Neural Activation Underlying Acute Grief in Women After the Loss of an Unborn Child
Abstract
Objective: The traumatic loss of an unborn child by induced termination of pregnancy because of fetal malformation is a major life event that causes intense maternal grief. Increasing evidence supports the hypothesis that the same neural structures involved in the experience of physical pain are involved in the experience of social pain and loss. Method: To investigate neural activation patterns related to acute grief, the authors conducted a functional MRI study of 12 post-termination women and 12 noninduced women who delivered a healthy child. Brain activation was measured while participants viewed pictures of happy baby, happy adult, and neutral adult faces. Results: Relative to comparison women, post-termination women showed greater activation in the middle and posterior cingulate gyrus, the inferior frontal gyrus, the middle temporal gyrus, the thalamus, and the brainstem in response to viewing happy baby faces. Functional connectivity between the cingulate gyrus and the thalamus during the processing of happy baby faces was significantly stronger in post-termination women. Conclusions: Overall, acute grief after the loss of an unborn child was closely related to the activation of the physical pain network encompassing the cingulate gyrus, the inferior frontal gyrus, the thalamus, and the brainstem. To the authors’ knowledge, the stronger functional thalamocingulate connectivity in post-termination women is the first in vivo demonstration of an involvement of the neural maternal attachment network in grief after the loss of an unborn child.
The loss of an unborn child after induced termination of pregnancy because of fetal anomaly in late pregnancy is a major life event that is accompanied by intense acute psychological reactions. Instead of joy and happiness, women have to face grief and mourning. Although they did not actually know the child, women experience the loss as intensely as that of a close and emotionally important person, sometimes with enduring reactions of grief and severe psychological problems (1 – 3) . Grief-related cues, such as baby buggies, toys, and other babies, provoke loss-related emotions in women after termination of pregnancy and are therefore often avoided in everyday life.
In general, grief as an instant and individual response to the loss of an emotionally important person refers to affective, physiological, and psychological reactions (4) . The loss of a loved one is often spoken of in terms of painful feelings, and increasing evidence has shown that such metaphors do indeed reflect what is happening in the brain. Psychological pain, especially the separation from or the loss of close others, seems to share the same neural pathways that are associated with physical pain (5 , 6) . One explanation for this phenomenon is the importance of close social ties for survival in mammals. Panksepp and colleagues (7) were the first to discuss the overlap in the systems underlying physical and social pain based on the effect of exogenous opiates on pain tolerance in socially isolated puppies.
Imaging studies of the physical pain system have most consistently produced activation in somatosensory cortices, the insula, the anterior cingulate cortex, the inferior frontal gyrus, and the thalamus (8) . The social pain network, as proposed by Eisenberger (6) , encompasses activations in the dorsal anterior cingulate cortex, the insula, the right ventral prefrontal cortex, and the periaqueductal gray matter in the brainstem during social rejection.
There have been limited functional imaging studies of grief-related activation patterns (9 – 11) . To our knowledge, no study has investigated acute grief after the loss of a child. In a study of women who had lost a first-degree relative in the past year, Gündel and colleagues (9) observed an increased activation in the anterior and posterior cingulate cortex, the insula, the inferior temporal and fusiform gyrus, the cuneus, and the superior lingual gyrus during the presentation of photographs of the deceased. O’Connor and colleagues (10) compared bereaved women with complicated or uncomplicated grief after the death of a mother or sister in the past 5 years. They observed activation in pain-related brain areas in both groups, although the women with complicated grief also showed activity in the nucleus accumbens, a subcortical structure that has been associated with reward.
In this study, we used functional MRI (fMRI) to investigate grief in women within 2 months after termination of pregnancy. To elicit acute grief, we used pictures of happy baby faces and compared results to those elicited by happy and neutral adult faces and a no-face baseline condition. We hypothesized that there would be greater activation in pain-related areas of the brain in post-termination women than in a comparison group of young women who had recently given birth to a healthy child. Building on nonhuman mammalian investigations, the thalamocingulate division has been regarded as important in mammalian mother-infant attachment as well as maternal behavior in humans (12 , 13) . We analyzed functional connectivity between the thalamus and the cingulate gyrus and hypothesized that a stronger thalamocingulate connectivity would be observed in post-termination women during the visualization of baby faces.
Method
Participants
Twelve women (mean age=30.2 years, SD=5.1) who had experienced the loss of an unborn child within the past 2 months by induced termination of pregnancy in the second or third trimester because of fetal malformation were included in the study. Termination of pregnancy was performed by induction of labor and delivery of a stillborn child at the Department of Gynecology and Obstetrics of the University of Muenster, Germany. Counseling was provided during the decision-making process for inducing termination of pregnancy and during the hospitalization period (14) . Specific loss-related therapeutic support was offered at the Department of Psychiatry. Grief was assessed by the German version of the Perinatal Grief Scale (15) . Post-termination women presented with a high level of grief (mean Perinatal Grief Scale score=3.4, SD=0.4)—higher than in women who suffered an early miscarriage before the 20th gestational week (mean Perinatal Grief Scale score=2.6, SD=0.7) (13) and higher than in post-termination women in comparable studies (see reference 16 , for example).
Our comparison group was 12 women (mean age=30.6 years, SD=4.2) who had a noninduced delivery of a healthy child in the past 12 months. None of the comparison women showed impaired mother-infant bonding according to the Postpartum Bonding Questionnaire (17 , 18) .
None of the participants had a history of psychiatric disorders according to the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) or neurological disorders according to clinical interview, and all had normal vision, were right-handed, were free of psychotropic medication, and were native German speakers. The study protocol was approved by the University of Muenster ethics committee according to the Declaration of Helsinki (1964), and written informed consent was obtained from all participants before they were enrolled in the study.
Post-termination women had significantly higher mean scores than comparison women on the Beck Depression Inventory (19) (17.0 [SD=9.5] compared with 2.4 [SD=1.9]; Mann-Whitney U=0.500, p<0.001) and the Montgomery-Åsberg Depression Rating Scale (20) (12.8 [SD=9.5] compared with 0.17 [SD=0.4]; U=7.000, p<0.001). Moreover, they had significantly higher mean state anxiety scores on the State-Trait Anxiety Inventory (21) (48.9 [SD=12.2] compared with 32.8 [SD=5.7]; U=9.000, p<0.001) and the Hamilton Anxiety Scale (22) (8.9 [SD=7.0] compared with 0.3 [SD=0.6]; U=1.5, p<0.001). Depressive symptoms shortly following the loss of an unborn child have been reported in other studies (see references 16 , 17 , for example). Given that none of the post-termination women had primary affective or anxiety disorders (according to the SCID), elevated scores for depression and anxiety were due to emotional distress.
Stimuli and Procedure
Facial stimuli consisted of gray-scale images of 10 babies with happy expressions as well as 10 images each of happy and neutral expressions in adults (23) . Pictures of baby faces were contributed by hospital staff and friends and rated by 168 medical students on a 7-point Likert scale to evaluate the emotional expression of the face. The 10 baby pictures that were rated highest for happiness were selected for the fMRI study. Participants saw alternating 30-second epochs of emotional faces (babies with happy expressions or adults with happy or neutral expressions) or a no-face control stimulus (a gray oval; Figure 1 ). Emotional stimuli were presented twice per second for 500 msec in a random sequence, and the no-face stimulus was presented 60 times per 30-second epoch (450 msec followed by a blank screen for 50 msec). The order of blocks was counterbalanced across participants, and each emotional epoch was preceded by a no-face condition and presented twice (overall presentation time, 6 minutes). Participants were told that they would see human faces and that they should pay attention to them. Images were presented via projection to the rear end of the scanner. The head position was stabilized with a vacuum head cushion. Immediately after the scanning, each participant rated the peak intensity of grief and happiness experienced during the presentation of the baby faces on a scale of 1–9, where 1=no grief/no happiness at all, and 9=most intense grief/happiness.
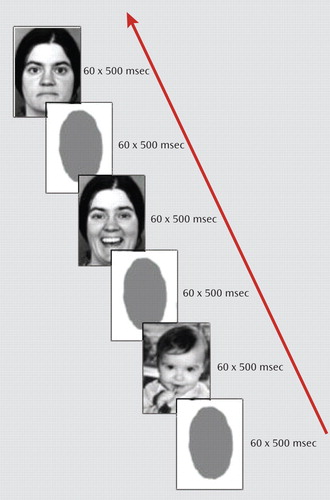
a The blocks were presented in random order to 12 women who had recently lost an unborn child by induced termination of pregnancy because of fetal malformation and 12 comparison women who had a noninduced delivery of a healthy child in the past 12 months.
fMRI
Transverse relaxation time [T 2 *] functional data were acquired with a 3-T scanner (Gyroscan Intera 3T, Philips Medical Systems, Best, the Netherlands) using a single-shot echo-planar sequence with parameters selected to minimize distortion in the regions of central interest while retaining an adequate signal-to-noise ratio and T 2 * sensitivity according to suggestions made by Robinson et al. (24) . Volumes consisting of 25 axial slices were acquired (matrix=128128, resolution=1.75 mm×1.75 mm×3.5 mm; repetition time=3 seconds, echo time=30 msec, flip angle=90°) 120 times in block designs, 10 times per condition. To optimize the following normalization procedures, the same sequence parameters were used to cover the entire brain with 43 slices. Additionally, two anatomical data sets were acquired: T 1 -weighted inversion recovery and a high-resolution T 1 -weighted three-dimensional sequence (isotropic pixel=0.5 mm 3 ).
Analysis
Functional imaging data were motion corrected using a set of six rigid body transformations determined for each image. Images were spatially normalized to standard Montreal Neurological Institute (MNI) space and smoothed (Gaussian kernel, 6 mm full width at half maximum) using statistical parametric mapping (SPM2, Wellcome Department of Imaging Neuroscience, London). Statistical analysis was performed by modeling the different conditions (babies with happy expressions, adults with happy or neutral expressions, and the no-face control condition) as variables within the context of the general linear model (convolved with a standard hemodynamic response function). A whole-brain analysis was conducted to determine brain regions that were differentially activated as a function of different emotional facial expressions.
On a single-subject level, we extracted contrast values for each of the three emotional face conditions relative to the no-face control condition. To differentially investigate the effect of emotion, we compared happy baby and happy adult versus neutral adult faces, and to assess the effect of presenting babies, we compared happy baby versus happy adult faces (and vice versa). First, one-sample t tests were performed on activation data. Random-effects analyses (t tests for independent samples) were then performed for emotional faces compared to the no-face baseline to examine brain activation differences between the two experimental groups. Since it has been shown that neutral faces are not a reliable baseline in group comparisons (25, 26), we applied the no-face baseline as the control condition in our between-group analyses. The significance threshold in the whole-brain analysis was set at 0.001, with clusters defined by at least eight contiguous voxels of significant response. This low cluster threshold was chosen to maximize sensitivity in the detection of activation differences between study groups. As noted in the tables, some of our results survived cluster-level correction (p<0.05). Anatomical labels of reported coordinates (transformed from MNI to Talairach space) of peak clusters were retrieved from the Talairach Daemon database (27) within a 5-mm cubical search range or from the SPM anatomy toolbox (28) .
Functional connectivity has been defined as the (undirected) correlation between two or more fMRI time series recorded from distributed brain regions. To evaluate interdependency of the thalamocingulate circuitry during the presentation of grief-related stimuli, correlations between MR signal time courses of the anterior, middle, and posterior cingulate gyrus and the thalamus were selected as regions of interest (29) and specified for happy baby faces, happy adult faces, and neutral adult faces separately using the MarsBaR toolbox (30) . Results were then compared between the two groups.
Results
Activations in Response to Emotional Faces
Relative to the no-face baseline, each face condition elicited differential significant activations in predominantly cortical but also subcortical emotion processing areas in both groups (see Figure S1 in the data supplement that accompanies the online edition of this article). Brain activation in response to happy baby faces was contrasted with activation in response to neutral adult faces in each group ( Table 1 ). Post-termination women showed increased activation patterns in the left middle and posterior cingulate gyrus (Brodmann’s area [BA] 31), the left superior frontal gyrus (BA 9), the right precentral gyrus (BA 6), and the temporo-occipital areas (BA 39/36/17 and 18). Increased activation in comparison women encompassed the fusiform gyrus bilaterally (BA 19/37), the cuneus bilaterally (BA 18/19), the left lingual gyrus (BA 18), the left superior, middle, and inferior temporal cortex (BA 38/39/20), the left superior and middle occipital gyrus (BA 19), and the cerebellum. In comparison women, however, no significant activation in the cingulate gyrus was observed. Happy adult versus neutral faces elicited an increased activation in the middle frontal gyrus (BA 46) in post-termination women and an increased activation in the inferior parietal lobule (BA 39) and precentral gyrus (BA 6) in comparison women. Contrasts of happy baby versus happy adult faces ( Table 1 ) revealed significantly increased baby-related activations in the posterior and anterior cingulate cortex (BA 30/31/32), the medial frontal gyrus (BA 11), and temporo-occipital areas (BA 19/18/31) in post-termination women, whereas in comparison women increased activations were restricted to the temporo-occipital cortex (BA 37/17/18/19).
Between-Group Activations in Response to Happy Baby Faces
A group comparison revealed significantly greater activation in post-termination women relative to comparison women in response to happy baby faces in the right middle anterior cingulate cortex (BA 24), the left posterior cingulate cortex (BA 23), the left and right inferior frontal gyrus (BA 47), the right middle temporal gyrus (BA 21), the left cuneus (BA 18), the left thalamus, and the right brainstem (periaqueductal gray matter) ( Table 2 , Figure 2 ). Comparison women showed increased activation of the right lingual gyrus (BA 17) relative to post-termination women.

a Two-sample t tests, p<0.001, uncorrected. All activations are rendered on a single subject template in Montreal Neurological Institute space.
Between-Group Activations in Response to Happy and Neutral Adult Faces
Post-termination women did not show any areas of greater brain activation relative to comparison women in response to happy and neutral adult faces. Comparison women showed significantly greater activation in the right fusiform gyrus (BA 19), the right lingual gyrus (BA 17), and the left middle temporal gyrus (BA 39) relative to post-termination women during presentation of happy adult faces ( Table 2 ). In response to neutral adult faces, comparison women showed a small cluster of greater activation in the right lingual gyrus (BA 17) relative to post-termination women ( Table 2 ).
Functional Connectivity Between Thalamocingulate Regions
Results from our time-series analyses suggest a stronger functional connectivity between the anterior, middle, and posterior cingulate gyrus and the thalamus in post-termination women relative to comparison women in response to happy baby faces ( Table 3 ). In the case of happy and neutral adult faces, no significant group differences were observed.
Subjective Ratings
After scanning, post-termination women rated the presentation of baby faces as inducing a significantly higher degree of sadness (t=4.710, df=22, p<0.001) and a significantly lower degree of happiness (t=–2.994, df=22, p<0.001) than did comparison women.
Discussion
The death of a child is an incomprehensible and devastating loss causing intense parental grief and mourning. In our study, after the loss of an unborn child due to malformation, women showed increased activation in a large number of brain areas related to the physical pain network while viewing happy baby faces. As psychological pain has recently been shown to share some of the same neural pathways that are associated with physical pain, our results are consistent with data from previous studies of grief and social rejection (6 , 9 – 10) . One mechanism that has been suggested as an explanation of why social separation is considered painful follows from the evolutionary advantages of close social ties for survival in mammalian species.
The dorsal anterior or middle cingulate cortex has been shown to activate in response to physical as well as social pain. It has been related to the affective component of physical pain and the distress of social rejection. In our study, increased activity in the middle cingulate cortex was distinctly observed for happy baby faces in post-termination women, thus underlining the assumption that the observed differences in activation are related to grief triggered by specific cues and are not caused by the depressed and mourning affective state of post-termination women.
Additionally, we observed an increased activation in the posterior cingulate gyrus during the processing of happy baby faces in post-termination women. Posterior cingulate gyrus activity as been shown to be elicited by an interaction between emotion and memory (31) , specifically by the arousal dimension of emotional stimuli. It appears to be important in monitoring the environment for emotionally salient events and for the comparison of these events to memories. Increased posterior cingulate gyrus activity has been demonstrated in individuals who had experienced a bank robbery when the robbery video was compared to a control video (32) . Gündel and colleagues (9) reported an increased activation in the posterior cingulate gyrus that was evoked by photographs of the deceased and by viewing grief-related words.
The inferior frontal gyrus, especially BA 47, has repeatedly been associated with regulating feelings of physical as well as psychological pain or negative affect (6 , 33 – 35) . Activity in this area is modified by voluntary regulation of emotional responses, such as using cognitive control to reduce negative emotions (33) , and in our study could be related to the attempt of post-termination women to control painful feelings.
The thalamus and periaqueductal gray matter are involved in pain processing as well as in attachment behavior in nonhuman mammals (36 – 38) . In studies of physical pain, attentional processes and vigilance have been shown to increase thalamic activity, and thus thalamic activation might reflect a general arousal reaction to pain, whereas the periaqueductal gray matter is regarded as the key structure for pain inhibition (8) . Furthermore, localized electrical stimulation of the dorsomedial thalamus and periaqueductal gray matter in the brainstem has been shown to provoke separation cries in mammals.
MacLean (39) hypothesized that the brain’s thalamocingulate division is important in mammalian mother-infant attachment behavior, such as infant crying and a mother’s caretaking response. Furthermore, in cingulate-lesioned mice, the degree of maternal behavior impairment appeared to correlate with the degree of accompanying anterior thalamic nuclei degeneration (40) . Thus, increased functional connectivity between the cingulate gyrus and the thalamus during the processing of baby faces in post-termination women strongly underlines the hypothesis of an involvement of the maternal attachment network in emotional distress after the death of a child.
Using unfamiliar baby faces as emotional cues in both groups, we attempted to minimize any contributions to grief-related activations other than the subjective experience of grief itself. In studies using photos of the deceased, familiarity could not be excluded as a relevant variable to influence activation patterns (9 , 10) .
Significantly stronger activations during the processing of happy baby and adult faces in comparison women relative to post-termination women were restricted to temporo-occipital areas. Recent neuroimaging studies have demonstrated that the visual presentation of emotionally charged compared to neutral stimuli activates not only emotion-specific brain areas but also extensive areas in the extrastriate cortex (41 , 42) Interestingly, whereas neuroimaging research reported a common effect of physiological arousal on perceptual cortical processing with greater overall magnitude of visual cortical response to both negative and positive stimuli, behavioral research suggests that positive affect broadens and negative affect narrows the distribution of one’s field of view. Thus, significantly stronger activations in comparison women relative to post-termination women might be related to an overall more positive affect in comparison women.
There are some limitations to our study. First, we used a passive viewing paradigm that does not provide behavioral data. Thus, one might assume that post-termination women withdrew their attention from the baby faces to avoid any emotional trigger reminding them of their loss. Increased activations in a large number of visual cortical areas, such as the lingual gyrus, the middle occipital gyrus, and the cuneus, however, provide evidence for the active viewing of emotional cues in post-termination women, since activation in visual cortical areas has been correlated with the emotional valence of stimuli (43) . Moreover, we were unable to monitor physiological parameters to control for changes in blood pressure, heart rate, and breathing.
Nevertheless, our data suggest an extensive overlap in neural networks of grief and physical pain and also suggest a close relationship between emotional distress in maternal attachment and the neural pain network. Improvement in our understanding of the neurobiological background of grief and mourning will aid us in the development of more efficient and effective strategies to prevent prolonged and complicated grief in individuals after social loss.
1. Salvesen KA, Oyen L, Schmidt N, Malt UF, Eik-Nes SH: Comparison of long-term psychological responses of women after pregnancy termination due to fetal anomalies and after perinatal loss. Ultrasound Obstet Gynecol 1997; 9:80–85Google Scholar
2. Korenromp MJ, Christiaens GC, van den Bout J, Mulder EJ, Hunfeld JA, Bilardo CM, Offermans JP, Visser GH: Long-term psychological consequences of pregnancy termination for fetal abnormality: a cross-sectional study. Prenat Diagn 2005; 25:253–260Google Scholar
3. Kersting A, Dorsch M, Kreulich C, Reutemann M, Ohrmann P, Baez E, Arolt V: Trauma and grief 2–7 years after termination of pregnancy because of fetal anomalies: a pilot study. J Psychosom Obstet Gynaecol 2005; 26:9–14Google Scholar
4. Prigerson HG, Frank E, Kasl SV, Reynolds CF III, Anderson B, Zubenko GS, Houck PR, George CJ, Kupfer DJ: Complicated grief and bereavement-related depression as distinct disorders: preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry 1995; 152:22–30Google Scholar
5. MacDonald G, Leary MR: Why does social exclusion hurt? the relationship between social and physical pain. Psychol Bull 2005; 131:202–223Google Scholar
6. Eisenberger NI: Identifying the neural correlates underlying social pain: implications for developmental processes. Hum Dev 2006; 49:273–293Google Scholar
7. Panksepp J, Nelson E, Bekkedal M: Brain systems for the mediation of separation-distress and social-reward: evolutionary and neuropeptide intermediaters. Ann NY Acad Sci 1997; 807:78–100Google Scholar
8. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK: Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9:463–484Google Scholar
9. Gündel H, O’Connor M-F, Littrell L, Fort C, Lane RD: Functional neuroanatomy of grief: an fMRI study. Am J Psychiatry 2003; 160:1946–1953Google Scholar
10. O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD: Craving love? enduring grief activates brain’s reward center. Neuroimage 2008; 42:969–972Google Scholar
11. Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS: Regional brain activity in women grieving a romantic relationship breakup. Am J Psychiatry 2004; 161:2245–2256Google Scholar
12. Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS: A potential role for thalamocingulate circuitry in human maternal behaviour. Biol Psychiatry 2002; 51:431–445Google Scholar
13. McLean PD, Newman JD: Role of midline frontolimbic cortex in production of the isolation call of squirrel. Brain Res 1988; 450:111–123Google Scholar
14. Kersting A, Fisch S, Baez E: Psychosocial care of mothers after stillbirth (letter). Lancet 2002; 360:1600Google Scholar
15. Beutel M, Will H, Volkl K, von Rad M, Weiner H: Assessment of grief exemplified by pregnancy loss: development and initial results on the validity of the Munich Grief Scale. Psychother Psychosom Med Psychol 1995; 45:295–302 (German)Google Scholar
16. Kersting A, Kroker K, Steinhard J, Lüdorff K, Wesselmann U, Ohrmann P, Arolt V, Suslow T: Complicated grief after traumatic loss: a 14-month follow up study. Eur Arch Psychiatry Clin Neurosci 2007; 257:437–443Google Scholar
17. Brockington IF, Fraser C, Wilson D: The Postpartum Bonding Questionnaire: a validation. Arch Womens Ment Health 2006; 9:233–242Google Scholar
18. Reck C, Klier CM, Pabst K, Stehle E, Steffenelli U, Struben K, Backenstrass M: The German version of the Postpartum Bonding Instrument: psychometric properties and association with postpartum depression. Arch Womens Ment Health 2006; 9:265–271Google Scholar
19. Beck AT, Ward CH, Mendelson M: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Google Scholar
20. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Google Scholar
21. Spielberger CD: State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1985Google Scholar
22. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Google Scholar
23. Ekman P, Friesen WV: Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
24. Robinson S, Windischberger C, Rauscher A, Moser E: Optimized 3 T EPI of the amygdalae. Neuroimage 2004; 22:203–210Google Scholar
25. Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, Blonder LX: Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res 2001; 11:213–226Google Scholar
26. Rojahn J, Gerhards F, Matlock ST, Kroeger TL: Reliability and validity studies of the facial discrimination task for emotion research. Psychiatry Res 2000; 95:169–181Google Scholar
27. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT: Automated Talairach atlas labels for functional brain mapping. Human Brain Mapp 2000; 10:120–131Google Scholar
28. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K: A new SPM toolbox for combining probabilistic cytoarchitectonic and functional imaging data. Neuroimage 2005; 25:1325–1335Google Scholar
29. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M: Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Google Scholar
30. Brett M, Anton JL, Valabregue R, Poline JB: Region of interest analysis using an SPM toolbox. Neuroimage 2002; 16:abstract 497Google Scholar
31. Maddock RJ, Garrett AS, Buonocore MH: Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 2003; 18:30–41Google Scholar
32. Fischer H, Wik G, Frederikson M: Functional neuroanatomy of robbery re-experience: affective memories studied with PET. Neuroreport 1996; 7:2081–2086Google Scholar
33. Hariri AR, Bookheimer SY, Mazziotta JC: Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 2000; 11:43–48Google Scholar
34. Petrovic P, Ingvar M: Imaging cognitive modulation of pain processing. Pain 2002; 95:1–5Google Scholar
35. Ochsner KN, Gross JJ: The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Google Scholar
36. Bandler R, Shipley MT: Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 1994; 17:379–389Google Scholar
37. Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang I, Tracey I: A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 2005; 10:7333–7341Google Scholar
38. Lonstein JS, Stern JM: Role of midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats: J Neurosci 1997; 17:3364–3378Google Scholar
39. MacLean PD: The Triune Brain in Evolution: Role in Paleocerebral Functions. New York, Plenum Press, 1990Google Scholar
40. Slotnick BM, Nigrosh BJ: Maternal behaviour of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol 1975; 88:118–127Google Scholar
41. Schmitz TW, De Rosa E, Anderson K: Opposing influences of affective state valence on visual cortical encoding. J Neurosci 2009; 29:7199–7207Google Scholar
42. Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V: Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology 1998; 35:199–210Google Scholar
43. Kober H, Barrett FL, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD: Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 2008; 42:998–1031Google Scholar






