. Parasite Immunol 2009; 31:706–715Crossref, Medline, Google Scholar
5. : Are some cases of psychosis caused by microbial agents? a review of the evidence. Mol Psychiatry 2008; 13:470–479Crossref, Medline, Google Scholar
6. : Infectious agents and gene-environmental interactions in the etiopathogenesis of schizophrenia. Clin Neurosci Res 2006; 6: 97–109Crossref, Google Scholar
7. : Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007; 33:729–736Crossref, Medline, Google Scholar
8. : Anti-Toxoplasma gondii antibodies in patients with schizophrenia: preliminary findings in a Turkish sample. Schizophr Bull 2007; 33: 789–791Crossref, Medline, Google Scholar
9. : The schizophrenia and Toxoplasma gondii connection: infectious, immune, or both? Adv Ther 2008; 25:703–709Crossref, Medline, Google Scholar
10. : Psychosis may be associated with toxoplasmosis. Med Hypotheses 2009; 73:799–801Crossref, Medline, Google Scholar
11. : Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis 2006; 6:178Crossref, Medline, Google Scholar
12. : Seropositivity of toxoplasmosis in patients with schizophrenia. J Egypt Public Health Assoc 2005; 80:509–524Medline, Google Scholar
13. : A controlled prospective study of Toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull 2007; 33:782–788Crossref, Medline, Google Scholar
14. : Selected infectious agents and risk of schizophrenia among US military personnel. Am J Psychiatry 2008; 165:99–106Link, Google Scholar
15. : Feasibility of neonatal screening for Toxoplasma infection in the absence of prenatal treatment (Danish Congenital Toxoplasmosis Study Group). Lancet 1999; 353:1834–1837Crossref, Medline, Google Scholar
16. : Detection by enzyme immunosorbent assay of Toxoplasma gondii IgG antibodies in dried blood spots on PKU-filter paper from newborns. Scand J Infect Dis 1995; 27:259–263Crossref, Medline, Google Scholar
17. : The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 2006; 53:441–449Medline, Google Scholar
18. : The Danish Psychiatric Central Register. Dan Med Bull 1997; 44:82–84Medline, Google Scholar
19. : Family history, place and season of birth as risk factors for schizophrenia in Denmark: a replication and reanalysis. Br J Psychiatry 2001; 179:46–52Crossref, Medline, Google Scholar
20. : Modern Epidemiology. Philadelphia, Lippincott Williams & Wilkins, 2008Google Scholar
21. : Statistical Models in Epidemiology. New York, Oxford University Press, 1993Google Scholar
22. : Gender differences in young adults with first-episode schizophrenia spectrum disorders at baseline in the Danish Opus Study. J Nerv Ment Dis 2007; 195:396–405Medline, Google Scholar
23. : Reliability of clinical ICD-10 schizophrenia diagnoses. Nord J Psychiatry 2005; 59:209–212Crossref, Medline, Google Scholar
24. : Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 2007; 33:745–751Crossref, Medline, Google Scholar
25. : Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation 2009; 16:122–133Crossref, Medline, Google Scholar
26. : Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet 2005; 6:7Crossref, Medline, Google Scholar
27. : Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr Bull 2009; 35:1163–1182Crossref, Medline, Google Scholar
28. : Parasitic brain infection, endocannabinoids, and schizophrenia. Med Hypotheses 2009; 72:220–222Crossref, Medline, Google Scholar
29. : Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull 2007; 33:652–653Crossref, Medline, Google Scholar
30. : Molecular mimicry in infectious encephalitis and neuritis: binding of antibodies against infectious agents on Western blots of human nervous tissue. J Infect 2000; 41:32–38Crossref, Medline, Google Scholar
31. : Molecular mimicry: anti-DNA antibodies bind microbial and nonnucleic acid self-antigens. Curr Top Microbiol Immunol 2005; 296:137–151Medline, Google Scholar
32. : Systemic lupus erythematosus. N Engl J Med 2008; 358:929–939Crossref, Medline, Google Scholar
33. : Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003; 349:1543–1554Crossref, Medline, Google Scholar
34. : Antibodies and neuronal autoimmune disorders of the CNS. J Neurol 2010; 257:509–517Crossref, Medline, Google Scholar
35. : Acute psychosis associated with anti-NMDA-receptor antibodies and bilateral ovarian teratomas: a case report. J Clin Psychiatry 2010; 71:504Crossref, Medline, Google Scholar
36. : Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect 2009; 11:1011–1018Crossref, Medline, Google Scholar



