Long-Term Use of Antidepressants for Depressive Disorders and the Risk of Diabetes Mellitus
Abstract
Objective: Use of antidepressants has been reported to cause considerable weight gain. The aim of this study was to assess the risk of diabetes mellitus associated with antidepressant treatment and to examine whether the risk is influenced by treatment duration or daily dose. Method: This was a nested case-control study in a cohort of 165,958 patients with depression who received at least one new prescription for an antidepressant between January 1, 1990, and June 30, 2005. Data were from from the U.K. General Practice Research Database. Patients were at least 30 years of age and without diabetes at cohort entry. Results: A total of 2,243 cases of incident diabetes mellitus and 8,963 matched comparison subjects were identified. Compared with no use of antidepressants during the past 2 years, recent long-term use (>24 months) of antidepressants in moderate to high daily doses was associated with an increased risk of diabetes (incidence rate ratio=1.84, 95% CI=1.35–2.52). The magnitude of the risk was similar for long-term use of moderate to high daily doses of tricyclic antidepressants (incidence rate ratio=1.77, 95% CI=1.21–2.59) and selective serotonin reuptake inhibitors (incidence rate ratio=2.06, 95% CI=1.20–3.52). Treatment for shorter periods or with lower daily doses was not associated with an increased risk. Conclusions: Long-term use of antidepressants in at least moderate daily doses was associated with an increased risk of diabetes. This association was observed for both tricyclic antidepressants and selective serotonin reuptake inhibitors.
Atypical antipsychotics have been linked to changes in body weight (1) and glucose homeostasis (2) , and there is growing evidence that these drugs may also increase the risk of newly diagnosed diabetes mellitus (3 – 6) . Similarly, several antidepressants have been reported to cause considerable weight gain (7) and impairment of glucose homeostasis, which has been observed in vitro (8) and in vivo (9 – 13) . However, data on the risk of diabetes associated with the use of antidepressants are sparse. A recent randomized Diabetes Prevention Program trial (14) found an increased risk of diabetes in high-risk patients who used antidepressants at baseline, whether continuously or intermittently during the study period, and who had received placebo or a lifestyle intervention during the study; this elevated risk was not observed in patients who had received the antidiabetic drug metformin as preventive treatment. Two observational studies produced ambiguous data. While one study did not find an increased risk of diabetes in antidepressant users as compared with nonusers (15) , the other found an increased risk of diabetes in patients concomitantly using selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants as compared with tricyclic antidepressant monotherapy (16) .
The aim of this study was to investigate whether use of antidepressants in depressive disorders is associated with an increased risk of diabetes mellitus in patients at least 30 years of age and whether the risk is influenced by treatment duration or daily dose.
Method
We performed a nested case-control study in a cohort of depressed patients treated with at least one new prescription for an antidepressant between January 1, 1990, and June 30, 2005. The study was approved by the Scientific and Ethical Advisory Group of the U.K. General Practice Research Database (GPRD).
Data Source
Data were obtained from the GPRD, which contains medical records for more than 6.4 million patients from some 450 general practices in the United Kingdom. Patients are enrolled by general practitioners who have agreed to provide data for research purposes to the GPRD and received instruction on the standardized computer recording of medical information. The recorded information includes the patient’s characteristics (e.g., age, sex, smoking, height, and weight), drugs prescribed, diagnoses, referrals to consultants and hospitals, and medical history. The completeness and validity of recorded information on diagnoses and drug exposure, checked on an ongoing basis by GPRD staff, have been confirmed in several studies (17 – 19) . The database has been used for the study of numerous diseases, including diabetes (4) .
Study Population
Cohort entry was defined as the date of the first prescription of an antidepressant. To be included in the study, patients had to be at least 30 years of age at the time of cohort entry; have had no diagnosis of diabetes or impaired glucose tolerance and no treatment with oral antidiabetics or insulin before cohort entry; have had a diagnosis of depression within 180 days before or 90 days after cohort entry; have had no treatment with antidepressants in the year prior to their first prescription of an antidepressant (cohort entry); have at least one database entry of body mass index (BMI) before cohort entry; and have registered with a practice with ensured GPRD quality standards of recorded data for at least 1 year prior to cohort entry. Follow-up was from cohort entry of each patient until the first date of any of the following: new onset of diabetes; switching to or combination with another antidepressant; death; end of registration with the practice; or end of the study period.
Potential cases of diabetes were identified using predefined diabetes codes and prescriptions of oral antidiabetics and insulin. To be included as a case subject, a patient had to have at least one prescription of an antidiabetic drug, or two diagnoses of diabetes on different calendar days, or a diagnosis of diabetes and a diabetes-specific test (e.g., glycosylated hemoglobin) on different calendar days. This case definition was used to exclude patients from the case group who had a suspected diagnosis of diabetes that was not confirmed later on (internal validation). Since differentiation between type 1 and type 2 diabetes is not always possible from the diagnostic diabetes codes, only patients ≥30 years of age were included in the cohort to ensure that incident cases of diabetes were most likely type 2 diabetes. The date of diabetes onset (index date) was defined as the first diagnosis of diabetes, the first prescription of an oral antidiabetic drug, or the first prescription of insulin, whichever came first. For each case, we randomly selected up to four comparison subjects among the risk set of cohort members who were still being followed up and had not developed diabetes (incidence density sampling). Comparison subjects were matched to case subjects on age (±2 years), sex, and year of cohort entry. The date that resulted in the same time of follow-up as for the respective case subject was designated as the index date for the comparison subject.
Identification of Exposure to Antidepressants
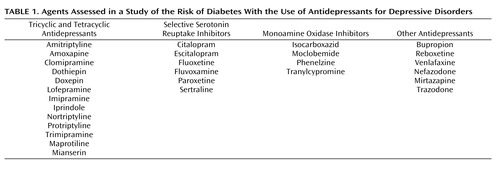
We identified all prescriptions of antidepressants during follow-up and determined the duration of each prescription by dividing the quantity of prescribed tablets by the number of tablets to be taken daily. We classified antidepressants into tricyclic and tetracyclic antidepressants, SSRIs, monoamine oxidase (MAO) inhibitors, and the heterogeneous group of mixed-action “other antidepressants” ( Table 1 ). We categorized exposure as “recent,” “former,” and “past” according to antidepressant use before the index date. Recent exposure was defined as a prescription that lasted into the 6-month period before the index date. If the prescription supply ended between 6 and 12 months before the index date, it was categorized as former exposure, and if it ended between 12 and 24 months before the index date, it was termed past exposure. “Nonuse” was defined as no use of an antidepressant during the 2 years before the index date. To investigate whether the definition of these time windows influenced the results substantially, we performed sensitivity analyses using time windows for recent use of 3 months, 1 month, 14 days, 7 days, and 0 days. In recent users, we calculated the cumulative duration of antidepressant use before the index date by adding the duration of all prescriptions of each antidepressant. We categorized cumulative use into short-term (<12 months), medium-term (12–24 months), and long-term (>24 months) use. We determined the daily dose of antidepressants by multiplying the tablet strength with the prescribed number of tablets per day. In recent users, we calculated the median daily dose during the 6 months prior to the index date. The median of the daily dose in comparison subjects was used as the cutoff value to classify recent users as “low” dose (≤ the median dose of comparison subjects) or “moderate/high” dose (> the median dose of comparison subjects) users. Calculated cutoff values were 38 mg for amitriptyline, 300 mg for bupropion, 20 mg for citalopram, 20 mg for clomipramine, 62.5 mg for dothiepin, 30 mg for doxepin, 10 mg for escitalopram, 20 mg for fluoxetine, 50 mg for imipramine, 140 mg for lofepramine, 25 mg for mianserin, 30 mg for mirtazapine, 200 mg for nefazodone, 30 mg for nortriptyline, 20 mg for paroxetine, 15 mg for phenelzine, 5 mg for protriptyline, 8 mg for reboxetine, 50 mg for sertraline, 100 mg for trazodone, 50 mg for trimipramine, and 75 mg for venlafaxine.
Statistical Analysis
The incidence rate ratios of diabetes for recent, former, and past use of antidepressants as compared with nonuse were estimated from odds ratios calculated by conditional logistic regression using the PHREG program in SAS, version 9.1 (SAS Institute, Cary, N.C.). We constructed individual models characterizing recent users according to the cumulative duration of use, median daily dose, and a combination of both. We conducted stratified analyses with respect to sex, age, and baseline BMI. In an explorative analysis, we investigated whether the depression was more severe in patients with long-term antidepressant intake using the number of days a patient was diagnosed with depression in the year before the index date as a proxy for depression severity. For all analyses, the reference category was no exposure to antidepressants during the 2 years before the index date. A p value below 0.05 (two-tailed) was considered significant, and 95% confidence intervals (CIs) were calculated for all incidence rate ratios. All multivariate models controlled for hyperlipidemia, hypertension, smoking (current, past, never, and unknown), BMI at cohort entry (<18.5, 18.5–24.99, 25–29.99, 30–34.99, 35–39.99, and ≥40), and prescriptions of other drugs within 3 months before the index date that may lead to weight gain and/or an increased risk of diabetes (beta-blockers, thiazides, antipsychotics, carbamazepine, phenytoin, valproate, lithium, and corticosteroids).
Results
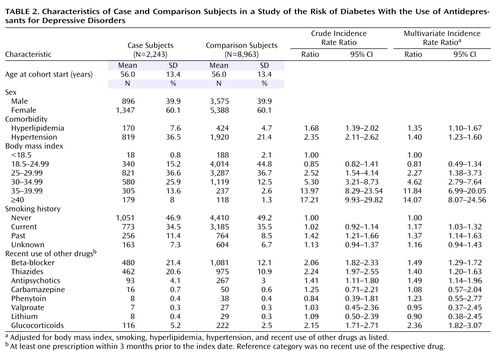
A total of 165,958 patients in the GPRD database fulfilled our cohort definition, summing up to 464,116 person-years of follow-up. The numbers of included and excluded patients are displayed in Figure 1 . The largest amount of exposed person-time was observed for fluoxetine (26,664 person-years), followed by dothiepin (17,886 person-years), paroxetine (15,197 person-years), citalopram (12,440 person-years), sertraline (7,153 person-years), and amitripyline (6,855 person-years). We identified 2,423 potential cases of diabetes, of which 2,243 patients (92.6%) fulfilled our case definition and were included in the final case-control analysis ( Figure 1 ). To these case subjects, we randomly matched 8,963 comparison subjects from the cohort risk set, thereby identifying four comparison patients for the majority of case subjects (99.8%). The mean follow-up time was 2.8 years for case and comparison subjects. Table 2 summarizes the baseline characteristics of both groups. Our analysis confirmed high BMI at baseline as the strongest predictor of new-onset diabetes. A total of 1,337 case subjects (59.6%) and 5,142 comparison subjects (57.4%) had been exposed to an antidepressant within 2 years before their index date. Of those, 851 case subjects and 3,036 comparison subjects were recent users. Because only one case subject and one comparison subject were exposed to MAO inhibitors, this drug category was not further analyzed.
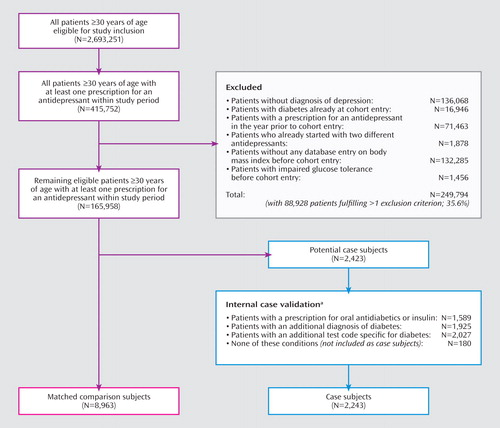
a Overlapping categories may not sum to 100%.
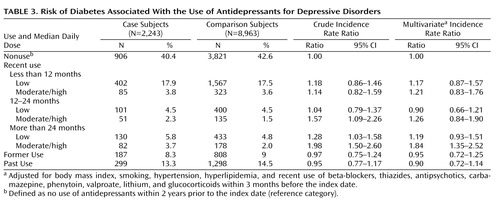
Recent long-term use of antidepressants in moderate or high daily doses was associated with an increased risk of diabetes (incidence rate ratio=1.84; 95% CI=1.35–2.52), whereas recent use of shorter duration, use in lower daily doses, former use, and past use were not ( Table 3 ). For users of tricyclic antidepressants and SSRIs as groups, increased risk was likewise observed only for recent long-term use of moderate or high daily doses (incidence rate ratio=1.77; 95% CI=1.21–2.59, and incidence rate ratio=2.06; 95% CI=1.20–3.52, respectively). The analysis for other antidepressants as a group was limited by the small number of exposed case and comparison subjects and revealed no increased risk with long-term use of moderate or high daily doses (incidence rate ratio=1.64; 95% CI=0.34–7.81).
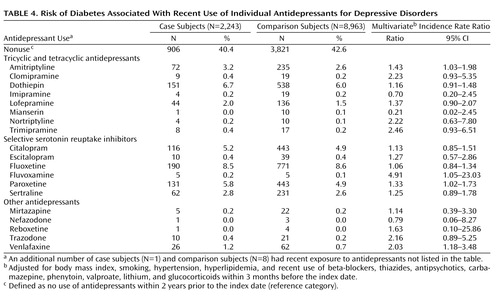
In the analysis of individual antidepressants, increased risk estimates were observed for recent use of amitriptyline, fluvoxamine, paroxetine, and venlafaxine ( Table 4 ). This effect was again evident only in long-term users (>24 months). The risk estimates (incidence rate ratios) associated with long-term use were 2.49 (95% CI=1.52–4.08) for amitriptyline, 9.05 (95% CI=1.08–75.58) for fluvoxamine, 1.75 (95% CI=1.13–2.72) for paroxetine, and 3.01 (95% CI=1.01–9.02) for venlafaxine.
The sensitivity analyses of different definitions of the time window for recent use resulted in similar risk estimates for grouped and individual antidepressants. The stratified analysis with respect to age, sex, and baseline BMI revealed similar risk estimates and no indication of an interaction. The explorative analysis did not indicate that recent long-term users of antidepressants had greater depression severity as compared with medium- or short-term users. Adjusting for depression severity in the multivariate models did not change the results substantially.
Discussion
In this large observational study, which included more than 160,000 patients with depressive disorder treated with antidepressants, recent long-term use of antidepressants in moderate to high daily doses was associated with an 84% increase in risk of diabetes. This association was present for both tricyclic antidepressants and SSRIs. Antidepressant treatment for shorter periods or with lower daily doses was not associated with an increased risk. Recent use of other antidepressants was associated with an 80% increase in risk of diabetes; however, a dose or duration effect could not be detected, probably because of the rather low number of exposed case and comparison subjects.
Our results are supported by recently published data from the randomized Diabetes Prevention Program trial, which investigated newly developing diabetes in patients at high risk of diabetes receiving the antidiabetic drug metformin, lifestyle intervention, or placebo (14) . Continuous antidepressant use over an average study duration of 3.2 years was associated with an increased risk of diabetes of 2.60 (95% CI=1.37–4.94) in the placebo arm and 3.39 (95% CI=1.61–7.13) in the lifestyle intervention arm. Intermittent use of antidepressants similarly increased the risk of diabetes in both treatment arms. There was no increased risk observed in the metformin arm of the study, possibly because of weight loss instead of weight gain under metformin (20) . Metformin also attenuated the effect of other risk factors for diabetes, such as baseline BMI (21).
Observational studies have produced conflicting data on the risk of diabetes in antidepressant users. Knol et al. (15) , using prescription data from the PHARMO database, did not identify an increased risk of diabetes in antidepressant users. Their study had several limitations. In contrast to our study, they lacked information on BMI, lifestyle factors, and the indication for antidepressant treatment, and hence they could not adjust for these factors in the statistical analysis. Their study included only patients with antidiabetic treatment as cases of diabetes, whereas we also included patients diagnosed with diabetes who had not yet received antidiabetic treatment. In contrast to our study, which included patients with new use of antidepressants, Knol et al. also included patients with prevalent use, thereby possibly overrepresenting patients who tolerate antidepressant treatment well. They also did not consider the duration or dose of antidepressant treatment, which was most important in our analysis.
Some other observational studies suggest an association between antidepressants and diabetes. Brown et al. (16) reported an increased risk of diabetes in concurrent users of tricyclic antidepressants and SSRIs as compared with those taking tricyclics alone. A similar analysis of combined use was not possible in our study, since we excluded users of more than one antidepressant to ensure pure exposure categories. In a Norwegian cross-sectional health survey, SSRIs were found to be associated with abdominal obesity and hypercholesterinemia, and a trend toward an association with diabetes was observed (22) . An analysis of spontaneous reports of adverse drug reactions recorded in the World Health Organization’s Adverse Drug Reaction Database showed increased reporting odds ratios of hyperglycemia and hypoglycemia associated with the use of antidepressants (23) . The association of hyperglycemia was most pronounced after more than 1 year of antidepressant use. We similarly observed an increase in the risk of diabetes only with long-term use of antidepressants.
What are possible mechanisms for the observed association? Weight gain is a common side effect in short- and long-term treatment with tricyclic antidepressants and was already described in the 1970s (24 , 25) . An initial rapid weight gain is followed by a lower but continuous gain at a steady rate (26) . There are conflicting data on the question of whether individual tricyclic antidepressants differ in potential for causing weight gain. While some studies indicated higher weight gain with amitriptyline than with other tricyclic antidepressants (27 , 28) , others did not (29 , 30) . We observed an increased risk of diabetes only with long-term use of amitriptyline. Risk estimates for long-term use of clomipramine, nortriptyline, and trimipramine were also elevated more than twofold but did not reach statistical significance, most likely because of the low number of case and comparison subjects exposed to these drugs.
Weight changes associated with SSRI treatment are complex. There is evidence for an initial stable weight or even weight loss with use of SSRIs followed by weight gain if used for longer periods (31 , 32) . Some studies have suggested differences in the potential of individual SSRIs to cause weight gain. In a randomized, double-blind 6-month trial of SSRI continuation therapy, weight gain was more often reported for patients treated with paroxetine than for those treated with sertraline (33.1% versus 20.2%, p<0.05) (33) . Another randomized trial also reported the greatest increase in weight with paroxetine as compared with sertraline or fluoxetine (34) . This corresponds to our finding of a fourfold increase in risk of diabetes associated with long-term therapy with paroxetine in daily doses above 20 mg/day but not with long-term use of fluoxetine, citalopram, or sertraline. Our finding of an increased risk with fluvoxamine, which has not been reported to cause marked weight gain, was based on four exposed case subjects and two comparison subjects and should thus be interpreted with caution.
From the heterogeneous group of other antidepressants, we observed an increased risk of diabetes associated with recent long-term use of venlafaxine. There are conflicting data on weight changes associated with venlafaxine treatment. While some studies reported no weight gain (35) or even weight loss (36 , 37) , others found weight gain with higher doses of venlafaxine (38) or at least reversal of initial weight loss during long-term treatment (39) . The prescribing information of venlafaxine addresses weight loss as a potential adverse effect but also mentions weight gain as a frequent adverse effect (40) . It should be noted, however, that the observed risk of diabetes associated with long-term use of venlafaxine was based on eight exposed case subjects and 10 exposed comparison subjects.
Other mechanisms beyond weight gain may have a role in the increased diabetes risk associated with antidepressants. It is interesting in this respect that in the Diabetes Prevention Program trial the increased risk of diabetes in users of antidepressants remained significantly elevated even after adjustment for the observed increase in body weight (14) . Thus, other mechanisms, such as hyperglycemic effects of noradrenergic activity of antidepressants, may play a role.
Could our results be explained by the fact that depression itself and not the antidepressant drug treatment increases the risk of diabetes? It has been reported that patients with depression have a 35% increase in risk of developing diabetes as compared with nondepressed individuals (15) . The underlying reasons for this association are unknown. One might argue that patients treated with antidepressants for >24 months are a special subgroup with an increased risk of diabetes only because of their active depressive disorder. There are several reasons why this explanation is rather unlikely. First, one would expect to see increased risks with most individual antidepressants used for >24 months if the increased diabetes were caused by the depression and not by the drug. In contrast, our results indicate that antidepressants differ with respect to their diabetogenic potential. Second, in the Diabetes Prevention Program, elevated Beck Depression Inventory scores at baseline were not associated with an increased risk of diabetes, but the use of antidepressants was (14) . Third, in our study, all cohort members were treated with only one antidepressant during the entire follow-up period. A high proportion of long-term users probably responded to treatment, as otherwise a switch to another antidepressant would have been indicated. Fourth, in an explorative analysis using the number of days with a diagnosis of depression in the year before the index date as a proxy for depression severity, there was no indication of increased severity in long-term users. Inclusion of this proxy variable into the multivariate models did not affect our main findings.
One strength of our study is that all information was recorded prospectively so that recall bias can be ruled out. Selection bias in the choice of comparison subjects is unlikely because the study was designed as a nested case-control study in a defined cohort of antidepressant users, providing both case and comparison subjects. We included only depressed patients to exclude other indications for antidepressant use and to ensure a more homogeneous study population. We excluded prevalent users of antidepressants to avoid overrepresentation of patients who tolerated antidepressant treatment well (“depletion of susceptible” bias). We ensured by study design that all patients were exposed to only one antidepressant during the entire follow-up period.
Some limitations of our study must also be considered. First, because of the new-user criterion in our study design, it was not possible to investigate second-line antidepressants, such as MAO inhibitors. Second, weight gain during follow-up was not systematically recorded, so it was not possible to analyze this factor. Third, antidepressants causing massive initial weight gain might have been withdrawn early before diabetes developed. For these drugs, we might have underestimated the true risks. Fourth, because of the naturalistic character of the GPRD, adjustment for the severity of depression was limited to an analysis considering an indicator for depression severity that might not have removed all residual confounding from this possible source of bias. If drugs were primarily prescribed to patients with atypical depression, we might have overestimated the risk associated with these drugs. This might apply, for instance, to venlafaxine, which has been reported to be associated with initial weight loss in clinical trials. Finally, because we used the GPRD database, we could consider only ambulatory prescriptions and had no information about in-hospital medication.
1. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Google Scholar
2. Hedenmalm K, Hagg S, Stahl M, Mortimer O, Spigset O: Glucose intolerance with atypical antipsychotics. Drug Saf 2002; 25:1107–1116Google Scholar
3. Kornegay CJ, Vasilakis-Scaramozza C, Jick H: Incident diabetes associated with antipsychotic use in the United Kingdom General Practice Research Database. J Clin Psychiatry 2002; 63:758–762Google Scholar
4. Koro CE, Fedder DO, L’Italien GJ, Weiss SS, Magder LS, Kreyenbuhl J, Revicki DA, Buchanan RW: Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002; 325:243Google Scholar
5. Lambert BL, Chou CH, Chang KY, Tafesse E, Carson W: Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005; 14:417–425Google Scholar
6. Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R: Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002; 159:561–566Google Scholar
7. Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmacher T: Epidemiology, implications, and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003; 37:193–220Google Scholar
8. Levkovitz Y, Ben-Shushan G, Hershkovitz A, Isaac R, Gil-Ad I, Shvartsman D, Ronen D, Weizman A, Zick Y: Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol Cell Neurosci 2007; 36:305–312Google Scholar
9. Carvalho F, Barros D, Silva J, Rezende E, Soares M, Fregoneze J, de Castro e Silva E: Hyperglycemia induced by acute central fluoxetine administration: role of the central CRH system and 5-HT 3 receptors. Neuropeptides 2004; 38:98–105 Google Scholar
10. Fisfalen ME, Hsiung RC: Glucose dysregulation and mirtazapine-induced weight gain (letter). Am J Psychiatry 2003; 160:797Google Scholar
11. Petty KJ: Hyperglycemia associated with paroxetine (letter). Ann Intern Med 1996; 125:782Google Scholar
12. Sugimoto Y, Inoue K, Yamada J: Involvement of serotonin in zimelidine-induced hyperglycemia in mice. Biol Pharm Bull 1999; 22:1240–1241Google Scholar
13. Yamada J, Sugimoto Y, Inoue K: Selective serotonin reuptake inhibitors fluoxetine and fluvoxamine induce hyperglycemia by different mechanisms. Eur J Pharmacol 1999; 382:211–215Google Scholar
14. Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, Haffner SM, Price DW, Knowler WC; Diabetes Prevention Program Research Group: Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the Diabetes Prevention Program. Diabetes Care 2008; 31:420–426Google Scholar
15. Knol MJ, Geerlings MI, Egberts AC, Gorter KJ, Grobbee DE, Heerdink ER: No increased incidence of diabetes in antidepressant users. Int Clin Psychopharmacol 2007; 22:382–386Google Scholar
16. Brown LC, Majumdar SR, Johnson JA: Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res Clin Pract 2008; 79:61–67Google Scholar
17. Jick H, Jick SS, Derby LE: Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 1991; 302:766–768Google Scholar
18. Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garcia Rodriguez LA, Ruigomez A, Meier CR, Schlienger RG, Black C, Jick H: Validity of the General Practice Research Database. Pharmacotherapy 2003; 23:686–689Google Scholar
19. Walley T, Mantgani A: The UK General Practice Research Database. Lancet 1997; 350:1097–1099Google Scholar
20. Hermansen K, Mortensen LS: Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142Google Scholar
21. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403Google Scholar
22. Raeder MB, Bjelland I, Emil VS, Steen VM: Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry 2006; 67:1974–1982Google Scholar
23. Derijks HJ, Meyboom RH, Heerdink ER, De Koning FH, Janknegt R, Lindquist M, Egberts AC: The association between antidepressant use and disturbances in glucose homeostasis: evidence from spontaneous reports. Eur J Clin Pharmacol 2008; 64:531–538Google Scholar
24. Kupfer DJ, Coble PA, Rubinstein D: Changes in weight during treatment for depression. Psychosom Med 1979; 41:535–544Google Scholar
25. Paykel ES, Mueller PS, de la Vergne PM: Amitriptyline, weight gain, and carbohydrate craving: a side effect. Br J Psychiatry 1973; 123:501–507Google Scholar
26. Garland EJ, Remick RA, Zis AP: Weight gain with antidepressants and lithium. J Clin Psychopharmacol 1988; 8:323–330Google Scholar
27. Fernstrom MH, Kupfer DJ: Antidepressant-induced weight gain: a comparison study of four medications. Psychiatry Res 1988; 26:265–271Google Scholar
28. Yeragani VK, Pohl R, Aleem A, Balon R, Sherwood P, Lycaki H: Carbohydrate craving and increased appetite associated with antidepressant therapy. Can J Psychiatry 1988; 33:606–610Google Scholar
29. Harris B, Young J, Hughes B: Comparative effects of seven antidepressant regimes on appetite, weight, and carbohydrate preference. Br J Psychiatry 1986; 148:590–592Google Scholar
30. Kazes M, Danion JM, Grange D, Pradignac A, Simon C, Burrus-Mehl F, Schlienger JL, Singer L: Eating behaviour and depression before and after antidepressant treatment: a prospective, naturalistic study. J Affect Disord 1994; 30:193–207Google Scholar
31. Harvey BH, Bouwer CD: Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin Neuropharmacol 2000; 23:90–97Google Scholar
32. Sussman N, Ginsberg DL, Bikoff J: Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry 2001; 62:256–260Google Scholar
33. Aberg-Wistedt A, Agren H, Ekselius L, Bengtsson F, Akerblad AC: Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. J Clin Psychopharmacol 2000; 20:645–652Google Scholar
34. Fava M, Judge R, Hoog SL, Nilsson ME, Koke SC: Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment. J Clin Psychiatry 2000; 61:863–867Google Scholar
35. Silverstone PH, Ravindran A: Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. Venlafaxine XR 360 Study Group. J Clin Psychiatry 1999; 60:22–28Google Scholar
36. Liebowitz MR, Gelenberg AJ, Munjack D: Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry 2005; 62:190–198Google Scholar
37. Rudolph RL, Fabre LF, Feighner JP, Rickels K, Entsuah R, Derivan AT: A randomized, placebo-controlled, dose-response trial of venlafaxine hydrochloride in the treatment of major depression. J Clin Psychiatry 1998; 59:116–122Google Scholar
38. Harrison CL, Ferrier N, Young AH: Tolerability of high-dose venlafaxine in depressed patients. J Psychopharmacol 2004; 18:200–204Google Scholar
39. Danjou P, Hackett D: Safety and tolerance profile of venlafaxine. Int Clin Psychopharmacol 1995; 10(suppl 2):15–20Google Scholar
40. Wyeth Pharmaceuticals: Effexor prescibing information. http://www.fda.gov/cder/foi/label/2007/020151s043,020699s069lbl.pdfGoogle Scholar