A PET Study Evaluating Dopamine D2 Receptor Occupancy for Long-Acting Injectable Risperidone
Abstract
OBJECTIVE: Long-acting injectable risperidone represents the first clinically available depot atypical antipsychotic. The present study used positron emission tomography (PET) to evaluate its dopamine D2 binding profile at doses of 25, 50, or 75 mg administered every 2 weeks. METHOD: After achieving stabilization with one of the doses, nine patients with a diagnosis of schizophrenia or schizoaffective disorder underwent [11C]raclopride PET to measure D2 occupancy. Participants were scanned twice during the 2-week injection interval: within 3 days after injection (postinjection) and within 5 days before the next injection (preinjection). At the same time, plasma was collected for measurements of risperidone plus 9-hydroxyrisperidone. RESULTS: Mean post- and preinjection D2 occupancy levels for the 25-, 50-, and 75-mg doses were 71.0% and 54.0%, 74.4% and 65.4%, and 81.5% and 75.0%, respectively. There was a significant correlation between dose and plasma concentrations of risperidone plus 9-hydroxyrisperidone, and the estimated plasma concentration associated with 50% D2 occupancy (ED50) was 11.06 ng/ml. Prolactin levels were not correlated with drug levels or D2 occupancy. CONCLUSIONS: All three doses of injectable risperidone showed peak D2 occupancy levels above the 65% threshold associated with optimal clinical response; the 75-mg dose approximated the 80% threshold linked to increased risk of extrapyramidal symptoms. Doses of 25 or 50 mg should provide therapeutic efficacy while minimizing the risk of extrapyramidal symptoms.
Long-acting depot antipsychotics were introduced for clinical use in the 1960s, and among their advantages compared with oral agents was the greater assurance of medication delivery, reflected in decreased relapse rates (1, 2). While the use of depot antipsychotics varied between countries and settings (3, 4), they have proven to be a useful and important option in our treatment armamentarium (2, 5).
A shift in practice patterns took place in the 1990s with the influx of a number of novel antipsychotics, and the clinical advantages of these newer “atypical” agents rapidly positioned them as the treatment of choice for psychotic conditions such as schizophrenia (6–8). Despite their identified benefits, however, adherence has remained an issue (9, 10).
It is only recently, with the introduction of long-acting, injectable risperidone, that clinicians have had access to a depot atypical antipsychotic. This formulation represents an aqueous suspension that contains the drug in a matrix of glycolic acid-lactate polymer, and gradual hydrolysis of the copolymer at the injection site allows slow but steady release of risperidone over several weeks (11, 12). Various trials have indicated that it is an effective and well-tolerated treatment when administered every 2 weeks (13–16).
Positron emission tomography (PET) studies of antipsychotics, focusing in particular on dopamine D2 receptor occupancy, have proven valuable in understanding the relationship between dose, clinical response, and D2-related adverse events (e.g., extrapyramidal symptoms). Most of this work has involved oral antipsychotics (17–20), although at least one report with haloperidol decanoate has suggested that peak occupancies for the depot are in line with what is observed in oral formulations (21).
Building upon the existing clinical evidence for long-acting risperidone, we set out to evaluate the D2 receptor occupancy of various doses using PET. The study was not designed as a clinical efficacy trial but rather was intended to provide occupancy data that, in the context of our current understanding, could provide additional in vivo evidence regarding appropriate dosing with this new formulation.
Method
Subjects were recruited from a multicenter study investigating the use of long-acting risperidone administered every 2 weeks at three different doses (25, 50, or 75 mg) in individuals with schizophrenia or schizoaffective disorder (13). The PET investigation was approved by the Human Subjects Review Committee of the University of Toronto, and subjects provided written informed consent after receiving detailed information about the protocol.
Details of the multicenter trial have been published elsewhere, including design and clinical results (13). Briefly summarizing, patients were candidates for a switch to long-acting risperidone if they met DSM-IV criteria for schizophrenia or schizoaffective disorder and had been stabilized on an oral antipsychotic regimen for at least 4 weeks. Exclusion criteria were current treatment with clozapine, presence of DSM-IV-defined substance abuse or dependence within the preceding 3 months, a neurological or medical condition that could adversely influence patient safety or evaluation, pregnancy, demonstrated lack of response to oral risperidone in the past, or concomitant medications that could compromise D2 evaluation (e.g., nonantipsychotic agents with dopamine agonist or antagonist properties).
During a 2-week run-in phase, antipsychotics other than oral risperidone were discontinued and replaced with oral risperidone at flexible doses ranging from 1 to 6 mg/day. Thus, all subjects were receiving oral risperidone during this phase, and the dose of long-acting risperidone that they received thereafter was dependent on the oral risperidone dose at the end of the run-in phase. Specifically, they were assigned to one of the three doses of long-acting risperidone as follows: those receiving 1–2 mg/day or 3–4 mg/day of oral risperidone were assigned to the 25-mg and 50-mg doses of long-acting risperidone, respectively; subjects assigned to the 75-mg dose of long-acting risperidone were those receiving 5–6 mg/day of oral risperidone. Supplementary oral risperidone doses (1–6 mg/day) could be used for the first 2–3 weeks following the switch, although this was not required in any of the cases reported here.
Long-acting risperidone was administered every 2 weeks, and at least five consecutive injections were administered before PET was carried out. Participants were scanned twice during the 2-week injection interval: within 3 days following injection (postinjection) and within 5 days before the next injection (preinjection).
D2 occupancy was established using [11C]raclopride following the same procedure employed at our center and detailed previously (22). PET scanning was conducted using a GEMS PC2048-Plus PET scanner (GE Medical Systems, Milwaukee) that produced 15 slices (thickness: 6.5 mm) with a resolution of about 5–6 mm in air. Patients were scanned lying down, with fixation of their heads achieved by use of a thermoplastic face mask (Tru-Scan Imaging, Annapolis, Md.).
Following injection of 10 mCi of high-specific-activity [11C]raclopride (>300 Ci/mmol) using a bolus plus infusion protocol (23–26), a series of emission scans were obtained for 75 minutes. The regions of interest were the caudate/putamen, with the cerebellum used as a reference region. The regions of interest were drawn directly on averaged PET images and transferred to the dynamic PET images to obtain a time activity curve. An average of the striatum/cerebellum ratio minus one obtained between 30 and 75 minutes of scanning was taken as a measure of the equilibrium binding potential (27). Receptor occupancy was then calculated as the percentage reduction of receptor binding potential with drug treatment relative to baseline, using age-corrected measures of binding potential from a previously collected dataset of unmedicated comparison subjects obtained with a similar methodology. This comparison group consisted of 22 healthy subjects and nine antipsychotic-naive patients with a diagnosis of schizophrenia or schizophreniform disorder (mean age=31.5 years, SD=8.51, range=19–47). Since the mean binding potential in the healthy subjects (mean=2.7, SD=0.4) did not differ significantly from that of the antipsychotic-naive subjects (mean=2.9, SD=0.4) (t=0.98, df=29, p=0.30), the data from the two groups were combined to obtain the regression equation for the age-matched estimate of binding potential in the occupancy calculation (binding potential=age ×0.03 + 3.8; R2=0.6).
Venous blood was drawn for drug and prolactin concentrations at the time of the PET scans. For risperidone, blood samples were collected in heparinized tubes and centrifuged for 10 minutes at 2500 rpm within 2 hours of collection. Separated plasma was stored at –20°C for transport, and concentrations of risperidone plus 9-hydroxyrisperidone were determined by means of a validated radioimmunoassay method (11). Prolactin levels were determined by using a two-site chemiluminometric immunoassay with a minimal detectable limit of 0.3 ng/ml and a coefficient of variance of 3.6%–4.5% (ACS, CIBA-Corning Diagnostics, East Walpole, Mass.).
Statistical analyses were carried out using SPSS (SPSS Inc., Chicago). Bivariate correlation analysis was used to examine the relationship between the primary variables of interest. Nonlinear regression analysis was used in the estimation of plasma risperidone plus 9-hydroxyrisperidone level associated with 50% D2 receptor occupancy.
Results
Nine participants (seven men and two women) completed the study. The group’s mean age was 34 years (SD=9, range=21–46), and the dosage breakdown was as follows: 25 mg (N=2), 50 mg (N=5), and 75 mg (N=2).
As seen in Figure 1, D2 occupancy levels, measured at the two time points (postinjection and preinjection) over the 2-week injection interval, increased in a dose-dependent fashion (25 mg: mean=71.0% [SD=5.7] and 54.0% [SD=1.4], respectively; 50 mg: mean=74.4% [SD=10.4] and 65.4% [SD=2.1]; 75 mg: mean=81.5% [SD=5.0] and 75.0% [SD=5.7]). Conversely, a comparison across all doses over the two time points indicated a significant reduction in both D2 occupancy (t=3.67, df=8, p=0.006) and plasma concentrations of risperidone plus 9-hydroxyrisperidone (t=3.35, df=8, p=0.01). Eight of nine participants showed an expected decline in both of these measures over the course of the injection interval (Table 1). One participant showed a small increase in occupancy that paralleled a modest increase in plasma levels (patient 4), but given the test-retest standard deviation of 6% in our lab this change is not significant.
Dose showed a significant correlation with plasma risperidone plus 9-hydroxyrisperidone concentrations (r=0.63, df=7, p=0.006), even after we controlled for time (r=0.69, p=0.002). The relationship between plasma concentration and D2 occupancy was captured by a saturating hyperbola (Bmax constrained to 100%), with the estimated plasma concentration associated with 50% D2 occupancy (ED50) being 11.06 ng/ml (95% confidence interval=9.15–12.96) (Figure 2).
Mean prolactin values at the two time points (postinjection and preinjection) over the 2-week injection interval were 57.11 μg/liter (SD=44.97) and 44.59 μg/liter (SD=38.30), respectively, representing a nonsignificant difference (t=0.74, df=23, p=0.47). There was no significant correlation between prolactin concentrations and plasma concentrations of risperidone plus 9-hydroxyrisperidone (r=0.32, p=0.12) or D2 receptor occupancy (r=0.34, p=0.10).
We sampled only a small subgroup of the larger clinical sample, and the specific findings of the latter have been reported elsewhere (13). All subjects participating in the PET study either remained stable or showed clinical improvement during the trial, with the exception of one individual (patient 8) who showed a 1-point Clinical Global Impression deterioration. Extrapyramidal symptom scores, as measured by the Extrapyramidal Symptom Rating Scale, remained low in all dose groups throughout the PET study.
Discussion
With any new medication, it is critical that guidelines regarding optimal dosing be established. Previous PET data have indicated that optimal clinical response occurs when at least 65% of striatal D2 receptors are occupied, while the risk of extrapyramidal symptoms increases notably at D2 occupancy levels above 80% (17, 18). The present findings indicate that long-acting injectable risperidone, even at a dose of 25 mg every 2 weeks, exceeds the threshold associated with clinical response. As expected, D2 occupancy was dose-dependent, with only the highest dose employed (75 mg) approximating the threshold that has been associated with extrapyramidal symptoms in previous studies.
These findings suggest that for most patients treated with long-acting risperidone, clinical efficacy should be expected at doses of 25–50 mg. This corroborates the existing clinical evidence showing no additional clinical benefit at higher doses (14). Moreover, our PET results suggest that dosing at 75 mg every 2 weeks may be associated with some degree of increased risk of extrapyramidal symptoms, supporting the observation of a dose-dependent risk of extrapyramidal symptoms in the clinical setting (14).
Mean D2 occupancy levels toward the end of the injection interval for the 50- and 75-mg doses exceeded 65%, while for the 25-mg dose the end-of-interval occupancy level was 54%. At first glance, these data might be seen as suggesting that individuals receiving 25 mg would be vulnerable to relapse toward the end of the injection interval. However, maintenance of D2 occupancy levels above the 65% threshold may not be necessary for clinical response. It has been shown, for example, that individuals can be stabilized on a regimen of haloperidol decanoate administered every 4 weeks, despite mean D2 occupancy levels decreasing from 73% (range=60%–82%) at week 1 to 53% (range=20%–74%) at week 4 (21). Indeed, there is a growing body of evidence to suggest that there may be clinical advantages to avoiding sustained, high D2 blockade (28).
Previous work from our center with oral risperidone, which evaluated the relationship between D2 occupancy and risperidone plus 9-hydroxyrisperidone, established an ED50 of 6.08 ng/ml (95% CI=4.8–7.3) (29). In contrast, the calculated ED50 here was 11.06 ng/ml (95% CI=9.15–12.96). This discrepancy may reflect study group size: in the first report the curve was established based on nine data points, including one significant outlier, whereas the current estimate was constructed from twice as many data points. There are well-recognized concerns regarding the impact of inter- and intraindividual variability in plasma antipsychotic levels and efforts to establish “therapeutic windows” (30–32). Bearing in mind this caveat, the current estimate actually dovetails nicely with a more recent review of this topic suggesting an optimal therapeutic range for risperidone plus 9-hydroxyrisperidone of 20–60 ng/ml (33). As seen in Figure 2, 20 ng/ml is associated with 65% D2 occupancy, while 60 ng/ml represents 84% D2 occupancy. Thus, the lower level meets the threshold associated with optimal clinical response, whereas the upper limit is just over the threshold linked to increased extrapyramidal symptom risk (18), which in turn may compromise clinical outcome.
The lack of correlation between prolactin and D2 occupancy was not entirely unexpected, since factors mediating prolactin secretion are complex (34). Our own examination of this issue has in the past provided mixed results (17, 22), and efforts to establish a threshold for hyperprolactinemia in the same fashion as clinical response and extrapyramidal symptoms have not met with the same degree of agreement (17, 35).
A comment is warranted regarding timing of the PET scans in this study and the actual pharmacokinetics of long-acting risperidone. The two points used here were chosen to reflect the time frame of the recommended 2-week injection interval, and in so doing provide a reflection of occupancy through the cycle of treatment at steady state. This needs to be distinguished, however, from the actual pharmacokinetics of single- and multiple-dose long-acting risperidone (11). According to single-dose data an injection’s peak occurs approximately 4–6 weeks later, meaning that the main release phase is not observed until after several injection intervals. Over the course of an injection interval at steady state, concentrations of the active moiety (Cmax to Cmin) decrease by approximately 50%–60%, and while this rate of decrease is notably greater than what is observed in terms of changes in D2 occupancy, we are reminded that a significant dissociation between brain and plasma kinetics in this regard has been reported with antipsychotics (36).
At least several limitations qualify our conclusions. PET studies of this sort involve limited numbers of patients, much smaller than what would be required to definitively analyze the relationship between dose/receptor occupancy and response/side effects. As a result, to make clinical inferences we must extrapolate the D2 occupancy data to other larger clinical trials (13, 14). In addition, by focusing on the D2 story we do not address the potential contribution of other receptors and systems in a class of medications that are best characterized by their pharmacological heterogeneity (37–39). Even confining our comments to D2, the use of [11C]raclopride as the ligand focuses on striatal D2 receptor occupancy. However, it can be argued that D2 receptor occupancy in other areas is involved in clinical response and that, further, there is evidence that atypical agents may show differential D2 occupancy between striatal and extrastriatal regions (40–47). However, having raised each of these concerns, we are somewhat reassured by the fact that the present data are in line with existing clinical trial and plasma drug data (13, 14, 33) as well as recently published PET data from another center (48).
In this particular study, patients were first switched to oral risperidone and then to the depot risperidone formulation on the basis of the final oral dose. Establishing comparable doses between the two formulations is complicated by the more complex pharmacokinetics of long-acting risperidone and differences in bioavailability. However, the PET data now available lend support to the position that long-acting risperidone doses of 25–75 mg are in line with oral risperidone doses of 2–6 mg (48).
In summary, the PET findings reported here support available clinical evidence indicating that dosing long-acting risperidone at 25–50 mg every 2 weeks is sufficient in attaining clinical response with minimal risk of extrapyramidal symptoms. In addition, they provide further data regarding the relationship between plasma drug levels and D2 occupancy. Having this information may assist clinicians as they familiarize themselves with long-acting injectable risperidone and incorporate it into their clinical practice.
 |
Received Jan. 19, 2005; revision received April 18, 2005; accepted May 13, 2005. From the Schizophrenia Program, Centre for Addiction and Mental Health. Address correspondence and reprint requests to Dr. Remington, Schizophrenia Program, Centre for Addiction and Mental Health, 250 College St., Toronto, Ont. M5T 1R8, Canada; gary_[email protected] (e-mail).Supported by the Janssen Research Foundation.The authors thank the patients for their participation, Dr. Alan Wilson for supervising the radiochemical synthesis, and the PET Centre staff for their assistance throughout.
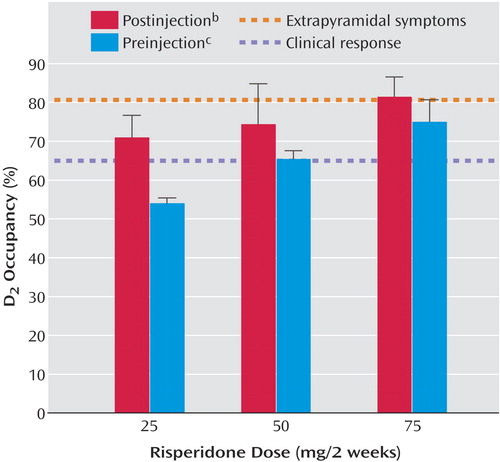
Figure 1. Dopamine D2 Receptor Occupancy Levels at Two Time Points Over the 2-Week Injection Interval for Nine Patients With Schizophrenia or Schizoaffective Disorder Receiving Long-Acting, Injectable Risperidone (25, 50, or 75 mg) a
aHashed lines represent D2 occupancy thresholds associated with clinical response and extrapyramidal symptoms from other reports (17, 18).
bWithin 3 days after the injection.
cWithin 5 days of the next injection.
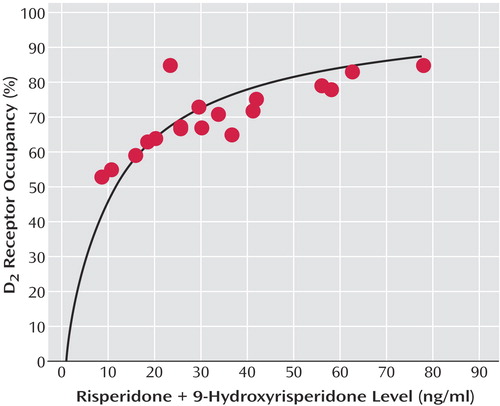
Figure 2. Relationship Between D2 Receptor Occupancy and Plasma Risperidone Plus 9-Hydroxyrisperidone Levels in Nine Patients With Schizophrenia or Schizoaffective Disorder Receiving Long-Acting, Injectable Risperidone (25, 50, or 75 mg) Every 2 Weeks a
aThe regression line was fit to the following rectangular hyperbolic equation: occupancy=α × [plasma level/(plasma level plus ED50)], where α is the maximal receptor occupancy, and ED50 is the plasma risperidone plus 9-hydroxyrisperidone level resulting in 50% maximal receptor occupancy. Maximal occupancy α was constrained to 100% to reflect the expected maximal occupancy at higher plasma levels. Each subject is represented by two points, representing the two scans carried out over the course of an injection interval.
1. Davis JM, Andriukaitis S: The natural course of schizophrenia and effective maintenance drug treatment. J Clin Psychopharmacol 1986; 6(suppl 1):2S-10SGoogle Scholar
2. Glazer WM, Kane JM: Depot neuroleptic therapy: an underutilized treatment option. J Clin Psychiatry 1992; 53:426–433Medline, Google Scholar
3. Citrome L, Levine J, Allingham B: Utilization of depot neuroleptic medication in psychiatric inpatients. Psychopharmacol Bull 1996; 32:321–326Medline, Google Scholar
4. Dencker SJ: Depot neuroleptics: a Scandinavian view. Acta Psychiatr Belg 1981; 81:115–120Medline, Google Scholar
5. Johnson DA: Observations on the use of long-acting depot neuroleptic injections in the maintenance therapy of schizophrenia. J Clin Psychiatry 1984; 45:13–21Medline, Google Scholar
6. Rothbard AB, Kuno E, Foley K: Trends in the rate and type of antipsychotic medications prescribed to persons with schizophrenia. Schizophr Bull 2003; 29:531–540Crossref, Medline, Google Scholar
7. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Schizophrenia, second edition. Am J Psychiatry 2004; 161(Feb suppl)Google Scholar
8. Emsley R, Oosthuizen P: Evidence-based pharmacotherapy of schizophrenia. Int J Neuropsychopharmacol 2004; 7:219–238Crossref, Medline, Google Scholar
9. Dolder CR, Lacro JP, Dunn LB, Jeste DV: Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002; 159:103–108; correction, 159:514Link, Google Scholar
10. Menzin J, Boulanger L, Friedman M, Mackell J, Lloyd JR: Treatment adherence associated with conventional and atypical antipsychotics in a large state Medicaid program. Psychiatr Serv 2003; 54:719–723Link, Google Scholar
11. Eerdekens M, Van Hove I, Remmerie B, Mannaert E: Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004; 70:91–100Crossref, Medline, Google Scholar
12. Ramstack M, Grandolfi GP, Mannaert E, D’Hoore P, Lasser RA: Long-acting risperidone: prolonged-release injectable delivery of risperidone using Medisorb microsphere technology. Schizophr Res 2003; 60(suppl 1):314Google Scholar
13. Fleischhacker WW, Eerdekens M, Karcher K, Remington G, Llorca PM, Chrzanowski W, Martin S, Gefvert O: Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003; 64:1250–1257Crossref, Medline, Google Scholar
14. Kane JM, Eerdekens M, Lindenmayer J-P, Keith SJ, Lesem M, Karcher K: Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160:1125–1132Link, Google Scholar
15. Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M: Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry 2004; 65:1084–1089Crossref, Medline, Google Scholar
16. Taylor DM, Young CL, Mace S, Patel MX: Early clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patients. J Clin Psychiatry 2004; 65:1076–1083Crossref, Medline, Google Scholar
17. Kapur SJ, Zipursky R, Jones C, Remington G, Houle S: Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000; 157:514–520Link, Google Scholar
18. Kapur S, Zipursky RB, Remington G: Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156:286–293Abstract, Google Scholar
19. Farde L, Wiesel FA, Nordstrom AL, Sedvall G: D1- and D2-dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology (Berl) 1989; 99(suppl):S28-S31Google Scholar
20. Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G: Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry 1992; 49:538–544Crossref, Medline, Google Scholar
21. Nyberg S, Farde L, Halldin C, Dahl M-L, Bertilsson L: D2 dopamine receptor occupancy during low-dose treatment with haloperidol decanoate. Am J Psychiatry 1995; 152:173–178Link, Google Scholar
22. Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, Remington G: A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry 2004; 161:818–825Link, Google Scholar
23. Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC: Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab 1997; 17:437–447Crossref, Medline, Google Scholar
24. Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M: Imaging human mesolimbic dopamine transmission with positron emission tomography, I: accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21:1034–1057Crossref, Medline, Google Scholar
25. Houle S, Kapur S, Hussey D, Jones C, Dasilva J, Wilson AA: Measurement of [11C]raclopride binding using a bolus plus infusion protocol, in Quantification of Brain Function Using PET. Edited by Myers R, Cunningham V, Bailey D, Jones T. London, Academic Press, 1996, pp 262-265Google Scholar
26. Fitzgerald PB, Kapur S, Remington G, Roy P, Zipursky RB: Predicting haloperidol occupancy of central dopamine D2 receptors from plasma levels. Psychopharmacology (Berl) 2000; 149:1–5Crossref, Medline, Google Scholar
27. Farde L, Hall H, Ehrin E, Sedvall G: Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231:258–261Crossref, Medline, Google Scholar
28. Kapur S, Seeman P: Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? a new hypothesis. Am J Psychiatry 2001; 158:360–369Link, Google Scholar
29. Remington G, Kapur S, Zipursky R: The relationship between risperidone plasma levels and dopamine D2 occupancy: a positron emission tomographic study (letter). J Clin Psychopharmacol 1998; 18:82–83Crossref, Medline, Google Scholar
30. Curry SH: The strategy and value of neuroleptic drug monitoring. J Clin Psychopharmacol 1985; 5:263–271Crossref, Medline, Google Scholar
31. McCreadie RG, Mackie M, Wiles DH, Jorgensen A, Hansen V, Menzies C: Within-individual variation in steady state plasma levels of different neuroleptics and prolactin. Br J Psychiatry 1984; 144:625–629Crossref, Medline, Google Scholar
32. McIntyre IM, Gershon S: Interpatient variations in antipsychotic therapy. J Clin Psychiatry 1985; 46:3–5Medline, Google Scholar
33. Hiemke C, Dragicevic A, Grunder G, Hatter S, Sachse J, Vernaleken I, Muller MJ: Therapeutic monitoring of new antipsychotic drugs. Ther Drug Monit 2004; 26:156–160Crossref, Medline, Google Scholar
34. Halbreich U, Kinon BJ, Gilmore JA, Kahn LS: Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 2003; 28(suppl 1):53-67Google Scholar
35. Nordstrom AL, Farde L: Plasma prolactin and central D2 receptor occupancy in antipsychotic drug-treated patients. J Clin Psychopharmacol 1998; 18:305–310Crossref, Medline, Google Scholar
36. Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S: Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 2002; 7:317–321Crossref, Medline, Google Scholar
37. Belmaker RH, Bersudsky Y: Mechanism of atypicality of antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:1067–1069Crossref, Google Scholar
38. Blin O: A comparative review of new antipsychotics. Can J Psychiatry 1999; 44:235–244Crossref, Medline, Google Scholar
39. Remington G: Understanding antipsychotic “atypicality”: a clinical and pharmacological moving target. J Psychiatry Neurosci 2003; 28:275–284Medline, Google Scholar
40. Bigliani V, Mulligan RS, Acton PD, Ohlsen RI, Pike VW, Ell PJ, Gacinovic S, Kerwin RW, Pilowsky LS: Striatal and temporal cortical D2/D3 receptor occupancy by olanzapine and sertindole in vivo: a [123I]epidepride single photon emission tomography (SPET) study. Psychopharmacology (Berl) 2000; 150:132–140Crossref, Medline, Google Scholar
41. Bigliani V, Mulligan RS, Acton PD, Visvikis D, Ell PJ, Stephenson C, Kerwin RW, Pilowsky LS: In vivo occupancy of striatal and temporal cortical D2/D3 dopamine receptors by typical antipsychotic drugs: [123I]epidepride single photon emission tomography (SPET) study. Br J Psychiatry 1999; 175:231–238Crossref, Medline, Google Scholar
42. Bressan RA, Erlandsson K, Jones HM, Mulligan RS, Ell PJ, Pilowsky LS: Optimizing limbic selective D2/D3 receptor occupancy by risperidone: a [123I]-epidepride SPET study. J Clin Psychopharmacol 2003; 23:5–14Crossref, Medline, Google Scholar
43. Bressan RA, Erlandsson K, Jones HM, Mulligan R, Flanagan RJ, Ell PJ, Pilowsky LS: Is regionally selective D2/D3 dopamine occupancy sufficient for atypical antipsychotic effect? an in vivo quantitative [123I]epidepride SPET study of amisulpride-treated patients. Am J Psychiatry 2003; 160:1413–1420Link, Google Scholar
44. Lidow MS, Williams GV, Goldman-Rakic PS: The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 1998; 19:136–140Crossref, Medline, Google Scholar
45. Pilowsky LS, Mulligan RS, Acton PD, Ell PJ, Costa DC, Kerwin RW: Limbic selectivity of clozapine (letter). Lancet 1997; 350:490–491Crossref, Medline, Google Scholar
46. Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc’h C, Maziere B, Paillere-Martinot ML: In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 2001; 21:207–214Crossref, Medline, Google Scholar
47. Xiberas X, Martinot JL, Mallet L, Artiges E, Loc’h C, Maziere B, Paillere-Martinot ML: Extrastriatal and striatal D2 dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry 2001; 179:503–508Crossref, Medline, Google Scholar
48. Gefvert O, Eriksson B, Persson P, Helldin L, Bjorner A, Mannaert E, Remmerie B, Eerdekens M, Nyberg S: Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta) in patients with schizophrenia. Int J Neuropsychopharmacol 2005; 8:27–36Crossref, Medline, Google Scholar



