Postdischarge Cannabis Use and Its Relationship to Cocaine, Alcohol, and Heroin Use: A Prospective Study
Abstract
OBJECTIVE: Research on the effects of cannabis on the brain and behavior has been surprisingly scarce. In humans, laboratory studies document toxicity and psychoactive effects of cannabinoids. However, among substance abuse patients, only a few studies have prospectively examined the relationship of cannabis use to remission or relapse of use of other substances. Because cannabis is a widely used substance, the authors examined whether cannabis use during follow-up after discharge from inpatient treatment affected cocaine, alcohol, and/or heroin use. METHOD: Two hundred fifty patients 18 years old or older from an inpatient psychiatric/substance abuse setting participated in a Psychiatric Research Interview for Substance and Mental Disorders. All patients were diagnosed according to DSM-IV as having current alcohol, cocaine, and/or heroin dependence. Sustained remission was defined as at least 26 weeks without use following hospital discharge. Data were analyzed with Cox proportional hazards models. RESULTS: About one-third of the patients (N=73) used cannabis after hospital discharge. Postdischarge cannabis use substantially and significantly increased the hazard of first use of any substance and strongly reduced the likelihood of stable remission from use of any substance. Examination of specific substances indicated that cannabis use affected first use of alcohol, stable remission, and subsequent relapse of alcohol use as well as first use of cocaine and stable remission but was unrelated to heroin outcomes. CONCLUSIONS: Potential negative clinical implications of cannabis use should be considered when treating dependence on other substances and planning aftercare. Clinical and laboratory research is needed to provide understanding of the mechanisms of cannabinoids in relapse to alcohol and cocaine use.
Although cannabis is the most commonly used illicit substance in the United States (1, 2), it remains among the least studied clinically (3, 4). Not until the past decade has growing empirical evidence demonstrated the wide range of detrimental effects of cannabis use. In humans, the pharmacologic effects of cannabis include symptoms such as tachycardia, antinociception, and impairment in cognitive and performance tasks (5). Cannabis use can cause impairment in interpersonal relations, motivation, and employment, and physical and psychological withdrawal symptoms have been reported, including appetite/weight loss, irritability, anxiety, and a variety of other less frequently reported symptoms (6–9).
Known physiological and psychological mechanisms involved in addiction are consistent with the possibility that continued cannabis use may worsen the course of dependence on other drugs. All substances of abuse have well-recognized reinforcing properties. Similar to other potent ingredients such as cocaine metabolites, ethanol, or opioids, the cannabinoids—specifically delta-9-tetrahydrocannabinol (Δ9-THC)—have reinforcing-rewarding properties. These reinforcing properties also play a role in relapse. In cannabis use, the cannabinoid reward effect is produced by increasing the activity of the dopaminergic neurons in the mesolimbic-dopamine system, similar to the effect of other addictive substances (10).
Because of the high comorbidity among drug use disorders, many patients treated for addiction to other substances are likely to use cannabis. Laboratory and animal studies suggest that the rewarding properties of cannabis appear similar in some ways to those of other drugs, raising important clinical issues. Cannabinoids, especially their psychoactive ingredient, Δ9-THC, may continue to trigger pleasant cues previously associated with other substances such as cocaine, alcohol, or heroin. If so, cannabinoids may increase the risk of relapse to other substances. In addition, the continued drug-seeking behavior necessary to obtain and use cannabis may continue exposure to generalized drug-related cues that increase the risk for relapse to the previously dependent substance. These possibilities have implications for treatment decisions concerning continued use of cannabis among patients being treated for abuse or dependence on other substances.
Most studies addressing this issue focused on opiate-dependent patients and had mixed results. Among opiate-dependent patients in buprenorphine detoxification (11), naltrexone maintenance (12), or methadone maintenance (13–15), concurrent cannabis use during treatment did not appear to worsen patients’ outcome. Two other studies with similar subjects reported that cannabis use increased the likelihood of negative outcome for drug use (16) and relapse at 6-month follow-up (17). There has been surprisingly little study of the impact of marijuana use on outcomes among subjects with either cocaine or alcohol addiction.
To gain a broader understanding of the effects of cannabis use on the posttreatment outcome for other substances, prospective follow-up studies are needed with high response rates to preclude bias caused by dropout among the patients with poor outcome. To our knowledge, in this article we present the first such study to address the effects of cannabis use on postdischarge outcome of inpatients treated for alcohol, cocaine, and/or heroin dependence. Inpatients were followed for up to 18 months after their hospital discharge from inpatient substance abuse treatment. Analysis addressed three outcomes: 1) time from hospital discharge to first use of the drug on which the patient had previously been dependent, 2) time from hospital discharge to the start of sustained remission (26 or more weeks of continuous abstinence), and 3) time to relapse after the start of sustained remission. We predicted that postdischarge cannabis use would decrease the time to first use of the other substances, decrease the probability of sustained remission, and increase the likelihood of subsequent relapse among those who remitted. Because we previously showed that primary and substance-induced major depression influence the outcome of substance dependence (18), we controlled for this and other factors in our analyses.
Method
Participants
As described elsewhere (18, 19), participants were inpatients in a New York dual-diagnosis facility who were not severely psychotic or medically ill. All patients were treated primarily for a substance use disorder. Of 379 patients invited to participate, 92% (N=349) participated in a baseline evaluation. Of these, 279 patients were of interest here because they had a current baseline DSM-IV diagnosis of alcohol, cocaine, and/or heroin dependence and never experienced mania or nonaffective psychosis. Of these, 90% (N=250) participated in at least one follow-up interview. These are the patients described here. Their mean age was 36.9 years (SD=9.2), 66% (N=165) were men, 57% (N=142) were white, and 15% (N=38) had not completed high school. Diagnostically, 15% (N=38) had lifetime antisocial personality, 22% (N=54) had current primary major depression, and another 22% (N=56) had current substance-induced major depression. At baseline, 75% (N=188) met DSM-IV criteria for current alcohol dependence; of these, 79 (42%) were also currently dependent on cocaine, six (3%) were also dependent on heroin, and 17 (9%) were dependent on both cocaine and heroin. For cocaine, 58% (N=144) met DSM-IV criteria for current dependence; of these, 79 (55%) were also currently dependent on alcohol, 14 (10%) on heroin, and 16 (11%) on both alcohol and heroin. For heroin, 20% (N=49) met criteria for current DSM-IV heroin dependence; of these, six (12%) were also currently dependent on alcohol, 14 (29%) on cocaine, and 16 (33%) on both alcohol and cocaine. Fifteen percent (N=38) of the 250 patients met DSM-IV criteria for current cannabis dependence.
Procedures
Following New York State Psychiatric Institute Institutional Review Board requirements, clinical staff identified eligible, sequentially admitted patients (who had completed acute withdrawal, if applicable) and obtained their agreement to meet with research staff, at which time the study was explained. Patients providing written informed consent participated in a baseline Psychiatric Research Interview for Substance and Mental Disorders (20–23). Follow-up longitudinal versions of this interview (21) were conducted at 6, 12, and 18 months. Participants were paid U.S. $35. Those not interviewed when scheduled were interviewed later whenever possible, at which point the interview covered the time since the previous interview.
Median length of follow-up was 91 weeks; 213 patients (85%) were followed for at least 1 year, and 167 (67%) completed all three follow-up interviews. Bias from loss to follow-up was unlikely because of the high follow-up rate and lack of demographic and clinical differences between subjects who were or were not followed (18). There was no difference in lifetime marijuana use (χ2=0.0003, df=1, p=0.98) or marijuana use in the 4 weeks preceding baseline (χ2=0.06, df=1, p=0.81) between those who were or were not followed.
Measures
At follow-up, subjects participated in a version of the Psychiatric Research Interview for Substance and Mental Disorders covering the period since the previous interview (21). The longitudinal Psychiatric Research Interview for Substance and Mental Disorders includes elements of the Longitudinal Interval Follow-Up Evaluation (24) as well as substance abuse timeline follow-back methods (25, 26). The timeline grids allow ratings of the course of separate conditions (including substance use, dependence, and depression) by week after study entry.
Interviewers obtained a history since the previous interview and then assessed the timing of alcohol and drug use, dependence and abuse symptoms, and psychiatric syndromes, referring to the timing of life events as needed. When the relative timing of substance and psychiatric disorders was unclear, semistructured probes aided systematic exploration. The timeframe for the timeline grids is flexible, allowing all time since the last interview to be covered rather than a fixed 6-month period. Interviewers had clinical experience and received extensive, structured training and supervision (19).
After data entry and cleaning, computer programs produced diagnoses as well as the follow-up onset and offset variables. We defined abstinence and substance-induced major depression during the follow-up (control variables in the analysis) as we did in an earlier study (18).
Outcome Measures Used in the Analysis
Three main outcomes were investigated. The first was any use of a substance after hospital discharge. The second was sustained remission from substance use, defined as 26 or more weeks during follow-up with no use of the substance. The third was relapse, defined as 1 or more weeks of substance use after the 26th week of remission from use. These three definitions were applied to a combined outcome including alcohol, cocaine, and/or heroin, a definition that guards against substance substitution and provides clinically meaningful periods of stability (27). The definitions were also applied to separate, specific outcomes of alcohol, cocaine, or heroin use. The substance-specific separate outcomes were examined only among patients who had been dependent on these substances at baseline. These outcomes were described in dichotomous form (occurrence versus nonoccurrence). They were analyzed by examining the time (weeks) from hospital discharge to their first occurrence.
Statistical Analysis
The bivariate association of postdischarge cannabis use with the outcomes described was first addressed with chi-square tests or Fisher exact tests. However, such tests do not address the time order of cannabis use relative to the outcomes or censoring due to different periods of follow-up. Therefore, survival analysis was used to investigate the outcomes more fully. For outcome 1, postdischarge use, we examined time (weeks) from hospital discharge to first use of the substance(s). For outcome 2, sustained remission, we examined time (weeks) from discharge to the first week of the 26 or more weeks remission required for our definition of sustained remission. For outcome 3, relapse, we examined the time (weeks) from establishment of stable remission (i.e., the 26th week of remission) to subsequent relapse into use. Data were censored if the subjects did not experience the event by the end of the follow-up, including those lost to death or follow-up.
The cumulative probabilities of remission and survival curves of relapse were obtained with Kaplan-Meier estimates. Cox proportional hazards models controlling for other variables were used to examine the effect of the time-varying predictor of cannabis use. Such models allow for variable length of follow-up time between subjects, making use of all available data for as long as subjects are followed. In these models, a hazard ratio of 1.00 indicates no effect, a hazard ratio greater than 1.00 indicates a positive relationship, and a hazard ratio less than 1.00 indicates an inverse relationship. The control variables included time-invariant and time-varying covariates. Time-invariant covariates included age; sex; race; education; baseline DSM-IV diagnoses of alcohol, cocaine, and heroin dependence; antisocial personality disorder with symptoms in the year preceding the interview; and a variable representing major depressive disorder beginning before the onset of substance abuse or dependence. Because we previously showed that abstinence and substance-induced major depressive disorder affect the outcome of substance dependence (18), we also included time-varying controls for each of these affective disorders. The resultant hazard ratios indicate the risk of occurrence of the outcome event.
Results
Description of Cannabis Use Before and After Hospital Discharge
Of the 250 patients, 92% (N=231) reported lifetime use of cannabis. Among the patients with current dependence diagnoses at baseline, these patients represented 90% (N=169) of the 188 patients with alcohol dependence, 99% (N=142) of the 144 patients with cocaine dependence, and 100% of the 49 patients with heroin dependence. Cannabis use in the 12 months before the baseline interview (current use) was reported by 41% (N=102) of the 250 patients. Among the patients with current dependence diagnoses at baseline, these patients represented 38% (N=72) of the patients with current alcohol dependence, 51% (N=73) of the patients with current cocaine dependence, and 59% (N=29) of the patients with current heroin dependence. Shortly before hospitalization (within 12 weeks), 38% (N=95) of the 250 patients used cannabis; 58 of these patients were using cannabis 1–2 times/week, 16 were using cannabis 3–4 times/week, and 21 were using cannabis 5–7 times/week.
After hospital discharge, 29% (N=73) of the patients used cannabis. Among these 73 patients, the maximum postdischarge frequency of cannabis use during the follow-up was 1–2 times/week for 48 of the patients, 3–4 times/week for six of these patients, and 5–7 times/week for 19 of the patients.
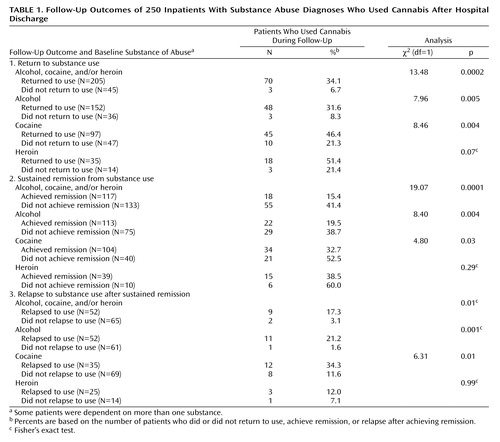
Postdischarge Cannabis Use and Outcome 1: Return to Use of Alcohol, Cocaine, or Heroin
The percent of cannabis users after hospital discharge and the substances on which patients were dependent at baseline among those who returned or did not return to substance use (outcome 1) are shown in Table 1. For example, among the 250 patients who were dependent on alcohol, cocaine, and/or heroin at baseline, 205 returned to use of alcohol, cocaine, or heroin during the follow-up. Of these 205 patients, 70 (34.2%) used cannabis after hospital discharge. Among the 45 patients who did not return to alcohol, cocaine, or heroin use during the follow-up, three (6.7%) used cannabis after hospital discharge. Thus, the percent of cannabis users after discharge was 5.10 times higher (34.2% divided by 6.7%) among patients who returned to using alcohol, cocaine, and/or heroin than among those who did not.
Among patients who were alcohol dependent at baseline, cannabis use was 3.81 times higher among patients who returned to alcohol use during the follow-up than among those who did not. Among patients who were cocaine dependent at baseline, cannabis use was 2.18 times higher among patients who returned to cocaine use during the follow-up than among those who did not. Although cannabis use was more frequent among heroin-dependent patients who returned to heroin use than among those who did not, the difference was not significant.
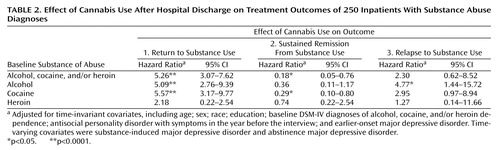
Results of the Cox proportional hazards models incorporate the important elements of time order and adjustment for covariates. Cannabis use after discharge substantially and significantly increased the hazard of subsequent use of substances on which a patient had been dependent (hazard ratio=4.83). Postdischarge cannabis use substantially and significantly increased the hazard of subsequent alcohol use among patients who were alcohol dependent at baseline and the hazard of subsequent use of cocaine among patients who were cocaine dependent at baseline. The magnitude of effect was smaller and not significant for return to heroin use among patients dependent on heroin at baseline.
Postdischarge Cannabis Use and Outcome 2: Sustained Remission From Alcohol, Cocaine, and/or Heroin Use
Among all patients in the study, 53.2% (N=133) achieved a sustained remission (≥26 weeks) from alcohol, cocaine, or heroin use, and 46.8% (N=117) did not. Among the patients who did not achieve sustained remission from alcohol, cocaine, or heroin use, 41.4% (N=55) used cannabis after hospital discharge, compared with 15.4% of the patients who did achieve sustained remission (Table 1). Thus, the percent of patients who used cannabis during the follow-up was 2.69 times higher among patients without a sustained remission from all three substances compared with patients with such a sustained remission, a highly significant difference (Table 1).
Among patients with alcohol dependence at baseline who did not achieve a sustained remission from alcohol use, 38.7% (N=29) used cannabis after discharge, compared with 19.5% (N=22) of those who did achieve sustained remission from alcohol. Thus, those who did not achieve sustained remission from alcohol were 1.98 times as likely to use cannabis after hospital discharge than those with a sustained remission from alcohol. Similarly, patients with cocaine dependence at baseline who did not achieve a sustained remission from cocaine use were 1.61 times more likely to use cannabis than patients with a sustained remission from cocaine use. However, cannabis use did not differ significantly between patients with or without a sustained remission from heroin use.
The results of the Cox proportional hazards models for time to achieving a sustained remission in substance use are shown in Table 2. Cannabis use after discharge substantially and significantly reduced the “hazard” of achieving a sustained remission from use of any type of substance on which a patient had been dependent at baseline (hazard ratio=0.18, indicating an inverse relationship between cannabis use and sustained remission). Postdischarge cannabis use also substantially and significantly decreased the possibility of achieving a sustained remission from alcohol use among those who had been alcohol dependent at baseline. Further, postdischarge cannabis use also substantially and significantly reduced the possibility of achieving sustained remission from cocaine use among those who had been cocaine dependent at baseline. Again, the magnitude of effect was weaker and not significant for sustained remission from heroin use among those who had been heroin dependent at baseline.
Postdischarge Cannabis Use and Outcome 3: Relapse Into Use of Alcohol, Cocaine, or Heroin After Sustained Remission
Among the 117 patients who achieved sustained remission from alcohol, cocaine, and/or heroin use, 44.4% (N=52) subsequently relapsed. The proportion of patients who used cannabis during the follow-up period was 5.58 times higher among those who relapsed to alcohol, cocaine, or heroin use after a sustained remission than among patients who did not relapse, a significant difference (Table 1). Among patients who were alcohol dependent at baseline, cannabis use was 13.3 times higher among those who relapsed to alcohol use after sustained remission than among those who did not relapse, a significant difference. Among patients who were cocaine dependent at baseline, cannabis use was 2.96 times higher among those who relapsed to cocaine use after sustained remission than among those who did not relapse, a significant difference. However, among patients who were heroin dependent at baseline, cannabis use did not differ significantly between patients who relapsed after sustained remission and those who did not.
The results of the Cox proportional hazards models for time to relapse after achieving a sustained remission are shown in Table 2. Cannabis use after discharge substantially and significantly increased the hazard of relapse to alcohol use after achieving a sustained remission from its use, and was at the borderline level of significance for relapse to cocaine use. However, postdischarge cannabis use was unrelated to heroin relapse.
Effects of Cannabis Use History
Bivariate chi-square tests of the relationship between a diagnosis of lifetime cannabis use or cannabis dependence at baseline (the 12 weeks before hospitalization) and the outcomes described above showed no significant effects of a history of cannabis dependence on any of the alcohol, cocaine, or heroin outcomes. Additionally, a variable was created to represent maximum days/week of cannabis use during the 12-week baseline period, categorized as 5–7 days/week, 3–4 days/week, 1–2 days a week, and 0 days a week. Kruskal-Wallis significance tests conducted with all 250 patients and with subsets of lifetime cannabis users or those who used cannabis during the follow-up period indicated that patients with more frequent cannabis use at baseline were not more likely than less frequent users to experience worse outcomes. These results suggest that proximal use of cannabis after discharge exerted the effect on outcome for the other substances, rather than a distal effect conferred by a prehospitalization history of dependence or more intensive use.
Discussion
Results from this prospective longitudinal study of cannabis use after discharge from inpatient treatment for cocaine, alcohol, and/or heroin dependence indicate that continued cannabis use, regardless of cannabis use history, has a significant negative impact on remission and relapse for other substances. Among cocaine-dependent patients, cannabis use significantly increased the hazard of return to cocaine use, decreased the likelihood of sustained remission from cocaine, and increased the hazard of relapse to cocaine use after sustained remission. Alcohol-dependent patients who used cannabis after discharge were also significantly more at risk to return to use and to relapse to use after sustained remission; the likelihood of achieving sustained remission followed the same negative trend. However, cannabis use after inpatient treatment did not significantly affect remission and relapse in heroin-dependent patients. The number of heroin-dependent patients was smaller than the number of those dependent on alcohol or cocaine, but the hazard ratios suggest that a significant relationship would not have been found even if the group had been larger.
Our main finding that continued marijuana use increases the risk of relapse to cocaine and alcohol use among patients previously dependent on these drugs is consistent with laboratory work in animals showing the possible function of the cannabinoid mechanism in its activation of the brain reward system. Using the reinstatement paradigm with rats, De Vries et al. (28) demonstrated that the synthetic cannabinoid agonist HU210 induced relapse to cocaine in rats after periods of abstinence and the cannabinoid antagonist SR141716A prevented relapse induced by cocaine but not relapse induced by stress. Similar findings concerning the differential effects (e.g., agonist versus antagonist) of the CB1 cannabinoid mechanism have been reported in ethanol preference and self-administration (29–31). Concerning heroin, the literature reveals more mixed results. Effects of synthetic cannabinoid agonists and antagonists on heroin relapse in rats were found by De Vries et al. (28) and Fattore et al. (32), but Fattore et al. did not find a similar effect for Δ9-THC itself. Although further studies are needed to elaborate these differences, the evidence from animal research suggests an involvement of the CB1 cannabinoid mechanism in reinstating the use of some substances after drug-free periods.
The finding that postdischarge cannabis use did not significantly increase the likelihood of relapse to heroin is consistent with previous clinical reports (15, 33). Researchers reported that among patients maintained on methadone (15) or treated with other medications (33), cannabis use did not negatively affect the outcome of heroin dependence. Surprisingly, only three prospective studies have been published on the effects of marijuana use on the course of treated cocaine dependence, and one was a case study of two patients (34). The second, a study of 85 patients published a number of years ago (33), suggested that cannabis use during treatment did not affect the outcome of cocaine dependence. In the third study (15), a diagnosis of cocaine dependence was not required for participation, potentially making an effect of cannabis use on cocaine outcome difficult to detect. Further, we were unable to find a single report that covered the effects of marijuana use on the outcome of alcohol dependence. This is a limited body of information on what is likely to be a common patient behavior that could have harmful effects.
One reason marijuana use may have adversely affected recovery from cocaine or alcohol dependence but not heroin dependence in this study is that the situation of patients who use cocaine or alcohol is usually different from that of patients who use opiates. In studies of patients maintained on methadone (15), the experimental treatment was followed by continued treatment with an opiate agonist. In contrast, treatment for cocaine or alcohol dependence is usually more limited because the option of a cocaine or alcohol agonist is currently not available. Therefore, it is important to address the effects of marijuana use on the outcome of cocaine or alcohol treatment separately. The gap in the literature concerning the relationship of cannabis use to the outcome of alcohol dependence was surprising. We were unable to find a single study that examined the effects of cannabis use on posttreatment outcome for alcohol dependence, despite the fact that the majority of patients now in treatment for alcohol dependence also abuse other drugs. Clearly, additional studies of this issue are warranted.
A clinical perception that cannabis use is harmless among patients in treatment for dependence on other drugs may be based on lack of knowledge about recent developments in the potency of marijuana in the United States. Data indicate a substantial increase in the potency (concentration) of Δ9-THC in marijuana since the early 1990s (35, 36). Marijuana abuse and dependence among users have increased over this time period (37). These developments suggest that cannabis use among patients in treatment for other problems, including dependence on other substances, may be more clinically relevant than previously thought. These results, considered in the current setting of high rates for comorbid cannabis and other substance use, underline the importance of patient’s cannabis use being taken seriously by mental health providers who are planning aftercare and administering treatment for dependence on cocaine, alcohol, and/or heroin.
Limitations of the study include the fact that we did not use urine tests, but empirical evidence suggests very high agreement between drug use self-reports and confirmed urine specimens (38–40). Participants in the follow-up had no penalties if they accurately reported their drug use, and the overwhelming majority did report drug use to us during the follow-up portion of the study (18). Therefore, bias due to underreporting appears minimal, although future studies should use biological specimens to verify self-report. In addition, longer follow-up periods would be informative. Almost all patients were followed for a year, ample time to detect short-term relapses. Concerning longer-range results, effects of cannabis on relapse to alcohol or cocaine after sustained remissions may have been underestimated, although the effects detected were quite strong. The lack of effect for cannabis on either short-term or longer-term heroin outcomes suggests that further follow-up would not have changed this, but additional studies would clarify the point. Failure to obtain treatment could not have confounded the results, since 97% of the patients received substance use treatment during the follow-up.
In conclusion, the results suggest that cannabis use during periods of sustained remission from dependence on another substance should be addressed as a possible risk or warning signal of impending relapse to use of substances on which patients were formerly dependent. A harm reduction model may regard abstinence from the primary substance (e.g., cocaine, alcohol) as improvement and cannabis use as acceptable. The data from this study suggest caution with this approach and that potential negative clinical implications of cannabis use should be considered when treating dependence on other substances and planning aftercare. Given the small body of research on this issue, however, further studies are clearly warranted. Additional longitudinal clinical research studies combined with laboratory studies involving human subjects are needed to further explore the mechanisms of cannabinoids and relapse and to assess the effects of continued cannabis use on relapse to use of primary substances.
 |
 |
Presented in part at the annual conference of the College for Problems on Drug Dependence, Bal Harbor, Fla., June 2003. Received April 9, 2004; revision received Aug. 31, 2004; accepted Oct. 26, 2004. From the Division on Substance Abuse, Columbia University. Address correspondence and reprint requests to Dr. Aharonovich, Division on Substance Abuse, 1051 Riverside Drive Unit 120, New York, NY 10032; [email protected] (e-mail). Supported by National Institute on Drug Abuse grant K23 DA-16743 (Dr. Aharonovich), National Institute on Alcohol and Alcohol Abuse grant K05 AA-00161 (Dr. Hasin), and the New York State Psychiatric Institute. The authors thank Valerie Richmond, M.A., for technical assistance.
1. National Institute on Drug Abuse: NIDA infofacts: marijuana. http://www.drugabuse.gov/Infofax/marijuana.htmlGoogle Scholar
2. Dennis M, Babor TF, Roebuck MC, Donaldson J: Changing the focus: the case for recognizing and treating cannabis use disorders. J Clin Pharmacol 2002; 42:28S-33SCrossref, Medline, Google Scholar
3. McRae AL, Budney AJ, Brady KT: Treatment of marijuana dependence: a review of the literature. J Subst Abuse Treat 2003; 24:369–376Crossref, Medline, Google Scholar
4. Westley-Clark H, MacNeill-Horton A, Dennis M, Babor TF: Moving from research to practice just in time: the treatment of cannabis use disorders comes of age. Addiction 2002; 97(suppl 1):1–3Google Scholar
5. Harrison GP Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D: Cognitive measures in long-term cannabis users. J Clin Pharmacol 2002; 42:41S-47SCrossref, Medline, Google Scholar
6. Wiesbeck GA, Schuckit MA, Kalmijn JA, Tipp JE, Bucholz KK, Smith TL: An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction 1996; 91:1469–1478Crossref, Medline, Google Scholar
7. Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK: Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend 1998; 50:27–37Crossref, Medline, Google Scholar
8. Budney AJ, Moore BA, Vandrey RG, Hughes JR: The time course and significance of cannabis withdrawal. J Abnorm Psychol 2003; 112:393–402Crossref, Medline, Google Scholar
9. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW: Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999; 141:395–404Crossref, Medline, Google Scholar
10. Wickelgren I: Marijuana: harder than thought? Science 1997; 276:1967–1968Crossref, Medline, Google Scholar
11. Budney AJ, Bickel WK, Amass L: Marijuana use and treatment outcome among opioid-dependent patients. Addiction 1998; 93:493–503Crossref, Medline, Google Scholar
12. Church SH, Rothenberg JL, Sullivan MA, Bornstein G, Nunes EV: Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. Am J Drug Alcohol Abuse 2001; 27:441–452Crossref, Medline, Google Scholar
13. Saxon AJ, Calsyn DA, Kivlahan DR, Roszell DK: Outcome of contingency contracting for illicit drug use in a methadone maintenance program. Drug Alcohol Depend 1993; 31:205–214Crossref, Medline, Google Scholar
14. Nirenberg TD, Cellucci T, Liepman MR, Swift RM, Sirota AD: Cannabis versus other illicit drug use among methadone maintenance patients. Psychol Addict Behav 1996; 10:222–227Crossref, Google Scholar
15. Epstein DH, Preston KL: Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? past findings and more evidence against. Addiction 2003; 98:269–279Crossref, Medline, Google Scholar
16. DuPont RL, Saylor KE: Marijuana and benzodiazepines in patients receiving methadone treatment (letter). JAMA 1989; 261:3409Crossref, Medline, Google Scholar
17. Wasserman DA, Weinstein MG, Havassy BE, Hall SM: Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend 1998; 52:183–192Crossref, Medline, Google Scholar
18. Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J: Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry 2002; 59:375–380Crossref, Medline, Google Scholar
19. Aharonovich E, Liu X, Nunes E, Hasin DS: Suicide attempts in substance abusers: effects of major depression in relation to substance use disorders. Am J Psychiatry 2002; 159:1600–1602Link, Google Scholar
20. Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J: Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry 1996; 153:1195–1201Link, Google Scholar
21. Hasin D, Trautman K, Endicott J: Psychiatric Research Interview for Substance and Mental Disorders: phenomenologically based diagnosis in patients who abuse alcohol or drugs. Psychopharmacol Bull 1998; 34:3–8Crossref, Medline, Google Scholar
22. Hasin D, Samet S, Nunes E, Meydan K, Matsoeane K, Waxman R: Diagnosis of comorbid disorders in substance users: Psychiatric Research Interview for Substance and Mental Disorders (PRISM-IV). Am J Psychiatry (in press)Google Scholar
23. Torrens M, Serrano D, Astals M, Pérez-Domínguez G, Martín-Santos R: Diagnosing comorbid psychiatric disorders in substance abusers: validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am J Psychiatry 2004; 161:1231–1237Link, Google Scholar
24. Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC: The Longitudinal Interval Follow-Up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987; 44:540–548Crossref, Medline, Google Scholar
25. Sobell LC, Sobell MB: Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods. Edited by Allen J, Litten RZ. Totowa, NJ, Humana Press, 1992, pp 41–72Google Scholar
26. Russell M, Marshall J, Trevisan M, Freudenheim J, Chan A, Markovic N, Vana J, Priore R: Test-retest reliability of the cognitive lifetime drinking history. Am J Epidemiol 1997; 146:975–981Crossref, Medline, Google Scholar
27. Hasin D, Tsai W-Y, Endicott J, Mueller TI, Coryell W, Keller MB: The effects of major depression on alcoholism: five-year course. Am J Addict 1996; 5:144–155Google Scholar
28. De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN: Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003; 168:164–169Crossref, Medline, Google Scholar
29. Hunglund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C: Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem 2003; 84:698–704Crossref, Medline, Google Scholar
30. Racz I, Bilei-Gorzo A, Toth ZE, Michel K, Palovits M, Zimmer A: A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci 2003; 23:2453–2458Crossref, Medline, Google Scholar
31. Lallemand F, Soubrie PH, De Witte PH: Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcohol Clin Exp Res 2001; 25:1317–1323Crossref, Medline, Google Scholar
32. Fattore L, Spano MS, Cossu G, Deiana S, Fratta W: Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 2003; 17:1723–1726Crossref, Medline, Google Scholar
33. Budney AJ, Higgins ST, Wong CJ: Marijuana use and treatment outcome in cocaine dependent patients. Exp Clin Psychopharmacol 1996; 4:1–8Crossref, Google Scholar
34. Budney AJ, Higgins ST, Delaney DD, Kent L, Bickel WK: Contingent reinforcement of abstinence with individuals abusing cocaine and marijuana. J Appl Behav Anal 1991; 24:657–665Crossref, Medline, Google Scholar
35. ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF III: Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forensic Sci 2000; 45:24–30Medline, Google Scholar
36. ElSohly MA: Practical challenges to positive drug tests for marijuana. Clin Chem 2003; 49:1037–1038Crossref, Medline, Google Scholar
37. Compton WM, Colliver J, Grant BF, Glantz MD, Stinson FS: Increases in the prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA 2004; 291:2114–2121Crossref, Medline, Google Scholar
38. National Institute on Drug Abuse: The validity of self-reported drug use data: the accuracy of responses on confidential self-administered answered sheets. NIDA Res Monogr 1997; 167:37–58Medline, Google Scholar
39. Simpson DD, Joe GW, Broome KM: A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry 2002; 59:538–544Crossref, Medline, Google Scholar
40. Weiss RD, Najavits LM, Greenfield SF, Soto JA, Shaw SR, Wyner D: Validity of substance use self-reports in dually diagnosed outpatients. Am J Psychiatry 1998; 155:127–128Link, Google Scholar



