Schizophrenia in the Offspring of Antenatally Depressed Mothers in the Northern Finland 1966 Birth Cohort: Relationship to Family History of Psychosis
Abstract
Objective
Maternal depression is relatively common during pregnancy. The authors examined whether maternal antenatal depressed mood increased the risk of schizophrenia and other psychoses among offspring with and without a familial history of psychosis.
Method
In the Northern Finland 1966 birth cohort, mothers of 12,058 children were asked at mid-gestation at the antenatal clinic if they felt depressed. The offspring were followed for over 30 years, and subsequent schizophrenia and other psychoses were detected using the Finnish Hospital Discharge Register, which was also used for identifying psychosis in the parents. Familial risk for psychosis was considered as a genetic risk factor and mothers' depressed mood as an environmental or genetic risk factor.
Results
The risk for schizophrenia was higher in the offspring with both maternal depressed mood during pregnancy and parental psychosis (OR=9.4, 95% CI=4.2–20.9 adjusted for sex and perinatal complications) than in those with a depressed mother but without parental psychosis (OR=1.0, 95% CI=0.6–1.8) or those without maternal depression and with a psychotic parent (OR=2.6, 95% CI=1.2–5.4). The reference group was birth cohort members without maternal antenatal depression and without parental psychosis.
Conclusions
Maternal depressed mood during pregnancy per se is unlikely to increase the risk for schizophrenia in the offspring but may affect subjects with a family history for psychosis. This finding could be an example of a gene-environment or possibly a gene-gene interaction in the development of schizophrenia. Mothers' antenatal depression may act as additive factor for subjects vulnerable to schizophrenia.
Today, schizophrenia is considered to be a neurodevelopmental disorder (1) acting in utero and in early childhood (2) with high genetic vulnerability (3). The strongest known risk factor for schizophrenia is familial risk, with a 10-fold increase in risk to siblings of probands (3).
There is evidence of gene-environment interaction in the origins of schizophrenia and other psychoses (4, 5). It is possible that some of the impact of genes on the occurrence of schizophrenia is mediated through environmental risk factors such as complications of birth and pregnancy, a dysfunctional early family rearing environment, stressful life events, and cannabis (6). There is also some evidence of gene-gene interaction causing additional risk for schizophrenia (7, 8).
Jones et al. (9) found that among the Northern Finland 1966 birth cohort, maternal depressed mood during pregnancy was elevated in schizophrenia patients. In subsequent analyses we found that depressed mood during pregnancy was more common in the mothers of schizophrenia patients who had a family history of psychosis compared with the mothers of schizophrenia patients without a family history of psychosis (10).
Maternal depression is common during both pregnancy and the postnatal period; it affects 10%–15% of mothers (11, 12). Clarification of the relationship between antenatal depression and risk for schizophrenia is important, not least because elucidation of this relationship could ultimately influence the treatment of antenatal depression.
Method
Data were gathered on maternal depressed mood during pregnancy, parental psychosis (familial risk), and hospital-treated schizophrenia and other psychoses among subjects of the Northern Finland 1966 birth cohort. Data collection of the offspring started antenatally, and the cohort was followed until the year 2001 when the subjects were 35 years of age.
Subjects
The Northern Finland 1966 birth cohort is an unselected, population-based sample of 12,058 children born alive (13). It covers 96.3% of all live births in the provinces of Oulu and Lapland in Finland with an estimated date of birth between January 1, 1966, and December 31, 1966. This general population birth cohort has been followed for more than 30 years. Data include all subjects alive and living in Finland at age 16 years (N=11,017). Permission to gather data was obtained from the Ministry of Social and Health Affairs, and the study has been approved by the Ethics Committee of the Faculty of Medicine of the University of Oulu.
Maternal Depressed Mood During Pregnancy
Data concerning the mother and offspring were gathered antenatally and at birth in 1965–1966. As part of the antenatal data collection, the mothers were asked by the interviewing nurse at the antenatal clinic during midgestation (mainly between the 24th and 28th gestational week) whether they felt that their mood during pregnancy had so far been normal, depressed, or very depressed; 13.9% of the mothers rated themselves as depressed (11.8%) or very depressed (2.1%) during pregnancy (10). In the analyses, these two categories were considered as "depressed." The information regarding the mothers' antenatal mood was available for 10,658 (96.7%) offspring living in Finland at the age of 16 years.
Maternal Later Depression
Data on mothers' immediate postnatal mood were not available, but we did have information on later severe, hospital-treated maternal depression, dating from the time when offspring were 6 years of age and onward. Of all the cohort members, mothers appearing on the Finnish Hospital Discharge Register between 1972–1997 for any depression (i.e., ICD-8 codes 2960, 2968, 3004; ICD-9 codes 2961, 2968, 3004; and ICD-10 codes F32-F34) were identified. Diagnoses of depression included both nonpsychotic and psychotic episodes.
Parental Psychosis
All the cohort members' mothers and fathers appearing on the Finnish Hospital Discharge Register between 1972–1997 for any psychosis (i.e., ICD-8 and ICD-9 codes 295–299 and ICD-10 codes F20-33, except nonpsychotic mood disorders) were identified. We grouped cohort members into those with and without a psychotic parent to reflect the familial risk for psychosis.
Diagnosis of Schizophrenia or Other Psychosis in Offspring
All cohort members over 16 years appearing on the Finnish Hospital Discharge Register between years 1982–1997 for any mental disorder were identified (14, 15). The Finnish Hospital Discharge Register covers all mental and general hospitals, beds in local health centers, and private hospitals nationwide. We used all available information to provide diagnostic information. All case records were scrutinized, and diagnoses were validated against DSM-III-R criteria, after which the diagnoses were rereviewed by a professional panel. There were 160 subjects with a psychotic episode prior to age of 31 years, of whom 146 were living in Finland in 1999.
Those 146 subjects were asked to participate in a field study during 1999–2001, and 91 subjects agreed to participate. The Structured Clinical Interview for DSM-III-R (SCID-I) (16) and all available anamnestic information of the subjects with hospital case records were used for diagnostic assessment: 61 cases with a diagnosis of schizophrenia and 12 subjects with other psychoses were detected (17). The diagnoses of the subjects who did not participate in the field study were based on validation of hospital notes against DSM-III-R criteria (15, 18).
Through these methods, a total of 111 cases of DSM-III-R schizophrenia and 45 cases of other psychosis were identified; the cumulative incidence of schizophrenia in the members of the Northern Finland 1966 birth cohort was 1.0%. The other psychoses included both affective and nonaffective psychoses, except schizophrenia. Data on maternal depressed mood were missing in four cases of schizophrenia (schizophrenia cases with information on maternal depressed mood: N=107) and in one case of other psychosis (N=44).
Potentially Confounding Variables
Perinatal complications.
Mothers with schizophrenia or affective disorder have been found to have increased risks of pregnancy, birth, and neonatal complications (19). In an earlier report of the Northern Finland 1966 birth cohort, perinatal insults were linked to elevated risk for schizophrenia in the offspring (9); therefore, in the present study, perinatal complications were treated as a potentially confounding factor. The classification of this factor for perinatal complications was dichotomized (1=no complications, 2=low birth weight [<2500 g] or short gestation time [<37 weeks] or perinatal brain damage as described by Rantakallio et al. [20]).
Sex. The risk for schizophrenia has been found to be higher in men than in women (21), hence we considered sex a covariate in our analyses.
Maternal smoking.
Although maternal smoking did not predict schizophrenia in the offspring in this birth cohort (9), another Finnish study indicated that mothers with schizophrenia smoke more during pregnancy than comparison groups (22). Therefore an additional logistic regression analysis was performed that also took maternal smoking in pregnancy into account. The classification of this factor for maternal smoking was dichotomized (1=none or the mother stopped before pregnancy, 2=smoked more than one cigarette daily during the entire duration of pregnancy) (23). Data on mothers' smoking were available for 10,355 cohort members living in Finland at age 16 years.
Statistical Analysis
Cross-tabulations and chi-square tests (Pearson two-sided and Wald) were used to assess the relationships between maternal depressed mood during pregnancy, parental psychosis, and cumulative incidence of schizophrenia (and other psychoses) in the offspring. Unless otherwise stated, we treated the offspring without parental psychosis and without maternal depression as a reference group. We also conducted additional analyses in the subgroups with a family history of psychosis where offspring with parental psychosis but no maternal antenatal depression were the reference group. Crude and adjusted odds ratios with 95% confidence intervals (95% CI) were calculated for the different groups of offspring. Since there was little difference between crude and adjusted odds ratio and confidence interval data, adjusted results are presented in this article with crude odds ratios and confidence intervals included in a data supplement that accompanies the online version of this article. Estimates were calculated for offspring with hospital-treated schizophrenia, other psychosis and any psychosis, and these were all compared with the category of nonpsychotic offspring (healthy offspring or offspring with nonpsychotic mental disorder). Logistic regression analyses (24) were conducted to examine the association of maternal antenatal mood, parental psychosis, and schizophrenia in the offspring and were adjusted for sex and obstetric complications. Analyses were performed using SPSS 16.0 for Windows statistical software.
Results
Cumulative Incidence of Schizophrenia in Offspring
The cumulative incidence of schizophrenia was 0.9% among children of nondepressed mothers and 1.3% among offspring of mothers with antenatal depressed mood (Table 1). The prevalence of other psychosis was 0.4% in both groups.
 |
Of the offspring with parental psychosis, 3.3% had schizophrenia, compared with 0.9% of those without a psychotic parent (adjusted odds ratio=3.9, 95% CI=2.2–6.8). Fifteen of the 107 schizophrenia patients (14.0%) had a history of psychosis in one or both parents. Ten schizophrenia patients had a history of maternal psychosis and six had a father with psychosis (one had a history of psychosis in both parents).
Of the other psychosis patients, 15.9% (N=7 of 44) had a history of parental psychosis. Five of them had a history of maternal psychosis, and three had a father with psychosis (one had a history of psychosis in both parents). There were 92 (86.0%) schizophrenia cases and 37 (84.1%) other psychosis patients without a known psychotic episode in their parents.
Risk of Schizophrenia in Offspring
Among offspring of mothers with depressed mood during pregnancy, the risk for schizophrenia was higher where there was a history of psychosis in a parent (adjusted odds ratio=9.4, 95% CI=4.2–20.9; p<0.001) than in those with only a depressed mother but without parental psychosis (adjusted odds ratio=1.0; 95% CI=0.6–1.8) and was also higher than the risk in those without maternal depression and with only a psychotic parent (adjusted odds ratio=2.6; 95% CI=1.2–5.4; p=0.011) (Table 1). The reference group was birth cohort members without maternal antenatal depression and without parental psychosis. The pattern of results for risk of other psychoses in the offspring was very similar to that for risk of schizophrenia in the offspring.
The adjusted odds ratio for schizophrenia was 14.2 (95% CI=4.9–41.2) among offspring of mothers with depressed mood and fathers with psychosis (Table 1). The respective adjusted odds ratio was 8.0 (95% CI=2.8–22.8) among offspring of mother with both antenatal depression and psychosis.
When we restricted our analysis to only the offspring of parents with psychosis, maternal antenatal depressed mood was associated with increased risk for schizophrenia (adjusted odds ratio=3.6, 95% CI=1.3–10.2; Wald χ2=5.7, p=0.017 [Table 2]). Here the reference group is offspring with a family history of psychosis but no maternal antenatal depression. The risk for schizophrenia was statistically significantly higher in the offspring with psychosis in the father and antenatal depression in the mother than in the subjects with paternal psychosis but no maternal depression (adjusted odds ratio=9.0, 95% CI=1.6–51.7; Wald χ2=6.1, p=0.013).
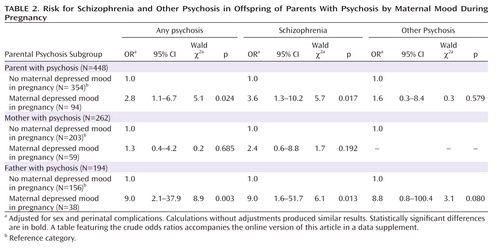 |
The cumulative incidences of schizophrenia in the offspring by presence of maternal antenatal depression and familial risk for psychosis are presented in Figure 1, which shows the effect of the combination of maternal depressed mood and familial psychosis risk on the age at onset of psychotic symptoms in schizophrenia.

Other Potential Confounding Variables
In further logistic regression analyses, we made additional adjustments for maternal smoking in pregnancy. The risks for schizophrenia in the offspring remained at the same level. Among offspring of mothers depressed during pregnancy, the risk of schizophrenia was higher among those with at least one psychotic parent (adjusted odds ratio=8.7, 95% CI=3.7–20.8) than in those with no parental psychosis (odds ratio=1.0, 95% CI=0.5–1.8) or those with only a psychotic parent and no maternal depressed mood (odds ratio=2.3, 95% CI=1.0–5.0).
Maternal antenatal depressed mood was linked to later hospitalization of the mothers due to depression. Of mothers with antenatal depressed mood, 77 (5.2%) had later hospital-treated depression, whereas 261 (2.8%) mothers without depression during pregnancy were later hospitalized due to depression (odds ratio=1.9, 95% CI=1.4–2.4). Of the offspring with schizophrenia, 12 had mothers with hospital-treated depression later on, two with both antenatal depressed mood and later severe depression.
Discussion
Mothers' depressed mood during pregnancy per se is unlikely to increase the risk for schizophrenia in the offspring but may affect subjects at risk for psychosis. Of those individuals with an antenatally depressed mother, the risk for schizophrenia was significantly higher in those individuals with a history of psychosis in one or more parents relative to those without a family history of psychosis. Of the risk factors we considered, the combination of the father having psychosis and an antenatally depressed mother resulted in the highest risk of schizophrenia, with an odds ratio of 14.2 (95% CI=4.9–41.2).
The results of the study can be interpreted in more than one way. One interpretation is that our findings reflect gene-environment interaction in the development of schizophrenia. A history of psychosis in one or both parents is usually considered to be indicative of a genetic risk for schizophrenia, and antenatal depression could be an environmental exposure acting adversely on fetal neurodevelopment and rearing during childhood in those who already have a genetic vulnerability. Under this interpretation maternal depressed mood in pregnancy may act as an additive factor for subjects with a preexisting vulnerability for schizophrenia. On the above assumption, familial psychosis is considered to be a genetic factor and maternal depression an environmental risk factor. However, familial psychosis may not be only genetic and maternal depressed mood may not be entirely an environmental factor.
An alternative explanation for what appears to be a gene-environment interaction is an underlying gene-gene interaction. In our study, depression among mothers may have been the consequence of genetic vulnerability. There is evidence that subjects with schizophrenia may inherit risk for the illness through at least two different loci or that a gene-gene interaction can raise the risk of schizophrenia (7, 8). It may be that genes predisposing to depression together with genes predisposing to schizophrenia have an additive effect on the vulnerability to psychosis. The increase in risk for schizophrenia in offspring of depressed mothers may be a psychosis gene-depression gene interaction.
Maternal antenatal depression is a risk factor for perinatal complications, such as low birth weight (25) and preterm delivery (26), which may be risk factors for subsequent schizophrenia (27). However, in the present study the increased risk for psychosis in the offspring of antenatally depressed mothers and one parent with psychosis was present both before and after adjustment for perinatal complications.
There is evidence of increased rates of schizophrenia in the children of mothers exposed to antenatal stress (28). One possibility is that depression is associated with overactivity of the hypothalamic-pituitary-adrenal (HPA) axis and consequent secretion of glucocorticoids that resembles the neuroendocrine response to stress (29). Oversecretion of glucocorticoids during pregnancy has been shown to elevate the glucocorticoid levels in the fetus (29, 30), which can alter the function of the HPA axis in the fetus and disturb fetal brain development (31). The dysfunction of the HPA axis can cause short- and long-term abnormalities such as reduced birth weight, increased infant morbidity, locomotion and cognition retardation, increased anxiety, and sleep disturbances (31). The association between prenatal exposure to maternal stress and later schizophrenia may also be mediated by the direct impact of stress, such as fetal hypoxia induced by vasoconstriction (30). Early stress may lead to dopaminergic hypersensitivity, which is connected to psychosis (32).
Maternal antenatal depression also increases the risk for postnatal depression (33), which is connected to adverse cognitive and emotional infant outcome (34). Depression can directly affect the mother's responsiveness and sensitivity during mother-infant interaction, resulting in either intrusive interactions or withdrawal (26). Maternal depression and patterns of parental care may increase stress reactivity in offspring and may mediate the effects of environmental adversity on neural development (35).
The strongest known risk factor for schizophrenia is familial risk with genetic loading, which has an association with a 10-fold increase in risk to siblings of probands (3). Caspi et al. (5) found that adolescent cannabis exposure is associated with increased risk of schizophreniform disorders among individuals with certain cathecol-O-methyltransferase (COMT) genotypes. In that study, cannabis exposure was considered as an environmental risk factor and Val/Val or Val/Met COMT genotype as a genetic risk factor for schizophreniform disorder. In the present study, the risk for psychosis and schizophrenia was the highest—approximately 14-fold—in the offspring whose father had a history of psychosis (e.g., genetic vulnerability) and whose mother additionally had antenatally depressed mood (environmental risk factor). Adverse psychosocial circumstances in early life—such as antenatal depressed mood—may interact with genetic vulnerability to increase the risk of psychosis (36). On the other hand, maternal depression might also be a proxy for mothers' poor diet or parental substance use or father's depression during pregnancy.
In this study, the prevalence of maternal depressed mood, asked mainly between the 24th and 28th gestational week, was 13.9%. The prevalence is in the same range as in previous studies dealing with the rates of antenatal depression in the second or last trimester (11, 12). In fact, it was almost the same as in the British study with 12,059 pregnant women reported by Evans et al. (12), where 13.5% of the women had probable depression at 32 weeks of pregnancy scored by the Edinburgh postnatal depression scale.
The cumulative incidence of schizophrenia in this study was 1.0%, which is relatively high when compared to the estimated overall (21 studies) lifetime prevalence of 0.4% (10%–90% quantile: 0.2–1.2%) reported by a recent meta-analysis (37). It is still in line with earlier findings of schizophrenia in Finland, such as 1.2% (38) and 1.3% (39). In the Finnish Health-2000 study, the lifetime prevalence for schizophrenia was recently reported to be 0.87% (40). In the present study 1.8% of the offspring with antenatally depressed mothers and 1.4% of the rest of the cohort had had a hospital-treated psychotic episode; the prevalence of all psychoses has been found to be 2.2% in general population in Finland in a former study (39) and 3.1% in a recent study (40).
The odds ratio for parental psychosis in this study is lower than often reported (3). Maybe we have missed psychotic illness in some of the parents, since the follow-up of the Finnish Hospital Discharge Register began when the child was 6 years, and no data were available at the time of pregnancy. The risk might have been somewhat higher if we had used the category of parental schizophrenia, but we used the broader category of psychosis in the parent.
Limitations of the Study
There are some limitations in the study. First, it is notable that maternal depressed mood did not signify a clinical condition but was the mothers' self-reported depressed mood. However, the prevalence of antenatally depressed mood was in the same range as in earlier reports (11, 12). Furthermore, mothers reported their mood to the nurse who had met with them on their earlier visits to the antenatal clinic, i.e., who had known them earlier (10). Another limitation of this study is the absence of information about maternal postnatal mental state. Antenatal depression is a risk factor for postnatal depression. However, information on later hospital-treated maternal depression was available from the offspring's age of 6 years onward. Many subjects with schizophrenia have comorbid depression and may describe their negative symptoms or disorganization as depression—the self-report of depression may represent more active or more significant disease (perhaps both a genetic and environmental risk factor). Data on maternal use of psychiatric medication and on parental psychoses during pregnancy and early childhood were lacking. Information was not available on mothers' nutrition and parents' substance use during pregnancy. The lack of fathers' depression history is a major limitation that makes it difficult to discriminate gene-gene versus gene-environmental hypotheses.
Strengths of the Study
A major strength of the study is that this was a prospective project with a long follow-up time, started antenatally. The subjects were representative, with all cohort members born in the same year and in a geographically defined area. The topic of the study is novel; to the authors' knowledge, this is the first report of schizophrenia in the offspring of antenatally depressed mothers where familial vulnerability was taken into account in a population-based sample.
Conclusions
Maternal depressed mood during pregnancy per se is unlikely to increase the risk for schizophrenia in the offspring but may affect subjects with increased risk for psychosis. This finding is an example of a gene-environment or gene-gene interaction in the development of schizophrenia. The study suggests that mothers' antenatal depressed mood may act as an additive factor for subjects vulnerable to schizophrenia.
1 : From neuropathology to neurodevelopment. Lancet 1995; 346:552–557 Crossref, Medline, Google Scholar
2 : Neonatal origins of schizophrenia. Arch Disease in Childhood 1998; 78:1–3 Crossref, Medline, Google Scholar
3 : Schizophrenia Genesis: The Origins of Madness. New York, WH Freeman and Company, 1991 Google Scholar
4 : Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry 1997; 154:355–362 Link, Google Scholar
5 : Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene x environment interaction. Biol Psychiatry 2005; 57:1117–1127 Crossref, Medline, Google Scholar
6 : The ecogenetics of schizophrenia: a review. Schizophr Res 1998; 32:127–135 Crossref, Medline, Google Scholar
7 : Evidence for the multigenic inheritance of schizophrenia. Am J Med Genetics part B: Neuropsychiatric Genetics 2001; 105:794–800 Crossref, Google Scholar
8 : Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behavioral and Brain Functions 2007; 3:31 (available from: http://www.behavioralandbrainfunctions.com/content/3/1/31) Crossref, Medline, Google Scholar
9 : Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 North Finland general population birth cohort. Am J Psychiatry 1998; 155:355–364 Link, Google Scholar
10 : Schizophrenia in the offspring of antenatally depressed mothers: a 31-year follow-up of the Northern Finland 1966 birth cohort. Schizophr Res 2004; 66:79–81 Crossref, Medline, Google Scholar
11 : Predicting depressive symptomatology: cognitive-behavioral models and postpartum depression. J Abnorm Psychol 1982; 91:457–461 Crossref, Medline, Google Scholar
12 : Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260 Crossref, Medline, Google Scholar
13 : Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scan 1969; 193:1–71 Medline, Google Scholar
14 : A comparison of clinical and research DSM-III-R diagnoses of schizophrenia in a Finnish national birth cohort. Soc Psychiatry Psychiatr Epidemiol 1997; 32:303–308 Crossref, Medline, Google Scholar
15 : Reasons for the diagnostic discordance between clinicians and researchers in schizophrenia in the Northern Finland 1966 birth cohort. Soc Psychiatry Psychiatr Epidemiol 2003; 38:305–310 Crossref, Medline, Google Scholar
16 : Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989 Google Scholar
17 : Outcome and its predictors in schizophrenia within the Northern Finland 1966 birth cohort. Eur Psychiatry 2007; 22:129–136 Crossref, Medline, Google Scholar
18 : Recovery from schizophrenic psychoses within the Northern Finland 1966 birth cohort. J Clin Psychiatry 2005; 66:375–383 Crossref, Medline, Google Scholar
19 : Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry 2005; 162:79–91 Link, Google Scholar
20 : Prognosis of perinatal brain damage: a prospective study of a one year birth cohort of 12,000 children. Early Hum Dev 1987; 15:75–84 Crossref, Medline, Google Scholar
21 : Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 2003; 60:565–571 Crossref, Medline, Google Scholar
22 : The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res 2007; 93:229–236 Crossref, Medline, Google Scholar
23 : Criminality in the offspring of antenatally depressed mothers: a 33-year follow-up of the Northern Finland 1966 birth cohort. J Affect Disord 2003; 74:273–278 Crossref, Medline, Google Scholar
24 : Generalized Linear Models, 2nd ed. New York, Chapman & Hall, 1989, pp 89–148 Google Scholar
25 : Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand 2007; 115:481–486 Crossref, Medline, Google Scholar
26 : Effects of parental mental illness on children. Psychiatry 2006; 5:10–12 Crossref, Google Scholar
27 : Perinatal risk factors for schizophrenia: diagnostic specificity and relationships with maternal psychopathology. Am J Med Gen 2002; 114:898–905 Crossref, Medline, Google Scholar
28 : Prenatal exposure to maternal stress and subsequent schizophrenia: the May 1940 invasion of the Netherlands. Br J Psychiatry 1998; 172:324–326 Crossref, Medline, Google Scholar
29 : Depression, stress and the adrenal axis. J Neuroendocrin 2003; 15:811–812 Crossref, Medline, Google Scholar
30 : Maternal stress or anxiety in pregnancy and emotional development of the child. Br J Psychiatry 1997; 171:105–106 Crossref, Medline, Google Scholar
31 : Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 2003; 27:119–127 Crossref, Medline, Google Scholar
32 : Risk factors for schizophrenia: all roads lead to dopamine. Eur Neuropsychopharmacology 2007; 17(suppl 2):S102–S107 Crossref, Medline, Google Scholar
33 : Correlates of postnatal depression in mothers and fathers. Br J Psychiatry 1996; 169:36–41 Crossref, Medline, Google Scholar
34 : Postpartum depression and child development. Psychol Med 1997; 27:253–260 Crossref, Medline, Google Scholar
35 : Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 2001; 24:1161–1192 Crossref, Medline, Google Scholar
36 : Developmental brain abnormalities in the offspring of schizophrenic mothers, 1: contributions of genetic and environmental factors. Arch Gen Psychiatry 1993; 50:551–564 Crossref, Medline, Google Scholar
37 : A systematic review of the prevalence of schizophrenia. PLoS Med 2005; 2(5);e141:413–433. Epub (
38 : Schizophrenia in the genetic isolate of Finland. Am J Med Genet 1997; 74:353–360 Crossref, Medline, Google Scholar
39 : Prevalence of mental disorders among adults in Finland: basic results from the mini Finland health survey. Acta Psychiatr Scand 1990; 81:418–425 Crossref, Medline, Google Scholar
40 : Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 2007; 64:19–28 Crossref, Medline, Google Scholar



