An Event-Related Brain Potential Study of Direct and Indirect Semantic Priming in Schizophrenia
Abstract
Objective: Following a meaningful prime stimulus, schizophrenia patients have been hypothesized to exhibit impaired neurophysiological activation of related concepts in general, and/or supranormal activation of weakly related concepts in particular, within semantic memory. The former abnormality may occur at longer intervals, and the latter at shorter intervals, after the prime. The authors tested these hypotheses using the N400 event-related brain potential as a probe of activation of concepts in semantic memory. Method: Event-related potentials were recorded in 16 schizophrenia patients and 16 normal comparison subjects who viewed prime words, each followed by a target that was a directly (strongly) related word, indirectly (weakly) related word, unrelated word, or nonword, in a lexical-decision task. Equal numbers of each target type were presented 300 and 750 msec after the prime. Results: In the comparison subjects, N400 amplitude was largest (most negative) following unrelated targets, intermediate after indirectly related targets, and smallest after directly related targets. In contrast, patients’ N400 amplitudes did not differ between these target types, reflecting larger amplitudes following both directly and indirectly related targets in patients than in comparison subjects; these findings held regardless of prime-to-target stimulus-onset asynchrony. Within patients, at the longer asynchrony, larger N400 amplitudes after directly and indirectly related targets correlated with positive psychotic symptoms. Conclusions: The results suggest hypoactivation of strongly and weakly related concepts following a meaningful stimulus, regardless of interval, in schizophrenia. An N400 index of this hypoactivation correlated with severity of delusions, suggesting a role for abnormal semantic processing in their pathogenesis.
A “disturbance of associations” was identified as a core feature of schizophrenia by Bleuler (1) , who observed that in schizophrenic language “associations lose their continuity” and appear “bizarre” or “senseless.” Bleuler’s notion of abnormalities in “pathways of association” presaged more recent theories that disorganized speech in schizophrenia results from abnormalities in the activations of conceptual representations in semantic long-term memory (2 – 4) . These theories assume a model of semantic memory in which concepts are nodes in a neural network and meaningful relationships between concepts are connections between nodes (5) . In this model, when a concept node is activated, as by its corresponding word stimulus, its subsequent processing is facilitated. This activation then spreads through the network to connected nodes, falling off with decreasing relatedness. The degree to which one concept activates another is thus presumably proportional to their relatedness in the semantic network.
One hypothesis for how disorganized speech in schizophrenia may result from abnormal activation in semantic memory postulates that schizophrenia patients are impaired in using contextual information to activate related items (4 , 6) . There is evidence supporting this hypothesis from semantic priming studies, in which the response to a target stimulus—e.g., reaction time in a lexical-decision or pronunciation task—is facilitated when it is preceded by a meaningfully related rather than an unrelated prime. Greater priming is thought to reflect greater activation of the target by the prime. In contrast to normal comparison subjects, schizophrenia patients have exhibited less or no semantic priming, consistent with low activation of related items by primes (7 – 10) . Although Barch et al. (8) reported no association between thought disorder and priming effects within patients, others observed smaller priming effects in patients with thought disorder than in those without thought disorder or normal comparison subjects (7 , 9 , 10) . In another study, although all participants were slower to recognize semantically congruent than incongruent words at the ends of sentences, schizophrenia patients with more thought disorder showed smaller congruity effects than patients with less thought disorder or normal participants (11) ; this difference is consistent with impaired use of sentence contexts to activate related items.
Some results have led to the suggestion that in schizophrenia, activation of related items might become subnormal only after some time following a prime stimulus has elapsed. In fact, Barch et al. (8) found smaller priming effects on word-pronunciation reaction time when the interval between the onsets of the prime and the target, or stimulus-onset asynchrony, was 950 msec but not when it was shorter (200–700 msec). Others, however, have observed smaller reaction time priming effects in schizophrenia in lexical-decision tasks even with stimulus-onset asynchronies of 500 msec (10) . Taken together, these results are consistent with the possibility that activation of related items may be subnormal in schizophrenia only approximately 500 msec or more after a prime.
A contrasting, albeit not mutually exclusive, hypothesis for how abnormal activation in semantic memory may cause disorganized speech postulates a broader spread of activation to weakly related items in particular, leading to production of minimally related concept sequences (2) . Consistent with this hypothesis, abnormally large priming effects on reaction time for indirectly related words (those related only through at least one other concept, such as “cat” and “cheese,” mediated by “mouse”) have been observed in schizophrenia patients with thought disorder (12 – 14) . Moritz et al. (13) suggested that these greater indirect priming effects may occur only when the stimulus-onset asynchrony is approximately 500 msec or less. Indeed, the studies that found these effects used 200-msec asynchronies. (Priming has been proposed to reflect automatically spreading activation at these short intervals and consciously controlled processes, e.g., prediction [ 7 , 8 , 13 ], at longer stimulus-onset asynchronies. Although this distinction does not appear clear-cut [ 8 , 15 , 16 ], to the extent that it is valid schizophrenia patients could exhibit hyperactivation of weakly related items in the former processes and/or deficient activation of all related items in the latter.)
Semantic priming effects also have been investigated by using the N400 component of electroencephalographic event-related brain potentials. The N400 is a negativity approximately 300–500 msec after stimulus onset, peaking around 400 msec, in response to any potentially meaningful stimulus, such as a word or picture. N400 amplitude is reduced (made less negative) by factors facilitating an item’s processing, such as greater linguistic word frequency or stimulus repetition (17) . N400 amplitude elicited by a target is also reduced by increasing semantic relatedness with a preceding prime (18 , 19) . Accordingly, N400 amplitude has been used to index the degree to which concepts activate one another in semantic memory, with lower amplitude associated with greater activation (all else held constant).
Evidence from a number of N400 studies in schizophrenia is also consistent with less use of context to activate related items. Pairs of prime and target words with stimulus-onset asynchronies of 450 msec (20 , 21) and 600 msec (22) yielded larger (more negative) N400 amplitudes in response to related targets in schizophrenia patients than in comparison subjects, while amplitudes following unrelated targets did not differentiate the two groups. These results suggest less activation of related targets due to impaired context use in schizophrenia. In another word-pair experiment, Condray et al. (23) found smaller N400 amplitude relatedness effects in patients than in comparison subjects, at stimulus-onset asynchronies of 350 and 950 msec (although N400 amplitudes for either related or unrelated targets did not reliably distinguish the groups). Studies employing sentence stimuli (21 , 24) yielded larger N400 amplitudes in response to sentence-congruent final words in patients than in comparison subjects; this finding is likewise consistent with impairment in using sentence contexts to facilitate processing of related items.
In contrast, at a stimulus-onset asynchrony of 250 msec, target words from the same semantic category as a picture prime elicited smaller N400 amplitudes in schizophrenia patients than in comparison subjects (25) , consistent with greater activation of related items. Although the relatedness of the prime and target in each word pair in this experiment was not quantified, if they were on average weakly related, then the results could reflect greater spread of activation to weakly related concepts. Taken together, the N400 results in schizophrenia patients, such as the reaction time priming data, are broadly consistent with less use of context to activate related items at longer stimulus-onset asynchronies, along with greater activation of weakly related items at shorter asynchronies.
Whereas these N400 studies compared priming effects only for unrelated and related items in general, we aimed to use the N400 to test for greater spread of activation to weakly related items—specifically, indirectly related word pairs. Thus, we recorded event-related potentials in schizophrenia patients and comparison subjects while they viewed prime-target pairs in which the targets were words directly related, indirectly related, or unrelated to the prime word or pronounceable nonwords. The participants’ task was to indicate by pressing a button whether or not the target was a real word. This response was delayed to minimize motor effects on the event-related potential. Equal numbers of directly related, indirectly related, and unrelated word pairs were presented at each of two stimulus-onset asynchronies (300 and 750 msec) for each participant.
We predicted that in the comparison subjects, at both asynchronies, N400 would be largest (most negative) in response to unrelated targets, intermediate following indirectly related targets, and smallest after directly related targets, a pattern consistent with previous findings (16 , 26 , 27) . Furthermore, we hypothesized that if schizophrenia is accompanied by broader spread of activation to weaker associates at short stimulus-onset asynchronies, then at the 300-msec asynchrony, prime words would activate indirectly related targets more strongly in patients than in comparison subjects, resulting in smaller N400 amplitudes following these targets in the patients. In addition, if schizophrenia patients make less effective use of context at longer stimulus-onset asynchronies to activate related items, then at the 750-msec asynchrony, both directly and indirectly related targets would be less activated; this would manifest as larger N400 amplitudes in patients than in comparison subjects. We also predicted that since the hypothesized abnormalities in schizophrenia patients are taken to cause their disorganized speech, these abnormalities would correlate with severity of disorganization.
Method
Participants
The participants included 16 outpatients with schizophrenia and 16 normal comparison subjects. Patients were recruited through community residential facilities and physician referral. Comparison subjects were recruited through newspaper advertisements and posted flyers. Participants were assessed for capacity to provide informed consent and, after receiving a detailed description of the study, gave written consent. The protocol was approved by the University of California, San Diego, institutional review board. Participants received cash compensation.
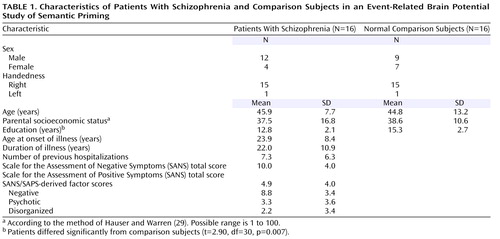
Patients were assessed with the Structured Clinical Interview for DSM-IV (SCID) and were screened to rule out other axis I diagnoses, including substance abuse. Comparison subjects were assessed with the SCID (nonpatient version) to rule out past or present axis I or II diagnoses, including substance abuse. Exclusion criteria for all participants included exposure to a language other than English as a child and current or past neurological disorder. Comparison subjects were matched to patients on the basis of age, gender, handedness (28) , and parental socioeconomic status (29) . Demographic characteristics of the study group are shown in Table 1 .
Ten patients were prescribed second-generation antipsychotics, three were prescribed first-generation antipsychotics, and three were prescribed a combination of these; one patient in the first group reported not taking any antipsychotic for at least 2 weeks before testing.
Assessments
Patients were assessed with the Scale for the Assessment of Negative Symptoms (SANS) (30) and the Scale for the Assessment of Positive Symptoms (SAPS) (31) . From these ratings, we calculated scores for a psychotic symptom factor (hallucinations, delusions), negative symptom factor (affective flattening, avolition/apathy, anhedonia/asociality), and disorganized symptom factor (positive formal thought disorder, bizarre behavior) (32) . Patients’ clinical characteristics are also shown in Table 1 .
Stimuli
The stimuli included 52 directly related, 52 indirectly related, and 52 unrelated prime-target word pairs. For each directly related pair (e.g., “metal” and “steel”), the target was among the words most commonly given as associates to the prime by participants in the University of South Florida word-association norms (33) ; the mean response probability of directly related targets (i.e., proportion of individuals producing that word in response to the prime) was 0.36 (SD=0.23). Indirectly related pairs (e.g., “birthday” and “pie,” mediated by “cake”) included 35 pairs from previous studies (34 – 36) and 17 new pairs. For each indirectly related pair, the prime and target were not associates in the norms (i.e., the target was not among words produced by more than one individual in response to the prime), but the mediating word was an associate of the prime (mean response probability=0.38, SD=0.22) and the target was an associate of the mediating word (mean response probability=0.19, SD=0.18). For each unrelated pair (e.g., “donkey” and “purse”), the prime and target were not associates in the norms. Across these three conditions, targets were matched for mean length and log frequency (37) . Across the conditions, primes were also matched for mean length and log frequency. The stimuli also included 156 word-nonword prime-target pairs (e.g., “dress” and “zores”), whose targets were pronounceable nonwords. No word occurred more than once among the stimuli.
The 312-trial stimulus list included all prime-target pairs in a fixed randomized order, in four blocks of 78 trials each. The list had two versions, in which the order of prime-target stimulus-onset asynchronies across blocks was counterbalanced. In version A, the asynchrony was 300 msec in blocks 1 and 3 and 750 msec in blocks 2 and 4; in version B, the order of stimulus-onset asynchronies was reversed.
Task
The participants were seated 100 cm in front of a video monitor on which the stimuli were visually presented, with each letter subtending on average 0.36° of visual angle horizontally and up to 0.55° vertically. Words were displayed in yellow letters on a black background.
Each participant was presented with the stimulus list, with short rest breaks between blocks. Half the participants received each version of the list. Each trial consisted of 1) a row of preparatory fixation crosses for 500 msec, 2) a blank screen for 250 msec, 3) a prime word for 175 msec, 4) a blank screen for 125 msec (in the trials with 300-msec stimulus-onset asynchrony) or 575 msec (in trials with 750-msec asynchrony), 5) a target for 250 msec, 6) a blank screen for 1250 msec, 7) the prompt “Yes or No?” until the participant responded by pressing a button, and 8) a blank screen for 3000 msec, until the onset of the next trial. All stimuli were centrally presented.
At the prompt, the participants were required to press one of two buttons, positioned under their right and left thumbs, respectively. One button (labeled “Yes”) signaled that the target was a word, while the other button (labeled “No”) signaled that it was a nonword. Assignment of buttons was divided equally among participants, counterbalanced across the two versions of the stimulus list.
Electroencephalographic Data Collection and Analysis
The EEG was recorded from 34 sites (FP1, FP2, F7, F3, Fz, F4, F8, T1, FC5, FC1, FC2, FC6, T2, T7, C3, Cz, C4, T8, TP9, CP5, CP1, CP2, CP6, TP10, P7, P3, Pz, P4, P8, PO9, O1, Iz, O2, PO10) referenced to the nose, bandpass filtered at 0.05–100 Hz, and continuously digitized at 1 kHz. Blinks and eye movements were monitored by means of electrodes on the supraorbital and infraorbital ridges of the left eye and on the outer canthi of both eyes. Electrode impedances were below 5 kW. The EEG was re-referenced off-line to the algebraic mean of the mastoids (TP9, TP10). Continuous data were algorithmically corrected for eye blink artifact (38) . Event-related potentials were computed for epochs from 100 msec prestimulus to 924 msec poststimulus. Individual trials containing artifacts due to eye movement, excessive muscle activity, or amplifier blocking were rejected off-line by visual inspection before time-domain averaging; the mean percentage of trials lost to such artifacts was 18% for patients and 11% for comparison subjects.
For each trial type, N400 latency was defined as the interval between target onset and the largest negative peak from 300 to 500 msec poststimulus. N400 amplitude was defined as the mean voltage from 300 to 500 msec poststimulus.
Statistical Analysis
The p values in analyses of variance (ANOVAs) with within-subject factors underwent Greenhouse-Geisser epsilon correction. Pairwise comparisons of factor-level means were made with Tukey simultaneous comparisons, with a family confidence coefficient of 0.95. All p values are two-tailed.
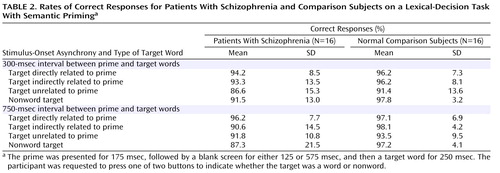
Percentage of correct responses was analyzed by ANOVA, with group (schizophrenia versus comparison) as the between-subject variable and with stimulus-onset asynchrony (300 msec versus 750 msec) and target (directly related versus indirectly related versus unrelated versus nonword) as within-subject variables.
N400 latency and amplitude were analyzed in ANOVAs with group (schizophrenia versus comparison) as the between-subject variable and with stimulus-onset asynchrony (300 msec versus 750 msec), target (directly related versus indirectly related versus unrelated), and electrode (34 levels, corresponding to all recording sites) as within-subject variables.
To examine the relationship between symptoms and N400 amplitude in the patients, we calculated coefficients for the correlations of N400 amplitude at Pz (midline parietal) for each target type at each stimulus-onset asynchrony to the psychotic, negative, and disorganized factor ratings. For correlations involving the ratings for the psychotic and disorganized factors, we used Spearman’s rank-order coefficient (r s ), since these variables were not normally distributed; otherwise, Pearson’s correlation (r) was used.
Results
Behavioral Data
The rates of correct responses by the schizophrenia patients and comparison subjects ( Table 2 ) indicate that overall, the participants were attending to the stimuli. There was a target effect (F=4.53, df=3, 60, p=0.005), with lower accuracy for unrelated targets than for directly or indirectly related targets. There was no effect of stimulus-onset asynchrony (F=2.31, df=1, 30, p=0.14) and no group effect (F=2.31, df=1, 30, p=0.14) or group-by-target interaction (F=0.63, df=3, 60, p=0.60), suggesting that the patients and comparison subjects did not differ significantly in their attention to the stimuli.
Grand Average Event-Related Potentials
Grand average event-related potentials of the schizophrenia and comparison groups are shown for midline electrodes with stimulus-onset asynchronies of 300 msec ( Figure 1 ) and 750 msec ( Figure 2 ).
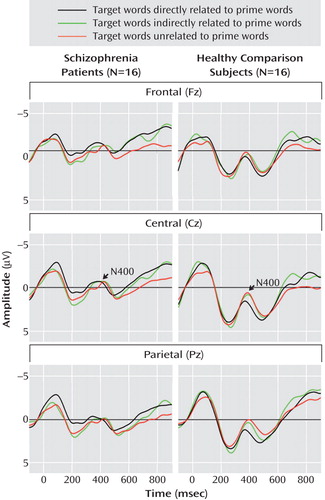
a The prime word was presented for 175 msec, followed by a blank screen for 125 msec and then a target word for 250 msec. The participant was requested to press one of two buttons to indicate whether the target was a word or nonword. Event-related potentials were recorded at midline electrode sites. Negative amplitudes are plotted upward.
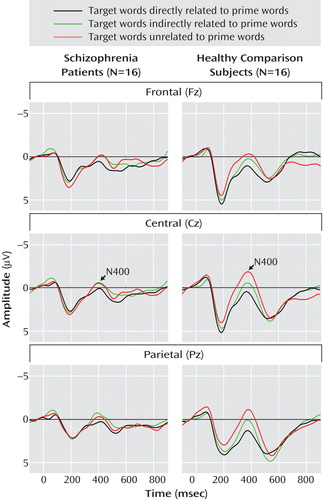
a The prime word was presented for 175 msec, followed by a blank screen for 575 msec and then a target word for 250 msec. The participant was requested to press one of two buttons to indicate whether the target was a word or nonword. Event-related potentials were recorded at midline electrode sites. Negative amplitudes are plotted upward.
N400 Latency
The mean N400 latency across the patient and comparison groups for all target types was 396 msec (SD=50). Mean latency did not vary significantly by group (F=1.97, df=1, 30, p=0.17), stimulus-onset asynchrony (F=0.23, df=1, 30, p=0.64), or target (F=0.67, df=2, 60, p=0.52), and there was no interaction between group and stimulus-onset asynchrony (F=0.90, df=1, 30, p=0.35) or between group and target (F=0.64, df=2, 60, p=0.53).
N400 Amplitude
N400 amplitudes for the schizophrenia patients and comparison subjects are shown in Table 3 . Across these two groups, N400 amplitude was largest (most negative) for unrelated targets, intermediate for indirectly related targets, and smallest for directly related targets (target main effect: F=3.38, df=2, 60, p=0.04). N400 effects were broadly distributed over the scalp, although they were the largest medially and centroparietally (target-by-electrode interaction: F=9.86, df=66, 1980, p=0.02). There was no effect of group (F=2.18, df=1, 30, p=0.15) or stimulus-onset asynchrony (F=1.02, df=1, 30, p=0.32) and no interaction between group and stimulus-onset asynchrony (F=0.70, df=1, 30, p=0.41). There was, however, a group-by-target interaction (F=3.77, df=2, 60, p=0.03)—for the comparison subjects, the N400 amplitudes for the three target types all differed significantly from one another, whereas for patients there were no differences among them. In addition, N400 amplitudes were larger for patients than for comparison subjects for both directly and indirectly related targets, but they did not differ between groups for unrelated targets.
Correlation of N400 Amplitudes with Symptom Ratings
Within the patient group (N=16), at a stimulus-onset asynchrony of 300 msec the N400 amplitudes in response to each target type were not significantly correlated with scores on the SAPS- and SANS-derived symptom factors (p>0.10 in all cases). At a stimulus-onset asynchrony of 750 msec, however, higher psychotic factor scores correlated with larger (more negative) N400 amplitudes following directly related targets (r s =–0.64, p=0.007) and indirectly related targets (r s =–0.64, p=0.007). Larger N400 amplitudes after directly and indirectly related targets correlated with scores on both the delusions subscale (directly related: r s =–0.66, p=0.005; indirectly related: r s =–0.68, p=0.003) and hallucinations subscale (directly related: r s =–0.55, p=0.03; indirectly related: r s =–0.53, p=0.04). At a stimulus-onset asynchrony of 750 msec, the N400 amplitudes following each target type were not significantly correlated with scores on the disorganized or negative symptom factor (p>0.64 in all cases).
Discussion
This study used the N400 component of the event-related potential as a brain-based method of assessing direct and indirect semantic priming in schizophrenia. Schizophrenia patients and matched comparison subjects viewed prime words followed by target words that were directly related, indirectly related, or unrelated to the primes. As hypothesized, the N400 amplitude of the comparison subjects was largest (most negative) in response to unrelated targets, intermediate for indirectly related targets, and smallest for directly related targets. By contrast, in the patients there was no significant difference in N400 amplitude elicited by these three target types, suggesting a lack of direct and indirect semantic priming. The absence of reliable priming effects in the patients reflected larger N400 amplitudes following both directly and indirectly related targets, compared to the comparison subjects, at both stimulus-onset asynchronies between the prime and the target (300 and 750 msec). In addition, within the patients, at the 750-msec asynchrony higher scores for psychotic symptom factors correlated with larger N400 amplitudes after directly and indirectly related targets.
Overall, our results do not provide evidence for greater spread of activation to weakly related items in schizophrenia. According to this hypothesis, we would expect smaller N400 amplitudes after indirectly related targets in schizophrenia patients compared to normal comparison subjects, but these were not seen at either stimulus-onset asynchrony. Rather, consistent with a number of previous studies (20 – 24) , the results provide further evidence for less activation in response to related items in schizophrenia. Specifically, the patients exhibited larger than normal N400 amplitudes following both directly and indirectly related targets, such that the amplitudes following related items were not statistically distinguishable from those following unrelated items (i.e., no priming effects). Notably, this abnormality occurred across both stimulus-onset asynchronies, suggesting that deficient activation of related items is in effect by 300 msec after a prime stimulus and that it persists thereafter. This finding fits with the view that schizophrenia is associated with impaired use of contextual stimuli even at the shortest time delays, consistent with deficient information consolidation in working memory (39) .
Further research is needed to clarify the dissociation between our finding of smaller than normal N400 priming effects in schizophrenia and the greater than normal N400 priming effects (25) and indirect priming effects on reaction time (12 – 14) previously reported, at least at short stimulus-onset asynchronies. If greater N400 priming is associated with relatively severe thought disorder, then perhaps we did not detect this abnormality because our patient group was on average insufficiently high in thought disorder. Nevertheless, we also did not find a correlation across patients between greater thought disorder (measured by the SAPS/SANS disorganized symptom factor) and greater N400 priming effects, as might have been expected. Further studies are needed to assess the degree to which greater N400 priming effects in schizophrenia are contingent on diagnostic subgroup and on characteristics of the semantic stimuli used. The contrast between our results and those of studies on reaction time priming could also stem from a dissociation between N400 and reaction time priming effects. Such dissociations have previously been reported (40) and may arise if N400 is a more direct measure of semantic activation than reaction time, which by its nature also incorporates response-related (e.g., decision-making) processes. For example, if patients rely more than healthy subjects on checking for a relationship between the prime and target in order to decide that the target is indeed a word (a strategy proposed to occur by Balota and Lorch [34] ) and detect some semantic relationship between indirectly related but not unrelated words, this could increase reaction time differences between indirectly related and unrelated targets more in patients than in comparison subjects. However, because our study was not designed to measure reaction time priming and used a delayed response, we cannot ascertain whether reaction time and N400 priming effects were dissociated in our study group.
Most patients in our study were taking a second-generation antipsychotic, either alone or in combination with a first-generation agent. Therefore, our data do not allow us to definitively assess whether and how each of these medication classes affects N400 abnormalities in schizophrenia; further research comparing N400 priming in patients taking first- versus second-generation antipsychotics and unmedicated patients is needed to do so.
In this study, following target stimuli that were related to their primes, schizophrenia patients scoring higher on the psychotic symptom factor had more negative N400 amplitudes, i.e., N400 amplitudes that were more similar to those following targets unrelated to their primes. This finding supports the view that abnormally similar neurophysiological processing of stimuli more and less meaningfully related to their context may play a role in the pathogenesis of delusions (41 , 42) . These results fit with our previous finding that psychosis in schizophrenia correlates with smaller differences in N400 amplitude between high- and low-typicality exemplars following category primes (42) . A smaller difference in the degree to which a stimulus facilitates processing of items more and less meaningfully related to it might produce the subjective experience that unrelated stimuli occurring in proximity have some meaningful connection or, conversely, that stimuli congruent with their context are unexpected or unusual, perhaps prompting an ultimately delusional explanation.
1. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
2. Spitzer M: A cognitive neuroscience view of schizophrenic thought disorder. Schizophr Bull 1997; 23:29–50Google Scholar
3. Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW: Word recall in schizophrenia: a connectionist model. Am J Psychiatry 1998; 155:1685–1690Google Scholar
4. McCarley RW, Niznikiewicz MA, Salisbury DF, Nestor PG, O’Donnell BF, Hirayasu Y, Grunze H, Greene RW, Shenton ME: Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. Eur Arch Psychiatry Clin Neurosci 1999; 249(suppl 4):69–82Google Scholar
5. Collins AM, Loftus EF: A spreading-activation theory of semantic processing. Psychol Rev 1975; 82:407–428Google Scholar
6. Cohen JD, Servan-Schreiber D: Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Google Scholar
7. Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR, Goldberg TE: Cognitive substrates of thought disorder, II: specifying a candidate cognitive mechanism. Am J Psychiatry 1998; 155:1677–1684Google Scholar
8. Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer SS, van Kammen DP: Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol 1996; 105:592–601Google Scholar
9. Besche C, Passerieux C, Segui J, Sarfati Y, Laurent JP, Hardy-Bayle MC: Syntactic and semantic processing in schizophrenic patients evaluated by lexical-decision tasks. Neuropsychology 1997; 11:498–505Google Scholar
10. Passerieux C, Segui J, Besche C, Chevalier JF, Widlocher D, Hardy-Bayle MC: Heterogeneity in cognitive functioning of schizophrenic patients evaluated by a lexical decision task. Psychol Med 1997; 27:1295–1302Google Scholar
11. Kuperberg GR, McGuire PK, David AS: Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from on-line monitoring for words in linguistically anomalous sentences. J Abnorm Psychol 1998; 107:423–434Google Scholar
12. Moritz S, Mersmann K, Kloss M, Jacobsen D, Wilke U, Andresen B, Naber D, Pawlik K: ‘Hyper-priming’ in thought-disordered schizophrenic patients. Psychol Med 2001; 31:221–229Google Scholar
13. Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M: Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophr Res 2002; 59:181–186Google Scholar
14. Spitzer M, Braun U, Hermle L, Maier S: Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry 1993; 34:864–877Google Scholar
15. Deacon D, Uhm TJ, Ritter W, Hewitt S, Dynowska A: The lifetime of automatic semantic priming effects may exceed two seconds. Brain Res Cogn Brain Res 1999; 7:465–472Google Scholar
16. Hill H, Ott F, Weisbrod M: SOA-dependent N400 and P300 semantic priming effects using pseudoword primes and a delayed lexical decision. Int J Psychophysiol 2005; 56:209–221Google Scholar
17. Kutas M, Federmeier KD: Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci 2000; 4:463–470Google Scholar
18. Holcomb PJ, Neville HJ: Auditory and visual semantic priming in lexical decision: a comparison using event-related brain potentials. Lang Cogn Process 1990; 5:281–312Google Scholar
19. Kutas M, Hillyard SA: Reading senseless sentences: brain potentials reflect semantic incongruity. Science 1980; 207:203–205Google Scholar
20. Kostova M, Passerieux C, Laurent JP, Hardy-Bayle MC: N400 anomalies in schizophrenia are correlated with the severity of formal thought disorder. Schizophr Res 2005; 78:285–291Google Scholar
21. Kostova M, Passerieux C, Laurent JP, Saint-Georges C, Hardy-Bayle MC: Functional analysis of the deficit in semantic context processes in schizophrenic patients: an event-related potentials study. Neurophysiol Clin 2003; 33:11–22Google Scholar
22. Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Guthrie D, Harper R, Yee CM, Nuechterlein KH: Event-related potential correlates of linguistic information processing in schizophrenics. Biol Psychiatry 1997; 42:596–608Google Scholar
23. Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR: Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol Psychiatry 2003; 54:1134–1148Google Scholar
24. Ohta K, Uchiyama M, Matsushima E, Toru M: An event-related potential study in schizophrenia using Japanese sentences. Schizophr Res 1999; 40:159–170Google Scholar
25. Mathalon DH, Faustman WO, Ford JM: N400 and automatic semantic processing abnormalities in patients with schizophrenia. Arch Gen Psychiatry 2002; 59:641–648Google Scholar
26. Kreher DA, Holcomb PJ, Kuperberg GR: An electrophysiological investigation of indirect semantic priming. Psychophysiology 2006; 43:550–563Google Scholar
27. Silva-Pereyra J, Harmony T, Villanueva G, Fernandez T, Rodriguez M, Galan L, Diaz-Comas L, Bernal J, Fernandez-Bouzas A, Marosi E, Reyes A: N400 and lexical decisions: automatic or controlled processing? Clin Neurophysiol 1999; 110:813–824Google Scholar
28. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Google Scholar
29. Hauser RM, Warren JR: A Socioeconomic Index for Occupations in the 1990 Census, Working Paper 96–01. Madison, University of Wisconsin, Center for Demography and Ecology, 1996Google Scholar
30. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
31. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1983Google Scholar
32. Miller DD, Arndt S, Andreasen NC: Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry 1993; 34:221–226Google Scholar
33. Nelson DL, McEvoy CL, Schreiber TA: The University of South Florida Word Association, Rhyme and Word Fragment Norms. Tampa, University of South Florida, Department of Psychology, 1998 (http://w3.usf.edu/FreeAssociation)Google Scholar
34. Balota DA, Lorch RF: Depth of automatic spreading activation: mediated priming effects in pronunciation but not in lexical decision. J Exp Psychol Learn Mem Cogn 1986; 12:336–345Google Scholar
35. McNamara TP, Altarriba J: Depth of spreading activation revisited: semantic mediated priming occurs in lexical decisions. J Mem Lang 1988; 27:545–559Google Scholar
36. Richards L, Chiarello C: Depth of associated activation in the cerebral hemispheres: mediated versus direct priming. Neuropsychologia 1995; 33:171–179Google Scholar
37. Francis WN, Kucera H: Frequency Analysis of English Usage. Boston, Houghton Mifflin, 1982Google Scholar
38. Semlitsch HV, Anderer P, Schuster P, Presslich O: A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 1986; 23:695–703Google Scholar
39. Fuller RL, Luck SJ, McMahon RP, Gold JM: Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol 2005; 114:279–290Google Scholar
40. Heinze HJ, Muente TF, Kutas M: Context effects in a category verification task as assessed by event-related brain potential (ERP) measures. Biol Psychol 1998; 47:121–135Google Scholar
41. Hemsley DR: The schizophrenic experience: taken out of context? Schizophr Bull 2005; 31:43–53Google Scholar
42. Kiang M, Kutas M, Light GA, Braff DL: Electrophysiological insights into conceptual disorganization in schizophrenia. Schizophr Res 2007; 92:225–236Google Scholar