Frequency of Provider Contact After FDA Advisory on Risk of Pediatric Suicidality With SSRIs
Abstract
Objective: The Food and Drug Administration (FDA) issued a public health advisory in October 2003 on the risk of suicide in pediatric patients taking antidepressants and advised maintaining “close supervision” of such patients. In this study, the authors compared trends in the frequency of provider contacts for patients with depression before and after the advisory was issued. Method: Retrospective cohorts of children (N=27,370) and adults (N=193,151) with new episodes of depression treated with antidepressants were created from a national claims database of managed care plans (1998–2005). Two standards were used in measuring patient monitoring: the Health Plan Employer Data and Information Set (HEDIS) quality-of-care criterion calling for three contacts in 3 months and the FDA-recommended contact schedule totaling seven visits in 3 months. Time-series models compared postadvisory trends to the expected trend based on preadvisory measures. Results: Less than 5% of all patients met FDA contact recommendations before the advisory, and the rate did not change after the advisory. A greater proportion of patients met the HEDIS contact criterion before the advisory (60% for children and 40% for adults), and the rate did not change after the advisory. A greater proportion of pediatric patients seen by a psychiatrist (80%) met the HEDIS criterion than those seen by a pediatrician (60%) or a non-pediatrician primary care physician (54%), and than adults seen by a psychiatrist (65%) or a primary care physician (37%). The proportions of pediatric patients who met the FDA recommendations did not differ by specialty. Conclusions: Contrary to expectations, the frequency of visits by patients with new episodes of depression treated with antidepressants did not increase after the October 2003 FDA advisory was issued.
In October 2003, the U.S. Food and Drug Administration (FDA) issued a public health advisory on reports of suicidal ideation and suicide attempts in pediatric patients being treated with antidepressant medications for major depressive disorder (1) . The FDA reminded prescribers that “close supervision of high-risk patients should accompany initial [antidepressant] drug therapy” (1) . In 2005, the FDA strengthened its recommendations by requiring that a black box warning be included in the labeling for antidepressants (2) and that a patient medication guide (3) be distributed with prescriptions for children and teenagers. The medication guide provides information for parents, including a frequency schedule suggesting that pediatric patients should have a total of seven visits with their physician during the first 3 months of antidepressant drug therapy—once a week for the first month, every 2 weeks for the second month, and a visit at 3 months. Between the initial advisory and the black box warning, the FDA also issued a public health advisory about use of antidepressants in adults and strengthened warnings about the need to monitor all patients for worsening of depression, especially early in treatment and after dosage changes (4) .
The FDA’s intention was to “strengthen safeguards for children treated with antidepressant medications,” an important component of which was close monitoring of patients as a way of managing the risk of suicidality (5) . The actual effect of the advisory on antidepressant medication management and frequency of provider contact has not been reported.
The purpose of this study was to evaluate the impact of the FDA warnings on market-level patterns of provider monitoring, using data from a national community-based cohort of pediatric and adult managed care patients with new episodes of depression. We hypothesized that the frequency of physician visits after initiation of antidepressant medication would increase as a result of the advisory and the publicity attending it. We evaluated two measures of physician contact: the National Committee on Quality Assurance’s (NCQA’s) Health Plan Employer Data and Information Set (HEDIS) metric for measuring the quality of outpatient depression care and antidepressant management in adults (6) and the FDA’s recommended frequency of physician visits as specified in the medication guide on antidepressant use in children and adolescents (3) . Although the FDA did not issue these specific visit frequency recommendations until 2005, we use them in this study as one benchmark for monitoring frequency before and after the 2003 advisory.
Over the past decade, the NCQA has used HEDIS measures in its reports on the state of health care quality (7) . The HEDIS quality indicators for antidepressant medication management, which were derived from expert consensus on treatment research and clinical care (6) , have been tracked by NCQA since 1998 (7) ; they have also been used in published depression treatment and outcomes research (6 , 8 – 10) . HEDIS defined three quality-of-care measures for antidepressant drug therapy: optimal provider contact, which was defined as three mental health-directed provider contacts during the first 12 weeks after initiation of treatment; duration of drug therapy during the first 12 weeks (acute treatment); and duration of therapy during the first 6 months (continuation treatment) (6) .
In the FDA’s medication guide, the agency recommended that pediatric patients visit their physician weekly during the first month of antidepressant therapy, biweekly during the second month, and again at 12 weeks (3) . The rationale for more frequent contacts early in treatment was based on observational studies and short-term placebo-controlled trials that suggested an elevated risk of suicidality in the first weeks after initiation of antidepressant medication (11 – 13) . To our knowledge, this advisory represents the first time the FDA has specified frequency-of-visit guidelines for clinicians.
The results of this study illustrate aggregate effects on antidepressant medication management and provider visits in community medical practice among U.S. managed care enrollees with depression. The study uses a data set that has unique strengths for evaluating the impact of drug warnings on patient care: a large national administrative claims database with information on patient diagnoses, prescriptions, and health care visits; a time span covering 5 years before and 1.5 years after the FDA advisory, allowing robust estimates of trends in patterns of care; and records of provider specialties, allowing comparison of trends by prescriber specialty. As a secondary objective, we specifically examined rates of monitoring among patients seen by a psychiatrist compared with patients seen by other providers.
In two previous studies (14 , 15) , we found decreased rates of diagnosis and treatment of depression in children and adults after the FDA advisory. This study broadens our understanding of physician behavior change after the advisory by addressing the effect of the advisory on frequency of follow-up visits among patients who received antidepressant drug therapy.
Method
Data
The data for this retrospective cohort study were from a commercially available administrative claims database acquired from PharMetrics, Inc. The database is made up of integrated medical and pharmacy paid claims data from more than 85 managed care plans nationally, representing more than 47 million covered lives with age, gender, and regional distributions similar to those in the 2000 U.S. Census data.
Enrollment and claims data were extracted from the PharMetrics database for enrollees of all ages who had a medical claim with a diagnosis of major depressive disorder or a related depressive disorder (ICD-9-CM codes 296.xx–300.xx or 311.xx) or had a paid claim for a filled prescription of any antidepressant drug (generic product identifier [GPI] code 58.xx). Because the data were deidentified, an expedited review was obtained, and the study was approved by the Colorado Multiple Institutional Review Board.
Study Population
Two study cohorts were created: pediatric patients (ages 5–18 years at depression diagnosis) and adult patients (older than age 18 at diagnosis) who had a new episode of depression between October 1998 and March 2005 and received antidepressant medication within 30 days of the diagnosis. The first antidepressant prescription filled within 30 days of the diagnosis was defined as the index antidepressant prescription. A new episode of depression was defined using the following HEDIS specifications for national tracking of the quality of antidepressant medication management: an ICD-9-CM code of 296.2, 296.3, 300.4, or 311 (i.e., major depressive disorder, single episode; major depressive disorder, recurrent episode; neurotic depression; and depression not otherwise specified, respectively); no other depression-related diagnoses during the 120 days before depression diagnosis; and no antidepressant medication claims during the 90 days before depression diagnosis (6 , 9) . Continuous enrollment for 120 days before and 180 days after diagnosis was required.
From the pediatric cohort of 65,349 unique depressive episodes, 27,370 patients received antidepressant medication within 30 days of diagnosis and met other inclusion criteria. From the adult cohort of 475,838 unique depressive episodes, 193,151 patients received antidepressant medication within 30 days of diagnosis and met other inclusion criteria.
FDA Advisory
The policy action of interest in this analysis was the October 2003 FDA public health advisory, a choice consistent with our previous reports examining the impact of the advisory on diagnosis and treatment of depression in children and adults (14 , 15) .
Measures
The measures described below for antidepressant medication management and provider contact were used for both the pediatric and adult cohorts. Monthly time series were created for each measure, with monthly observations representing an aggregate measure for all pediatric or adult patients with depression diagnosed in that month who filled an antidepressant prescription within 30 days of diagnosis.
HEDIS antidepressant treatment quality indicators
We derived three measures from individual components of the HEDIS antidepressant medication management technical specifications criteria (6) . Optimal practitioner contacts was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had three or more billable claims for contacts with a primary care or mental health practitioner coded with a mental health diagnosis during the 84 days following diagnosis (16) . Visits could include billable telephone interventions (current procedural terminology [CPT] codes 99371–99373). HEDIS specifications also state that “at least one of the three follow-up contacts must be with a prescribing practitioner” (6) . Effective acute phase treatment was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had antidepressant medication available for 84 of the 114 days following the first prescription (16) . Effective continuation phase treatment was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had at least a 180-day supply of any antidepressant during the 214 days following the first prescription (16) . Overall adherence was defined as meeting all three of these performance criteria.
FDA recommended monitoring
We derived three measures based on the FDA’s recommendations in its antidepressant medication guide (3) . Weekly month 1 monitoring was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had four physician visits during the 30 days after the index prescription fill date. Biweekly month 2 monitoring was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had two physician visits between 31 and 60 days after the index prescription fill date. Month 3 monitoring was defined as the percentage of new depressive episodes with a related antidepressant prescription for which the patient had a physician visit at 90 days (7 days) after the index prescription fill date. Overall adherence was defined as meeting all three of these patient contact measures, or as having had seven contacts in the 90 days after the index prescription fill date. To avoid an overly restrictive assessment of patient monitoring, we included any visit to a health care provider, not just to the prescribing provider.
Prescriber Specialty
Analyses were stratified by the specialty of the prescribing provider. Prescriber specialty was coded as pediatrician, primary care physician (family physician, general internist, or obstetrician-gynecologist), psychiatrist, other mental health provider (psychologist, social worker, or therapist), or other specialty not already listed.
Statistical Analysis
Segmented time-series regression analysis (17 – 19) was used to estimate changes in the HEDIS and FDA measures after the March 2003 FDA advisory was issued, controlling for preadvisory trends and other autocorrelation, as previously described (14 , 15) . All models were implemented in Stata (20) .
Results
Trends in HEDIS Measures
During the period before the FDA advisory was issued, about 60% of pediatric patients and 40% of adults met the HEDIS optimal provider contact criterion; 50%–60% received effective acute phase treatment; and 30%–40% received effective continuation treatment. Only 21% of children and 16% of adults met all three HEDIS criteria. There was no significant change after the FDA advisory in the proportion of pediatric or adult patients meeting any of the three HEDIS markers.
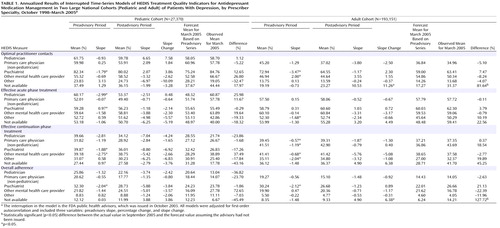
Table 1 summarizes the time-series regression results for the annualized HEDIS measures in the pediatric and adult cohorts stratified by the specialty of the prescribing provider. The percentages of children and adults treated by primary care physicians were 32% and 61%, respectively; by psychiatrists, 29% and 16%; by other mental health providers, 8% and 5%; and by other specialties, 14% and 12%. Twelve percent of children were treated by a pediatrician. Prescriber specialty was not available for 6% of children and adults.
The first four columns for each cohort in Table 1 report the annual mean percentage and trend (slope) for the preadvisory and postadvisory periods. The slope change from the preadvisory to the postadvisory period indicates an effect associated with the FDA advisory. Before the advisory was issued, the percentages of pediatric and adult patients who were seen by a psychiatrist and met the HEDIS criteria for optimal practitioner contact were declining at 1.8% and 3.5% per year, respectively. However, there were no significant changes in rates of receiving optimal practitioner contact after the advisory in any of the identified prescriber specialty groups, for pediatric or adult patients.
The baseline preadvisory trend was also used to forecast the percentages of patients who received optimal practitioner contact in March 2005, which were compared with the observed percentages for that month using a t test. The last column for each cohort in Table 1 lists the percentage difference between the observed and the predicted rate. A statistically significant positive value indicates that the rate observed 17 months after the advisory was issued was higher than predicted on the basis of preadvisory trends. Consistent with the trend analysis, there were no significant changes in projected versus actual HEDIS measures in children or adults for whom prescriber specialty was available.
Trends in FDA Measures
Before the FDA advisory was issued, the proportion of patients whose frequency of follow-up visits met FDA recommendations after initiation of antidepressant therapy was low. Among pediatric patients, less than 10% had four visits in month 1, less than 20% had two visits in month 2, and 30%–40% had a visit at month 3. Among adult patients, less than 10% had four visits in month 1, about 10% had two visits in month 2, and about 30% had a visit at month 3. Less than 5% of children and adults met criteria for all three measures (or had seven visits within 3 months of initiating therapy). There were no significant changes in the trend after the FDA advisory was issued in the proportion of pediatric or adult patients having the FDA-recommended frequency of visits in month 1 or month 2 or at month 3.
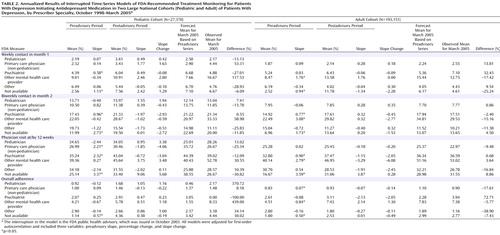
Table 2 summarizes the time-series regression results for the annualized FDA measures in the pediatric and adult patient cohorts, stratified by the specialty of the prescribing provider. In both children and adults, there was no significant change in testing trends or in the level of testing for any of the provider groups for any of the FDA measures.
Comparison of HEDIS and FDA Measures Across Prescriber Specialty
Figure 1 displays the mean postadvisory rates of meeting the HEDIS and FDA criteria among pediatric and adult patients whose initial antidepressant prescription was provided by psychiatrists, non-pediatrician primary care physicians, or pediatricians. A significantly higher proportion (p<0.05) of pediatric patients seen by a psychiatrist (80%) met the HEDIS contact criterion than pediatric patients seen by a pediatrician (60%) or a non-pediatrician primary care physician (54%) and than adults seen by a psychiatrist (65%) or a primary care physician (37%). There was no significant difference in the proportion of pediatric patients seen by a pediatrician, primary care physician, or psychiatrist who met all three FDA provider contact recommendations.

Discussion
To our knowledge, this is the first study to measure the effects of the FDA’s recommendation for “close supervision” of pediatric patients after initiation of an antidepressant medication in a pediatric community-based managed care population. It is also the first to assess follow-up visit frequency on the basis of the FDA-recommended numbers of visits during the first 3 months of antidepressant treatment as well as on the basis of the more permissive HEDIS treatment quality guidelines. For comparison, we also measured the level of provider contact for adult patients with depression, although a recent FDA analysis examining the association of suicidality with antidepressant treatment (21) indicates no need to change monitoring recommendations for adults 25 to 65 years of age on the basis of concerns about suicidality risk.
Overall, we did not find an increase in frequency of face-to-face visits after the FDA advisory was issued in October 2003. The proportion of pediatric patients meeting FDA frequency-of-visit recommendations was less than 5% before the advisory was issued, and it did not change significantly afterward. The low frequency of follow-up contact we observed was similar to preadvisory rates seen in a large managed-care organization (22) . HEDIS visit standards, which required at least three contacts with a primary care or mental health practitioner within 12 weeks, were met more frequently; about 60% of pediatric patients met this criterion, but the proportion did not rise after the FDA advisory was issued. A greater proportion of pediatric patients seen by a psychiatrist met the HEDIS contact criterion than pediatric patients seen by a pediatrician or a non-pediatrician primary care physician, although this association was not significant for the FDA contact criteria. In general, the frequency of follow-up contacts was higher for pediatric patients than for adults. The other HEDIS quality criteria, those regarding duration of drug therapy during the acute and continuation phases of treatment, showed no significant change from the roughly 60% and 40% rates in pediatric and adult patients, respectively.
These results are derived from a large, geographically diverse managed care claims data set containing 1.5 years of claims data during the period following the initial FDA advisory, which should be sufficient time to detect a resulting change in surveillance behavior. While specific visit recommendations for pediatric patients were not clearly presented and discussed until the FDA black box warning and patient medication guide were issued in 2005, it could be expected that visit frequency would increase given the publicity generated by the progressively more emphatic and global communications the FDA issued between the initial October 2003 advisory and the approved text of the black box warning and medication guide. Additional measurements in future research extending the time line beyond 2005 will be illuminating.
Managed care plans may have a differential capacity to respond to demands for more stringent chronic care management than clinicians operating in an unmanaged environment, and therefore these findings should not be generalized uncritically to the larger population of clinicians. Another limitation of administrative data sets relying on claims is that while they accurately reflect the numbers and timing of face-to-face contacts between clinicians and patients, they tell us nothing about the quality of the contact or the extent to which other innovative monitoring strategies may have been used. The chronic disease management model is an example of such an innovation that has been widely adopted by managed care companies and applied to depression care (23) . In this model, care is provided by a team that usually includes a care manager who is responsible for many of the monitoring follow-up contacts. These contacts are often done by telephone or by e-mail. In this study, we counted billed telephone contacts as an encounter, a permissive interpretation of the FDA recommendations. Managed care programs can also involve a psychiatrist and a primary care physician working collaboratively on behalf of a single patient, sometimes with alternating visits and sometimes discussing the patient together; under such a scenario, surveillance might be underestimated. However, the proportion of patients with depression in this managed care population whose care met the HEDIS criterion for provider contact was somewhat higher than in reports from the NCQA (7) and in other studies of administrative claims data (8 , 16) .
Limitations notwithstanding, the results of this study are strongly suggestive of a large gap between the FDA’s monitoring recommendations and actual practice. Although the FDA’s determinations regarding antidepressants have not been without controversy, there is little evidence that the community of mental health and general care clinicians reject the associations reported by the FDA for children and adolescents taking antidepressants. Indeed, APA supported the FDA’s recommendation for close monitoring of pediatric patients by physicians, families, and caregivers (24) . However, the FDA may have created an artificial gap between recommendations and practice by instituting a non-evidence-based standard. The schedule of seven visits over 12 weeks recommended by the FDA, including once-a-week visits for the first month, constitutes substantially more frequent contact than would be expected for most other medical disorders. For more than two decades, community-based practices have found it extremely difficult to increase face-to-face follow-up contact with newly diagnosed depressed patients by even one or two visits, and a variety of innovative protocols have been tested to achieve better postprescription monitoring (25 – 27) . Perhaps if data on the frequency of provider contact with children initiating antidepressant drug therapy had been available before the black box warning and patient medication guide were issued, a data-based discussion on expectations for changing monitoring frequency could have occurred before the FDA’s recommendations were set.
Motivation to adhere to the FDA’s recommendations may have been undermined by the fact that the recommended visit schedule appeared without supporting evidence that such close surveillance would confer protection or lead to improved outcomes, and physicians may have believed that their current level of monitoring was clinically sufficient. At the same time, many practitioners and practice managers may have been concerned that delivering care that fell short of FDA recommendations might leave them open to liability for practicing outside the established standard of care (even though the FDA lacks the legal authority to enforce its monitoring recommendations on physicians). Given the confusion surrounding the initial antidepressant advisory, it thus becomes plausible that clinicians and practices might have first responded with a reduction in the frequency of diagnosis and treatment of depression, as we have reported (14 , 15) .
While our findings in this study indicate that clinicians did not increase their frequency of monitoring for patients starting antidepressant drug therapy, the study’s limitations lead us to treat these results with caution until corroboration is provided. We should study more carefully the reasons why increased monitoring did not occur despite a well-publicized FDA advisory; this can be done with a more fine-grained analysis by studying monitoring in terms of practice, provider, patient, and disease characteristics. Finally, much good could come from ascertaining whether increased surveillance actually improves outcomes, and if so, what the follow-up frequency should be.
1. US Food and Drug Administration: FDA public health advisory: reports of suicidality in pediatric patients being treated with antidepressant medications for major depressive disorder, Oct 27, 2003. http://www.fda.gov/cder/drug/advisory/mdd.htmGoogle Scholar
2. US Food and Drug Administration: Class suicidality labeling language for antidepressants, 2005. http://www.fda.gov/cder/drug/antidepressants/PI_template.pdfGoogle Scholar
3. US Food and Drug Administration: Medication guide: about using antidepressants in children and teenagers, 2005. http://www.fda.gov/cder/drug/antidepressants/MG_template.pdfGoogle Scholar
4. US Food and Drug Administration: FDA Public Health Advisory: Worsening Depression and Suicidality in Patients Being Treated With Antidepressant Medications. Washington, DC, US Food and Drug Administration, Mar 22, 2004 (available at www.fda.gov/cder/drug/antidepressants/antidepressanstpha.htm)Google Scholar
5. US Food and Drug Administration: FDA News Release: FDA launches a multi-pronged strategy to strengthen safeguards for children treated with antidepressant medications, Oct 15, 2004. http://www.fda.gov/bbs/topics/news/2004/NEW01124.htmlGoogle Scholar
6. National Committee for Quality Assurance: HEDIS Volume 2: Technical Specifications. Washington, DC, 2004Google Scholar
7. National Committee for Quality Assurance: The State of Healthcare Quality. Washington, DC, 2006Google Scholar
8. Rost K, Dickinson LM, Fortney J, Westfall J, Hermann RC: Clinical improvement associated with conformance to HEDIS-based depression care. Ment Health Services Res 2005; 7:103–112Google Scholar
9. Scholle SH: NCQA behavioral health measurement efforts. J Manag Care Pharm 2005; 11(3 suppl):S9–S11Google Scholar
10. Busch SH, Leslie D, Rosenheck R: Measuring quality of pharmacotherapy for depression in a national health care system. Med Care 2004; 42:532–542Google Scholar
11. Jick SS, Dean AD, Jick H: Antidepressants and suicide. BMJ 1995; 310:215–218Google Scholar
12. Valuck RJ, Libby AM, Sills MR, Giese AA, Allen RR: Antidepressant treatment and risk of suicide attempt by adolescents with major depressive disorder: a propensity-adjusted retrospective cohort study. CNS Drugs 2004; 18:1119–1132Google Scholar
13. Mosholder AD, Willy M: Suicidal adverse events in pediatric randomized, controlled clinical trials of antidepressant drugs are associated with active drug treatment: a meta-analysis. J Child Adolesc Psychopharmacol 2006; 16:25–32Google Scholar
14. Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ: Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry 2007; 164:884–891Google Scholar
15. Valuck RJ, Libby AM, Orton HD, Morrato EH, Allen R, Baldessarini RJ: Spillover effects on treatment of adult depression in primary care after FDA Advisory on risk of pediatric suicidality with SSRIs. Am J Psychiary 2007; 164:1198–1205Google Scholar
16. Robinson R, Long S, Chang S, Able S, Baser O, Obenchain R, Swindle R: Higher costs and therapeutic factors associated with adherence to NCQA HEDIS antidepressant medication management measures: analysis of administrative claims. J Manag Care Pharm 2006; 12:43–54Google Scholar
17. Cook TD, Campbell DT: Quasi-Experimentation: Design and Analysis Issues for Field Settings. Chicago, Rand McNally, 1979Google Scholar
18. Wagner AK, Sourmerai SB, Zhang F, Ross-Degnan D: Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309Google Scholar
19. Catalano R, Libby A, Snowden L, Cuellar AE: The effect of capitated financing on mental health services for children and youth: the Colorado experience. Am J Public Health 2000; 90:1861–1865Google Scholar
20. Stata: Intercooled Stata for Windows. College Station, Tex, Stata Corp, 2006Google Scholar
21. Stone M, Jones M: Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults, Nov 16, 2006. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdfGoogle Scholar
22. Stettin G, Yao J, Verbrugge R, Aubert R: Frequency of follow-up care for adult and pediatric patients during initiation of antidepressant therapy. Am J Manag Care 2006; 12:453–461Google Scholar
23. Wagner E, Austin B, Von Korff M: Organizing care for patients with chronic illness. Milbank Q 1996; 74:511–544Google Scholar
24. American Psychiatric Association: News release: APA responds to FDA’s new warning on antidepressants, Oct 15, 2004Google Scholar
25. Grembowski DE, Martin D, Patrick DL, Diehr P, Katon W, Williams B, Engelberg R, Novak L, Dickstein D, Deyo R, Goldberg HI: Managed care, access to mental health specialists, and outcomes among primary care patients with depressive symptoms. J Gen Intern Med 2002; 17:258–269Google Scholar
26. Katon W, Russo J, Von Korff M, Lin E, Simon G, Bush T, Ludman E, Walker E: Long-term effects of a collaborative care intervention in persistently depressed primary care patients. J Gen Intern Med 2002; 17:741–748Google Scholar
27. Hunkeler EM, Katon W, Tang L, Williams JW Jr, Kroenke K, Lin EH, Harpole LH, Arean P, Levine S, Grypma LM, Hargreaves WA, Unützer J: Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006; 332:259–263Google Scholar