Effect of a Triallelic Functional Polymorphism of the Serotonin-Transporter-Linked Promoter Region on Expression of Serotonin Transporter in the Human Brain
Abstract
OBJECTIVE: The authors examined effects of a triallelic functional polymorphism of the human serotonin-transporter-linked promoter region (5-HTTLPR) on in vivo expression of serotonin transporter in the brain in healthy volunteers and subjects with major depressive disorder. METHOD: Twenty-five medication-free subjects with DSM-IV major depressive disorder during a major depressive episode and 42 healthy volunteers were clinically evaluated and genotyped. Serotonin transporter binding potential (f1Bmax/Kd) was determined by using positron emission tomography with the radiotracer [11C]McN 5652 and metabolite-corrected arterial input functions. RESULTS: There was no difference in serotonin transporter binding potential by genotype in healthy volunteers or in subjects with major depressive disorder. Allelic frequencies did not differ between subjects with major depressive disorder and healthy volunteers. CONCLUSIONS: Associations of the 5-HTTLPR polymorphism to clinical phenotypes appear to be due to developmental effects of 5-HTTLPR on expression and not due to its direct effect on serotonin transporter binding in adulthood.
Alterations of serotonin (5-HT) neurotransmission and transporter binding have been implicated in major depressive disorder (1). A 44-base-pair insertion/deletion polymorphism in the serotonin-transporter-linked promoter region (5-HTTLPR) regulates serotonin transporter gene expression in cell lines wherein the short (S) allele was originally reported to be associated with lower expression levels, compared with the long (L) allele. Subsequently, functional variants were identified in the L allele and designated as LA and LG(2). Thus, 5-HTTLPR is a triallelic locus with alleles designated as LG, LA, and S (2). Because the LG and S alleles have comparable levels of serotonin transporter expression, both of which are lower than that of LA, we reclassified the alleles on the basis of lower and higher levels of expression. LG and S were reclassified as S′, and LA was reclassified as L′. Associations between 5-HTTLPR and psychiatric illnesses have been reported (3). However, serotonin transporter expression in humans in vivo and postmortem has not been clearly related to the genotype (4–9). In previous studies, a biallelic 5-HTTLPR genotype classification has been used. Use of this system could lead to a functional misclassification of cases with the LG allele, and some of the disagreement in the literature could be explained by the effects of this allele.
We used positron emission tomography (PET) with the radiotracer [11C](+)-6β-(4-methylthiophenyl)-1,2,3,5,6α,10β-hexahydropyrrolo[2,1-a]isoquinoline ([11C]McN 5652) to examine the relationship of the triallelic polymorphism to serotonin transporter binding in the amygdala, hippocampus, thalamus, dorsal putamen, anterior cingulate, and midbrain in healthy volunteers and in subjects with major depressive disorder during a major depressive episode. A companion article describes differences in serotonin transporter binding potential between subjects with major depressive disorder and healthy comparison subjects (see the next article in this issue of the Journal).
Method
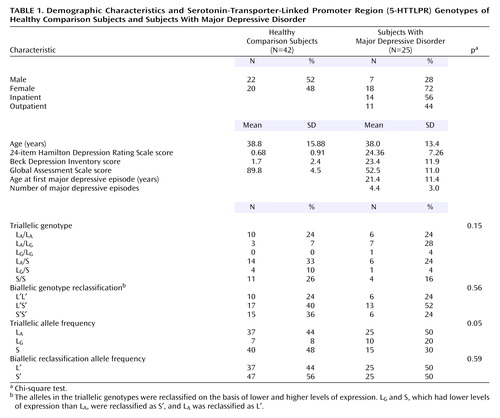
Twenty-five subjects with major depressive disorder with a current major depressive episode and 42 healthy comparison subjects (Table 1) were evaluated by clinical history, Structured Clinical Interview for DSM-IV (10), physical examination, and laboratory analyses. Detailed inclusion criteria are reported in the companion article. The Institutional Review Boards of the New York State Psychiatric Institute and Columbia University approved the protocol. All subjects gave written informed consent after explanation of the study.
Compound preparation (11), PET and magnetic resonance imaging data acquisition, region-of-interest determination, image analysis, and arterial input indices were as previously described (12). After a 10-minute transmission scan, [11C]McN 5652 was injected intravenously, and emission data were acquired for 130 minutes. The derivation of [11C]McN 5652 regional distribution volumes (VT) was performed by using likelihood estimation in a graphical approach (13). VT is the sum of specific (V3) and nondisplaceable (free plus nonspecific binding) (V2) distribution volumes. Binding potential (BP′) was calculated as follows: VT – V2, equivalent to f1Bmax/Kd, where f1 is the free concentration of the radiotracer in plasma, Bmax is the maximum number of binding sites, and Kd is the dissociation constant. The specific-to-nonspecific equilibrium partition coefficient (V3′′) was calculated as follows: BP′/V2.
Data were analyzed by using linear mixed-effects models with six regions and all subjects or with subjects segregated into two diagnostic groups (healthy comparison group and major depressive disorder patients) as fixed effects and subject as the random effect. To stabilize variance and satisfy modeling assumptions, natural log-transformed data were used in the analysis after a quantity was added to all measures to ensure positive values.
Subjects were genotyped with the biallelic classification (9) and with the triallelic classification of the same polymorphism in which the alleles act co-dominantly (2). We report the results of the triallelic classification, which were comparable with results obtained with the biallelic classification (data not shown). The triallelic genotypes were reclassified into a biallelic model by their level of expression as follows: LG/S, LG/LG, and S/S were reclassified as S′S′, LA/S and LA/LG were reclassified as L′S′, and LA/LA was reclassified as L′L′.
Results
Genotype distribution was in Hardy-Weinberg equilibrium in the healthy volunteers (χ2=0.38, df=1, p=0.54) and in the depressed subjects (χ2=1.75, df=1, p=0.19). Age (subjects with the L′L′ genotype: mean=37.05 years, SD=13.92; subjects with the L′S′ genotype: mean=38.96, SD=15.96; subjects with the S′S′ genotype: mean=40.61, SD=15.39) (F=0.27, df=2, 64, p=0.76) and sex (χ2=0.87, df=2, p=0.65) did not differ between genotype groups.
Genotype was not associated with diagnosis (χ2=3.71, df=2, p=0.16) (Table 1). There was no effect of genotype on BP′ and no effect of the interaction of genotype and brain region on BP′ in the healthy comparison subjects (F=0.14, df=2, 39, p=0.87), the subjects with major depressive disorder (F=0.47, df=2, 22, p=0.63), or the two groups combined (F=0.27, df=2, 63, p=0.77). No genotype effects were found in an analysis of the brain regions separately (Figure 1). No difference was found in the cerebellar VT between genotypes (F=0.89, df=2, 65, p=0.42). In analyses in which diagnostic group and genotype were independent variables, there was no main effect of genotype on BP′ (F=0.19, df=2, 58, p=0.83) and no effect of the genotype-by-group interaction on BP′ (F=0.37, df=4, 58, p=0.83). Adding the variables of sex and age to the model did not alter the results.
Discussion
We found no effect of 5-HTTLPR genotype on serotonin transporter binding potential in medication-free patients with major depressive disorder or in healthy volunteers. Previous in vivo imaging studies do not agree on the effect of 5-HTTLPR genotype on expression of serotonin transporter (4–8). Some of the previous studies demonstrated more (8) or less (4) binding with the S allele, and others found no differences in binding potential between genotypes (5–7). The newer triallelic genotype classification (2) was not used in these previous studies, which may have resulted in the classification of some L alleles as having higher levels of expression. In previous studies, the outcome measure was V3′′ (BP′/V2). Our results with this outcome measure were identical to those in which BP′ was calculated, which suggests that the lack of differences between the genotypes is not due to alterations in the free fraction of the tracer in the plasma or cerebrospinal fluid.
5-HTTLPR variants have been associated with clinical illness and with the function of the amygdala (14). If these findings are not due to altered serotonin transporter binding, they may reflect changes in maximum velocity in the enzymatic reaction (Vmax), effects of other polymorphisms (15), or developmental effects on neurogenesis or methylation patterns, perhaps in interaction with early-life stress (16). In our study, the error associated with determination of BP′ in the midbrain was greater than in other regions of interest (Figure 1), which increases the risk of a type II error. At least 10 allelic variants have been identified for 5-HTTLPR (17), and the range of variants in this important susceptibility gene should be examined more fully in future studies. Finally, [11C]N,N-dimethyl-2-(2-amino-4-cyanophenylthio)benzylamine ([11C]DASB) has a higher specific-to-nonspecific binding ratio and measurable concentration of free radioligand in plasma, compared to [11C]McN 5652, and future studies could use [11C]DASB to determine binding potential in some cortical regions (11).
 |
Received June 2, 2004; revisions received Jan. 11 and March 1, 2005; accepted April 13, 2005. From the Departments of Psychiatry and Radiology, Columbia University College of Physicians and Surgeons, New York; the Departments of Neuroscience and Analytic Psychopharmacology, New York State Psychiatric Institute; the Department of Biostatistics, Columbia University School of Public Health, New York; and the Laboratory of Neurogenetics, National Institute on Alcohol and Alcohol Abuse, Washington, D.C. Address correspondence and reprint requests to Dr. Parsey, Department of Neuroscience, New York State Psychiatric Institute, 1051 Riverside Dr., Box 42, New York, NY 10032; [email protected] (e-mail). This article is the first of two on serotonin transporters in the human brain in this issue of the Journal. The second article immediately follows this one. Supported by NIMH grants P30 MH-46745, MH-40695, MH-40210, and MH-62185, the National Alliance for Research on Schizophrenia and Depression, and the American Foundation for Suicide Prevention. The authors thank the staff of the Brain Imaging Division, Kreitchman PET Center. Drs. Anissa Abi-Dargham and Diana Martinez assisted by placing some arterial lines.
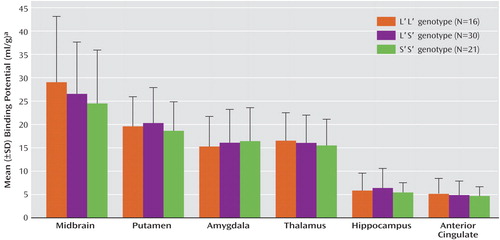
Figure 1. [11C]McN 5652 Binding Potential in Multiple Brain Regions in Healthy Comparison Subjects and Medication-Free Subjects With Major Depressive Disorder, by Serotonin-Transporter-Linked Promoter Region (5-HTTLPR) Genotypea
aNo significant difference in binding potential between brain regions.
1. Mann JJ: Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 1999; 21(2 suppl):99S-105SGoogle Scholar
2. Hu X, Zhu G, Lipsky R, Goldman D: HTTLPR allele expression is codominant, correlating with gene effects on fMRI and SPECT imaging intermediate phenotypes, and behavior (abstract). Biol Psychiatry 2004; 55(suppl 1):191SGoogle Scholar
3. Arango V, Huang YY, Underwood MD, Mann JJ: Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res 2003; 37:375–386Crossref, Medline, Google Scholar
4. Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR: A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 2000; 47:643–649Crossref, Medline, Google Scholar
5. Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J: Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry 2000; 157:1700–1703Link, Google Scholar
6. Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, Tauscher J, Fuchs K, Sieghart W, Hornik K, Aschauer HN, Brucke T, Kasper S: No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry 2001; 50:8–12Crossref, Medline, Google Scholar
7. Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S: No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse 2003; 48:184–188Crossref, Medline, Google Scholar
8. Van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J: Central serotonin transporter availability measured with [123I]β-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry 2004; 161:525–531Link, Google Scholar
9. Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V: A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000; 57:729–738Crossref, Medline, Google Scholar
10. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
11. Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M: Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med 2004; 45:682–694Medline, Google Scholar
12. Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M: In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 2000; 41:1465–1477Medline, Google Scholar
13. Parsey RV, Ogden RT, Mann JJ: Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. J Cereb Blood Flow Metab 2003; 23:1471–1478Crossref, Medline, Google Scholar
14. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403Crossref, Medline, Google Scholar
15. Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D: Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry 2004; 55:1090–1094Crossref, Medline, Google Scholar
16. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389Crossref, Medline, Google Scholar
17. Nakamura M, Ueno S, Sano A, Tanabe H: The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry 2000; 5:32–38Crossref, Medline, Google Scholar



