Pharmacotherapy and Pharmacogenetics of Nicotine Dependence
Abstract
The authors review recent advances in the pharmacotherapy and pharmacogenetics of nicotine dependence. Despite the negative health consequences of smoking, approximately 23% of adults in the United States are daily tobacco smokers and approximately 13% are nicotine dependent. Data for development of new medications for nicotine dependence are likely to come from animal models of the reinforcing value of nicotine, studies to identify proteins in transgenic rodents, and pharmacological studies of nicotine withdrawal. The initial pharmacogenetic studies of pharmacotherapies approved by the United States Food and Drug Administration for treatment of nicotine dependence—nicotine replacement (nicotine gum, nicotine nasal spray, and transdermal nicotine) and bupropion—have identified candidate alleles at the dopamine D2 receptor gene and μ opioid receptor gene that may predict therapeutic response. Because no one medication is likely to be safe and efficacious for a majority of persons with nicotine dependence, it will be useful to develop genetics-based methods and other tools to predict therapeutic response in subgroups of nicotine-dependent persons.
In this article, we briefly review the epidemiology of nicotine dependence, the efficacy of nicotine dependence pharmacotherapies, and the pharmacogenetics of nicotine dependence treatment. Nicotine dependence is a complex trait, the end result of genetic susceptibility interacting with environmental risk factors to produce the syndrome (1). Similarly, response to pharmacotherapy in nicotine dependence is a complex trait. Pharmacotherapy for nicotine dependence is important to psychiatrists, as epidemiological studies indicate that a majority of individuals with schizophrenia or affective disorders are daily smokers (2).
Epidemiology of Nicotine Dependence
Cigarette smoking is implicated in approximately 400,000 deaths annually (3). Cigarette smoking is the greatest preventable cause of cancer, accounting for at least 30% of all cancer deaths (4). Approximately 23% of American adults are cigarette smokers (5). The nicotine in tobacco is the primary rewarding compound that establishes and maintains tobacco use (5, 6), and most persons who smoke regularly (daily for at least 1 month) develop nicotine dependence (6–8). Regular smoking typically begins in adolescence (5, 9–11).
A complex set of factors allows for the initiation into smoking; those factors include the smoking status of friends and parents, economic status, and heritable factors (7, 12). Factors that facilitate continuation of smoking probably involve a complex interaction between aversive and rewarding influences of nicotine, as well as environmental variables, including peer group approval and economics. Continued regular smoking leads to nicotine dependence (7). For a DSM-IV diagnosis of nicotine dependence, a smoker must meet three or more of the following six major criteria:
| 1. | Tolerance (e.g., the absence of nausea or dizziness despite substantial levels of smoking) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. | Withdrawal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. | Repeated unsuccessful attempts to quit or reduce nicotine use | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. | Reduction or elimination of social or occupational activities because smoking tobacco is not allowed in those settings | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. | Continued use despite medical or psychological harm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6. | Use that is often greater than intended or more frequent than intended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
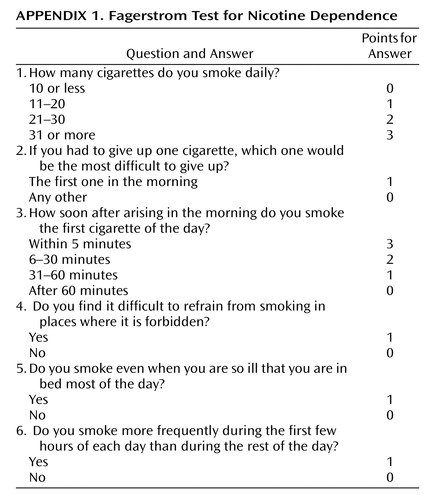
Once smoking becomes established (daily smoking for 1 month), approximately 20% of smokers develop nicotine dependence, based on responses to the Fagerstrom Test for Nicotine Dependence, which consists of six questions (13) (Appendix 1). Although the maximal score is 10, a score >4 indicates probable nicotine dependence.
Most nicotine-dependent persons report annual attempts to quit smoking, but less than 15% are successful over the long term (5). To reduce tobacco-related morbidity and mortality, new approaches to nicotine dependence treatment are needed. Some of these new approaches may be developed on the basis of findings from the study of animal models of nicotine dependence.
Animal Models of Nicotine-Related Phenotypes
The rewarding effects of nicotine are mediated, in part, by release of dopamine in the nucleus accumbens from ventral tegmental area neurons (14–16). Increasing synaptic dopamine in the nucleus accumbens is a mechanism of reward for many abused drugs (17). The mechanism through which nicotine mediates the increase in nucleus accumbens synaptic dopamine may involve the μ opioid receptor and its endogenous ligand, β-endorphin (18, 19).
Several animal models have been developed for the rewarding valence of nicotine. Rodents can be trained to self-administer nicotine in an operant chamber, where a lever must be pressed to receive an intravenous bolus of nicotine. Drugs that reduce operant self-administration may show promise as smoking cessation medicines in clinical trials. One promising compound is baclofen, a γ-aminobutyric acid type B receptor antagonist, which has been shown to decrease intravenous self-administration of nicotine in rats (20, 21). A second compound deserving additional study is a specific inhibitor of norepinephrine reuptake, reboxetine, which also reduced intravenous nicotine self-administration in rats (22).
Another behavioral model of the rewarding valence of nicotine in rodents is conditioned place preference. This paradigm uses an apparatus consisting of a box divided into two distinct compartments, with a communicating door. The two compartments are distinguished by floors and walls of different appearance and/or texture (e.g., one compartment might have a wire mesh floor and vertical stripes on walls, while the other compartment has a solid plastic floor and horizontal dashes). Immediately after drug administration, which is usually by injection, the rodent is placed into one compartment, and the communicating door is locked. Exposure to the drug is paired consistently with the same compartment, with the communicating door locked, and placebo is paired with the other compartment. After several pairings of the drug with one compartment and placebo with the other over several days, the rodent (without any drug for the past day) is placed in the box with the communicating door open. If the animal spends more time on the drug-paired side, this result is taken as evidence that the animal experienced the drug as rewarding. Similarly, avoidance of the drug-paired side is evidence that the drug was aversive.
Drugs that block nicotine place preference in rodents may be useful compounds for smoking cessation trials in humans. This conceptual leap is often assumed to be valid because cigarette smoking is valued in part for its rewarding effects (e.g., mild euphoria). If a medication blocks the rewarding effects, it may reduce cigarette consumption.
However, drugs that universally block experience of reward for any event would not be acceptable compounds for clinical trials in smoking cessation, for obvious reasons. For example, a partial agonist at the strychnine-insensitive glycine receptor site blocks nicotine, morphine, cocaine, and amphetamine place preference in mice, but it does not interfere with place preference for sucrose pellets (23). The absence of an effect on the natural reward of food suggests that the drug does not block all hedonic mechanisms in the brain.
Nicotine withdrawal may be studied by chronic nicotine administration in rodents, followed by a nicotinic antagonist injection (24). Compounds that attenuate the severity of nicotine withdrawal symptoms in rodents may have promise in treatment of nicotine dependence because smoking may be maintained in part through avoidance of withdrawal.
Implicit in these studies is the assumption that drugs that reduce the rewarding valence of nicotine, or reduce nicotine withdrawal symptoms, may be useful clinically in treating nicotine dependence. Although this assumption may not be correct, it is supported by the use of nicotine replacement therapy as a means to manage nicotine withdrawal symptoms.
Studies of transgenic mice have provided some insights into the rewarding mechanisms of nicotine, and these findings have suggested pharmacological approaches to the treatment of nicotine dependence. Nicotine has been found to induce release of β-endorphin (18, 19) and met-enkephalin (25) from neurons. Mice lacking the μ opioid receptor gene, for which β-endorphin and met-enkephalin are naturally occurring ligands, do not show nicotine place preference (26). Thus, some aspect of the rewarding valence of nicotine requires μ opioid receptors and (presumably) an endogenous ligand, either β-endorphin or met-enkephalin or both. These studies suggest a role for opioid antagonists in smoking cessation.
Mice lacking the β2 subunit of nicotinic receptors do not experience nicotine as rewarding (27). These data are consistent with the finding that the β2 subunit is essential for nicotine to elicit dopamine release in the nucleus accumbens (28). This assumption leads to the hypothesis that nicotine’s rewarding actions are mediated through binding to nicotinic receptors containing the β2 subunit in the nucleus accumbens, yielding increased release of dopamine, with an essential role for a functioning μ opioid receptor system.
Mice lacking cannabinoid type 1 (CB1) receptors do not show nicotine place preference (24). This study and other studies have led to the development of a promising compound for smoking cessation, the CB1 receptor antagonist rimonabant (29).
Promising compounds for smoking cessation may also be identified by screening for blockade of nicotine-induced increases in synaptic dopamine in the nucleus accumbens, an action of nicotine that is essential to its rewarding valence (15, 16). A recent example of this strategy is found in the observation that cannabinoid receptor antagonists can block the nicotine-induced release of presynaptic dopamine in the shell of the nucleus accumbens (30).
In multiple instances, animal model studies have yielded promising compounds for clinical trials in nicotine dependence. These clinical trials will most likely lead to new pharmacotherapies approved by the United States Food and Drug Administration (FDA) for nicotine dependence in the near future. At present, however, only two pharmacotherapies for nicotine dependence have FDA approval: nicotine replacement and bupropion.
Pharmacological Treatments for Nicotine Dependence
Nicotine Replacement Therapy
Nicotine replacement therapy increases smoking cessation rates significantly, compared to behavioral counseling (31, 32). However, nicotine gum is often not used properly and is not effective across all settings (33). Similarly, nicotine nasal spray is aversive for many smokers, and adherence rates tend to be lower than those for other forms of nicotine replacement therapy (34). Thus, transdermal nicotine (“the patch”) is the preferred first-line treatment (31).
Although transdermal nicotine is significantly more effective than placebo or behavioral counseling, only 20%–30% of smokers are able to quit with this form of treatment, and up to 95% relapse to their former smoking practices (35–37). Moreover, a short course of nicotine replacement therapy (e.g., 4 weeks) may not be effective in the long term (38). Although it has been suggested that the modest efficacy of transdermal nicotine is attributable to inadequate nicotine replacement with the 21-mg standard clinical dose, abstinence rates with a 44-mg dose are not maintained at 12-month follow-up, and significant side effects have been reported (39). Other studies have not found better outcomes with the 44-mg dose of transdermal nicotine, even in the short term (37, 40).
With regard to the optimal duration of transdermal nicotine treatment, the results of meta-analyses (36, 41) and clinical trials (42, 43) provide little support for improved outcomes with treatment longer than 8 weeks in duration or with tapering versus abrupt cessation of treatment (44). In a comparison of standard transdermal nicotine treatment and extended treatment (8 additional weeks of “maintenance” therapy with the 21-mg dose, followed by a tapering phase) in a group of 55 smokers with abstinence-induced depressed mood, significant differences in abstinence rates were not observed; however, when treatment dropouts were excluded, the extended treatment group had significantly lower rates of relapse (45). This effect of extended therapy was observed only during the 21-mg dose phase and not during the tapering phase, suggesting that the benefits of extended high-dose treatment may be achieved only if individuals continue taking this dose (45). Although these studies do not provide strong support for higher-dose or longer-duration nicotine replacement therapy overall, the optimal dosage and duration of treatment for subgroups of smokers remain to be determined.
Bupropion
Bupropion, an antidepressant used to treat nicotine dependence, has an unclear mode of action, although there is evidence that bupropion inhibits uptake of dopamine and norepinephrine (46–48). Bupropion is also a nicotinic receptor antagonist, which suggests the hypothesis that treatment may reduce the reinforcing effects of smoking (49). Sustained-release bupropion has been shown to produce significantly higher abstinence rates, compared both with placebo (50–52) and with transdermal nicotine (53, 54). Six-month abstinence rates of 18%–26.9% have been reported for bupropion, compared to rates of 7%–15.7% for placebo (50, 51). Bupropion is efficacious for smokers who may be more prone to relapse, such as women (55, 56), African Americans (57), and smokers with higher levels of nicotine dependence (58).
Bupropion’s efficacy in the treatment of nicotine dependence may be partly attributable to beneficial effects on abstinence-induced weight gain (51, 59) and negative mood symptoms (57, 60). The beneficial effects of bupropion have been observed only during active treatment (61, 62). The benefits of sustained bupropion treatment for nicotine dependence remain unexplored.
Medications Used for Other Indications
Several medications are being studied as treatments for nicotine dependence. Brief treatment with naltrexone, an opioid antagonist, has been shown to reduce cigarette consumption and levels of plasma nicotine, compared to placebo (63). Naltrexone in combination with transdermal nicotine attenuates cue-elicited craving in the laboratory (64), but an earlier report cited no effect of a single acute dose of naltrexone on smoking behavior (65). Clinical data on the efficacy of naltrexone as a smoking cessation therapy have been equivocal (66–70). Fluoxetine, a selective serotonin reuptake inhibitor, has been studied as a smoking cessation treatment, but largely negative results have been documented (71, 72). Preliminary evidence suggested that fluoxetine may be more effective than placebo among smokers with higher levels of pretreatment depression symptoms (73).
Pharmacogenetic Approaches to Nicotine Dependence Treatment
Despite progress made in the pharmacological treatment of nicotine dependence, the efficacy of available treatments is limited. Although current guidelines recommend transdermal nicotine as a first-line treatment for nicotine dependence (31), the vast majority of smokers receiving transdermal nicotine relapse to their former smoking practices (35, 36). Bupropion has been shown to produce higher quit rates than nicotine replacement therapy (53), yet it is effective for only a minority of smokers.
Several studies have attempted to identify pretreatment variables that can be used to individualize pharmacotherapies for nicotine dependence, but with limited success. Measures of nicotine dependence, such as smoking rate, level of dependence, and cotinine level, have predicted outcome in some nicotine replacement therapy studies (40, 74) but not in others (75). Smokers with low to moderate nicotine dependence levels, nonobese smokers, and Caucasian smokers may benefit more from transdermal nicotine, and smokers who are highly dependent, obese, or members of minority groups may benefit more from nasal spray (76). Bupropion may be a more effective treatment for women (55) and smokers with higher levels of nicotine dependence (58). These data do not provide an empirical basis on which to tailor the choice of treatment to individual smokers, but pharmacogenetic research may identify smokers for whom bupropion and nicotine replacement therapy will have the strongest benefit. Research on the role that inherited variation plays in the response to pharmacotherapy for nicotine dependence may yield individualized treatments based on genotype and may thereby improve efficacy.
A pharmacogenetic approach to nicotine dependence treatment may yield knowledge of DNA variants that influence treatment outcome, under the assumption that inherited differences in drug metabolism and drug targets influence toxicity and efficacy (77–79).
Pharmacogenetic Investigations of Nicotine Replacement Therapy
In a pharmacogenetic study conducted in the United Kingdom, 755 of 1,500 smokers participating in a placebo-controlled trial of transdermal nicotine also provided blood samples for DNA analysis (80, 81). The DNA analysis focused on genetic variation in the dopamine pathway, on the basis of previous evidence that nicotine’s rewarding effects are mediated, in part, by dopaminergic mechanisms (82). Transdermal nicotine was significantly more effective than placebo for carriers of the A1 allele of the D2 dopamine receptor gene (DRD2) but not for A2 homozygotes (80). The difference in the odds ratios for the treatment effect between the genotype groups was significant after the first week of treatment but not at the end of treatment. This study also examined a polymorphism in the dopamine β-hydroxylase gene (DBH). Transdermal nicotine was effective (odds ratio of 3.6 for transdermal nicotine versus placebo) in producing abstinence among smokers with both the DRD2*A1 allele and the DBH*A allele and was less effective for smokers with other genotypes. This genetic association with treatment response was significant at both 1 week and 12 weeks of treatment, suggesting that the efficacy of transdermal nicotine may be modulated by DRD2 and DBH. A follow-up study supported the association of the DRD2*A1 variant with abstinence at 6 and 12 months posttreatment; however, the effect was observed only among women. Results for DBH were not reported (81).
In an open-label pharmacogenetic trial of transdermal nicotine versus nicotine nasal spray, the role of the μ opioid receptor gene (OPRM1) gene was examined (83). The μ opioid receptor is the primary site of action for the rewarding effects of the endogenous opioid peptide, β-endorphin, which is released afer acute and short-term nicotine administration (18, 19). Exon 1 of the human OPRM1 gene includes a common Asn40Asp (A118G) mis-sense single nucleotide polymorphism. The Asp40 variant increases the binding affinity of β-endorphin for this receptor threefold, relative to the wild-type Asn40 OPRM1(84). The Asp40 variant is found in about 25%–30% of individuals of European ancestry (85, 86) and is therefore sufficiently common to explain clinically significant differences in response to different forms of nicotine replacement therapy.
Among 320 smokers of European ancestry, persons carrying the OPRM1 Asp40 variant (Asn/Asp or Asp/Asp, N=82) were significantly more likely than those homozygous for the Asn40 variant (Asn/Asn, N=238) to be abstinent at the end of the treatment (83). The differential treatment response was evident among smokers who received transdermal nicotine (quit rates of 52% versus 33% for the Asp40 and Asn40 groups, respectively; odds ratio=2.4) but was not significant among smokers who received nicotine nasal spray. Among smokers who received transdermal nicotine, the advantage for the Asp40 group was significantly greater during the 21-mg dose phase. Smokers with the Asp40 variant also reported significantly less severe withdrawal symptoms and mood disturbance during the first 2 weeks of abstinence and gained significantly less weight at the end of treatment, compared to those with the Asn40 genotype. Although these results must be validated in future research, the findings suggest a hypothesis that smokers with the OPRM1 Asp40 variant may achieve significant benefit from transdermal nicotine and may be candidates for extended high-dose patch treatment, or even maintenance therapy, to reduce risk of relapse.
Consistent with this pharmacogenetic hypothesis, a longitudinal analysis in the transdermal nicotine group revealed that the OPRM1 genotype effect in the Asp40 group was greatest during 21-mg patch treatment, declined as treatment was tapered, and disappeared after treatment was discontinued. In the Asn40 homozygotes, dose tapering did not appear to alter abstinence rates, which declined steadily from the quit date. Event history analysis of lapse and recovery events showed that transdermal nicotine-treated smokers with the Asp40 variant were significantly more likely to recover from lapses than were Asn40homozygotes during the 21-mg dose phase (83). There was no genotype effect on recovery from lapses during the 14-mg or 7-mg phase or after treatment was discontinued. Thus, nicotine-dependent persons with the Asp40 allele may benefit from extended higher-dose transdermal nicotine therapy.
Consistent with the treatment outcome data from this trial (83), smokers with the OPRM1 Asp40 variant reported significantly less severe withdrawal symptoms and mood disturbance during the first 2 weeks of abstinence, compared to the Asn40homozygotes. An increase in negative affect during this period strongly predicted relapse. Smokers with the Asp40 variant also had significantly less weight gain at the end of treatment than did the Asn40 homozygotes. OPRM1 genotype effects on these intermediate outcomes may be mediated by greater β-endorphin occupancy at the μ receptor. Although these results must be validated in future research, the findings suggest a hypothesis that smokers with the OPRM1 Asp40 variant may be candidates for extended high-dose patch treatment, or even maintenance therapy, as an alternative to smoking.
Pharmacogenetic Investigations of Bupropion
A placebo-controlled smoking cessation clinical trial of bupropion (87) was the source of pharmacogenetic analyses focused on the cytochrome P450 2B6 gene (CYP2B6), which has been implicated in bupropion kinetics (88) and in brain nicotine metabolism (89). In this trial, 426 smokers of European ancestry provided blood samples and received bupropion (300 mg/day for 10 weeks) or placebo, plus counseling. Smokers with decreased activity alleles of CYP2B6 (slower metabolizers) reported greater increases in cravings for cigarettes following the target quit date and had significantly higher relapse rates. These effects were modified by a significant gender-by-genotype-by-treatment interaction, suggesting that bupropion attenuated the effects of genotype among female smokers. The findings of a significant association of CYP2B6 genotype with smoking cessation in the placebo group and absence of a genotype association with bupropion side effects suggest that the genotype effect on treatment outcome is not attributable to bupropion pharmacokinetics. Rather, the greater relapse liability in the genetically slower metabolizers may be attributable to slower rates of inactivation of nicotine (by conversion to cotinine) in the central nervous system. Additional trials are warranted to confirm these results, as are studies to explore the neurobiological mechanisms of the observed genetic effect.
A second report from this clinical trial (87) examined genetic variation in the dopamine pathway, based on the premise that bupropion’s effects are attributable, in part, to inhibition of dopamine reuptake (46). The genetic analysis focused on common polymorphisms in the dopamine transporter (SLC6A3) gene and the DRD2 gene, both of which had been associated in previous studies with smoking behavior (90–93). Although the analysis did not support the hypothesis of genetic modulation of response to bupropion, the results revealed a significant gene-gene interaction effect on liability to relapse, mirroring results from a previous study of smoking status (90). Specifically, smokers with DRD2*A2 and SLC6A3*9 alleles had significantly higher abstinence rates at the end of treatment (53% versus 39%) and a longer latency to relapse at the end of treatment (28 versus 21 days) and at 6-month follow-up (83 versus 65 days). By contrast, among smokers with DRD2*A1 genotypes, the effect of SLC6A3 on abstinence rates and time to relapse was not significant.
On the basis of existing biological and epidemiological data on DRD2 and SLC6A3, a biobehavioral mechanism for the observed findings can be postulated. Data suggest that individuals with DRD2*A1 genotypes exhibit lower dopamine D2 receptor density and, therefore, may have lower levels of neuronal dopamine-dependent activity, compared to individuals with DRD2*A2 genotypes (94–96). This interpretation is consistent with epidemiological evidence for association of DRD2*A1 with cognitive function (97, 98), as well as with a variety of addictive behaviors (99). With regard to SLC6A3, the 9-repeat genotype has been associated with lower levels of dopamine transporter protein expression (100, 101). This lower level of expression may yield less neuronal dopamine reuptake and higher levels of synaptic dopamine. Thus, individuals with this 9-repeat allele may derive less reinforcement from nicotine-induced dopamine release, by virtue of a chronically high level of synaptic dopamine at baseline. Thus, one could speculate that in the presence of normal receptor function (i.e., in individuals with DRD2*A2 genotypes), lower dopamine transporter levels and higher levels of synaptic dopamine (i.e., in individuals with SLC6A3*9 genotypes) would minimize the phasic effects of nicotine on dopamine release, thereby reducing positive reinforcement from smoking. This effect, in turn, would make it easier for smokers with the DRD2*A2/SLC6A3*9 haplotype to maintain smoking abstinence. By contrast, individuals with lower synaptic dopamine levels (i.e., those with SLC6A3*10 genotypes) and normal receptor density (i.e., those with DRD2*A2 genotypes) might have the greatest need for and reinforcement from nicotine.
An association of the DRD2 Taq1 polymorphism with bupropion’s effects on subjective withdrawal symptoms has also been reported in a small investigation (102). The A1 allele of DRD2 has also been linked with smoking cessation and abstinence-induced negative mood symptoms following treatment with venlafaxine, a serotonin and norepinephrine reuptake inhibitor (103).
Weight gain occurs in a majority of daily smokers after quitting (104, 105), and weight gain predicts relapse to smoking (105). To investigate possible mechanisms, we examined genetic variations in the dopamine pathway as moderators of the effect of bupropion on abstinence-induced changes in the rewarding value of food (106). This analysis was based on the evidence described earlier for the beneficial effects of bupropion on weight gain after smoking cessation (51). Seventy-one smokers of European ancestry participated in this experiment; all of them were genotyped for the DRD2 Taq1 polymorphism and randomly assigned to treatment with bupropion (300 mg/day) or placebo. They participated in two behavioral laboratory sessions during which the rewarding value of food was assessed with a behavioral economics measure: session 1 occurred before medication and before cessation of smoking; session 2 occurred after 3 weeks of bupropion treatment and 1 week of sustained abstinence. Carriers of the DRD2*A1 allele exhibited significant increases in the rewarding value of food after abstinence from smoking, and these effects were attenuated by bupropion treatment (a significant medication-by-genotype interaction). Further, higher levels of food reward at session 2 (postquit) predicted a significant increase in weight by 6-month follow-up in the placebo group but not the bupropion-treated group. These results provide new evidence that the increase in body weight that occurs after smoking cessation is related to increases in food reward and that food reward is partly determined by genetic factors. Bupropion’s efficacy in attenuating abstinence-induced weight gain may be attributable, in part, to its effect in decreasing food reward.
Challenges in Pharmacogenetic Studies of Nicotine Dependence
Phenotype Assessment
Given the complexity of nicotine dependence, including its multiple genetic and environmental components, it is difficult to imagine that a single compound could provide effective pharmacotherapy for the millions of nicotine-dependent individuals in the United States. Rather, a given medication may be useful for a minority of nicotine-dependent persons. Thus, it becomes essential to optimize pharmacotherapy by using reliable predictors of therapeutic response. Genetic background will be one of those reliable predictors, but only one. It will be necessary to develop new phenotypes related to the multidimensional nature of nicotine dependence. These new phenotypes may provide better prediction of pharmacotherapy response.
As a complex trait, nicotine dependence has multiple facets, including molecular brain adaptations to chronic nicotine use, electrophysiological alterations, structural and functional brain abnormalities, and neurocognitive changes. Therapeutic response to pharmacotherapy has been measured typically in terms of reduction in the number of cigarettes smoked per day or in terms of absolute abstinence, confirmed by carbon monoxide in exhaled air and/or measures of plasma (or salivary) cotinine, a nicotine metabolite. Therapeutic response to pharmacotherapy for nicotine dependence might be more closely related to pharmacogenetics when that therapeutic response is also defined partly in terms of nicotine-evoked electrophysiological parameters, brain imaging findings for responses to nicotine, or nicotine-induced cognitive changes. Research in these areas is needed.
The overwhelming majority of daily smokers gain weight after smoking cessation (104), and the average increase may be as high as 5 kg. Weight gain is one predictor of relapse after smoking cessation (105). Thus, studies of weight gain after smoking cessation (107), as a nicotine-related phenotype, are needed for a more adequate response to this public health concern.
Sample Size
The complexity of the phenotype requires that pharmacogenetic studies of nicotine dependence have adequate statistical power to detect small gene effects, especially when the model includes gene-gene and gene-environment interactions in the presence of active (versus placebo) pharmacotherapy. It will be necessary to study hundreds to thousands of clinical trial participants to identify the key genetic variants predicting therapeutic and adverse responses.
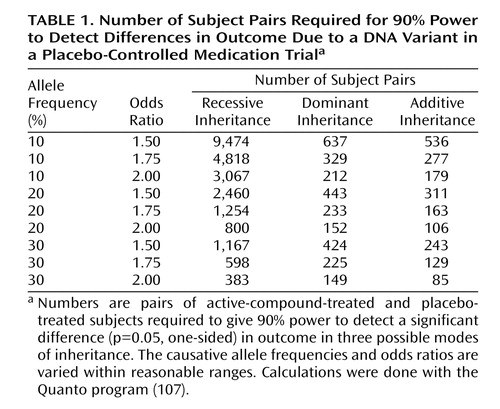
In any approach to estimating sample sizes for pharmacogenetic studies of nicotine dependence, several parameters must be estimated and several modes of inheritance must be assumed. One parameter estimated is the allele frequency of a hypothetical DNA variant that increases the probability that a nicotine-dependent person will stop smoking if given a certain medication. It does not seem reasonable to study alleles that are present at less than 10% frequency in the population, as these alleles will be too uncommon to influence the outcome for most treatment seekers. A second parameter estimated is the size of the effect of the variant in increasing the probability that a nicotine-dependent person will stop smoking if given the medication under study. Reasonable assumptions of effect size are in the range of 1.5 to 2 (expressed as the odds ratio). Several modes of inheritance (recessive, dominant, and additive) are included. Table 1 gives the sample sizes required for 90% power to detect a significant (p=0.05, one-sided) effect of an active compound under these reasonable assumptions for the typical study in which subjects are randomly assigned to receive either the active compound or placebo.
Implications of Pharmacogenetic Research in Nicotine Dependence for Psychiatry Practice
Individuals with psychiatric illness carry a disproportionate burden from cigarette smoking and tobacco-related mortality. Data from a nationally representative survey indicated that individuals with a current psychiatric illness are not only more likely to be regular smokers but also less likely to quit smoking (108). Ninety-two percent of persons with a diagnosis of schizophrenia have been estimated to have a lifetime history of smoking and 83% to be current smokers, compared to about one-quarter of the general population (109). Rates of current smoking are also elevated among persons with bipolar disorder (69%), major depression (37%), and generalized anxiety disorder (46%) (108). Further, there is evidence for a common genetic etiology for smoking and psychiatric illness and support for the role of the α7 neuronal nicotinic receptor in both schizophrenia and bipolar disorder (110).
Although smoking cessation treatments have not been extensively studied in persons with psychiatric illness, some pharmacotherapies have shown promise. For example, in a randomized clinical trial of a smoking cessation intervention for persons with schizophrenia, bupropion was found to be safe and significantly more effective than placebo (111). Evidence for the safety and efficacy of transdermal nicotine, especially in conjunction with atypical antipsychotic agents, has been reported (112).
Pharmacogenetics research in smoking has the potential to advance the science and practice of smoking cessation treatment in the general population and among persons with psychiatric illness. In addition to providing insights into targets for novel pharmacotherapies, information about smokers’ genotypes may allow practitioners to select the optimal type, dosage, and duration of treatment for individual patients. Although this research is still in its infancy, recent data suggested that health care providers are very favorably disposed toward providing genetically tailored treatment in practice; for example, among 1,120 physicians in the American Medical Association, the average reported likelihood of adoption of genetic testing to tailor smoking treatment (on a 0%–100% scale) was 73.5% (113). However, several barriers to clinical translation of pharmacogenetics research in smoking must be addressed, including a lack of preparedness of health care providers to counsel patients about genetic results and concerns regarding the potential for stigmatization and discrimination based on genetic findings (113, 114). Such findings highlight the importance of provider education in genetics, as well as the need to address broader health care policy issues.
Summary
Despite 40 years of government warnings regarding the negative health consequences of smoking, roughly 23% of adults in the United States are daily tobacco smokers. Nicotine produces a dependence syndrome that renders abstinence a difficult goal. Social pressures against smoking have increased, producing fewer opportunities to smoke, yet smoking represents the single largest preventable source of morbidity and mortality in the United States. Thus, the need for new medications is great.
New medications for nicotine dependence may be developed through animal models of the reinforcing value of nicotine as well as through pharmacological studies of nicotine withdrawal. Studies of transgenic rodents have been valuable in identifying specific genes whose proteins can be targeted for pharmacotherapy.
The initial pharmacogenetic studies of FDA-approved pharmacotherapies, including nicotine replacement and bupropion, have appeared in the literature. These studies have identified (in a preliminary manner) candidate alleles at the D2 dopamine receptor gene and μ opioid receptor gene that may predict therapeutic response. Given the complexity of the nicotine dependence phenotype, it seems that no one medication will show efficacy and safety for a majority of nicotine-dependent individuals. Thus, it will be essential to use genetics and other tools to predict therapeutic response in subgroups of nicotine-dependent persons.
This research is of particular importance to psychiatrists, as a large fraction of individuals with behavioral disorders are daily smokers, including the majority of persons with schizophrenia and affective disorders (2). Psychiatrists must consider smoking cessation therapy a high priority in their clinical practices, and newer, more efficacious medications will facilitate this change.
 |
Received July 12, 2004; revision received Aug. 31, 2004; accepted Sept. 10, 2004. From the Center for Neurobiology and Behavior and the Tobacco Use Research Center, Department of Psychiatry, University of Pennsylvania School of Medicine. Address correspondence and reprint requests to Dr. Berrettini, Center for Neurobiology and Behavior, Department of Psychiatry, University of Pennsylvania School of Medicine, Clinical Research Bldg., Room 111, 415 Curie Blvd., Philadelphia, PA 19104; [email protected] (e-mail). Supported in part by grant P5084718 from the National Cancer Institute and the National Institute on Drug Abuse (NIDA) and by NIDA grants R01 DA-14008 and P60 DA-05186.
 |
APPENDIX 1
1. Sullivan PF, Kendler KS: The genetic epidemiology of smoking. Nicotine Tob Res 1999; 1(suppl 2):S51-S57Google Scholar
2. Breslau N: Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995; 25:95–101Crossref, Medline, Google Scholar
3. Miller NS, Gold MS: Comorbid cigarette and alcohol addiction: epidemiology and treatment. J Addict Dis 1998; 17:55–66Crossref, Medline, Google Scholar
4. Cinciripini P, Gritz ER, Tsoh JY, Skaar KL: Smoking cessation and cancer prevention: current treatment recommendations, in Psycho-oncology. Edited by Holland J. New York, Oxford University Press, 1998, pp 27–44Google Scholar
5. Kessler DA, Natanblut SL, Wilkenfeld JP, Lorraine CC, Mayl SL, Bernstein IB, Thompson L: Nicotine addiction: a pediatric disease. J Pediatr 1997; 130:518–524Crossref, Medline, Google Scholar
6. The Health Consequences of Smoking—Nicotine Addiction: A Report of the Surgeon General. Rockville, Md, US Department of Health and Human Services, 1988Google Scholar
7. Breslau N, Johnson EO, Hiripi E, Kessler R: Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry 2001; 58:810–816Crossref, Medline, Google Scholar
8. Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M: Smoking typology profiles of chippers and regular smokers. J Subst Abuse 1994; 6:21–35Crossref, Medline, Google Scholar
9. Rojas NL, Killen JD, Haydel KF, Robinson TN: Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998; 152:151–156Crossref, Medline, Google Scholar
10. Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R: Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996; 21:117–127Crossref, Medline, Google Scholar
11. Woolf AD: Smoking and nicotine addiction: a pediatric epidemic with sequelae in adulthood. Curr Opin Pediatr 1997; 9:470–477Crossref, Medline, Google Scholar
12. Boomsma DI, Koopmans JR, Van Doomen LJ, Orlebeke JF: Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction 1994; 89:219–226Crossref, Medline, Google Scholar
13. Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF: Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav 1994; 19:33–39Crossref, Medline, Google Scholar
14. Corrigall WA, Coen WM, Adamson KL: Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 1994; 653:278–284Crossref, Medline, Google Scholar
15. DiChiara G: Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 2000; 393:295–314Crossref, Medline, Google Scholar
16. Nisell M, Nomikos GG, Svensson TH: Systemic nicotine-induced dopamine release from the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 1994; 16:36–44Crossref, Medline, Google Scholar
17. DiChiara G, Imperato A: Drugs abused by humans preferentially increase synaptic dopamine concentrations in the limbic system of freely moving rats. Proc Natl Acad Sci USA 1998; 85:5274–5278Crossref, Google Scholar
18. Boyadjieva NI, Sarkar DK: The secretory response of hypothalamic beta-endorphin neurons to acute and chronic nicotine treatments and following nicotine withdrawal. Life Sci 1997; 61:PL59-PL66Google Scholar
19. Davenport KE, Houdi AA, Van Loon GR: Nicotine protects against mu-opioid receptor antagonism by beta-funaltrexamine: evidence for nicotine-induced release of endogenous opioids in brain. Neurosci Lett 1990; 113:40–46Crossref, Medline, Google Scholar
20. Fattore L, Cossu G, Martellotta MC, Fratta W: Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol 2002; 37:495–498Crossref, Medline, Google Scholar
21. Paterson NE, Froestl W, Markou A: The GABAB receptor antagonist baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004; 172:179–186Crossref, Medline, Google Scholar
22. Rauhut AS, Mullins SN, Dwoskin LP, Bardo MT: Reboxetine: attenuation of intravenous nicotine self-administration in rats. J Pharmacol Exp Ther 2002; 303:664–672Crossref, Medline, Google Scholar
23. Papp M, Gruca P, Willner P: Selective blockade of drug-induced place preference conditioning by ACPC, a functional NMDA receptor antagonist. Neuropsychopharmacology 2002; 27:727–743Crossref, Medline, Google Scholar
24. Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O: Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology 2002; 43:857–867Crossref, Medline, Google Scholar
25. Dhatt RK, Gudehithlu KP, Wemlinger TA, Tewani GA, Neff NH, Hadjiconstantinou M: Preproenkephalin mRNA and met-enkephalin content are increased in mouse striatum after treatment with nicotine. J Neurochem 1995; 64:1878–1883Crossref, Medline, Google Scholar
26. Berrendero F, Keiffer BL, Maldonado R: Attenuation of nicotine-induced antinociception, rewarding effects and dependence in mu opioid receptor knock-out mice. J Neurosci 2002; 22:10935–10940Crossref, Medline, Google Scholar
27. Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changieux JP: Acetylcholine receptors containing the beta 2 subunit are involved in the reinforcing properties of nicotine. Nature 1998; 391:173–177Crossref, Medline, Google Scholar
28. Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP: Subunit composition of functional nicotinic receptors on dopaminergic neurons investigated with knock-out mice. J Neurosci 2003; 23:7820–7829Crossref, Medline, Google Scholar
29. Foulds J, Burke M, Steinberg M, Williams JM, Ziedonis DM: Advances in pharmacotherapy for tobacco dependence. Expert Opin Emerg Drugs 2004; 9:39–53Crossref, Medline, Google Scholar
30. Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P: SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in the rat. Behav Pharmacol 2002; 13:451–463Crossref, Medline, Google Scholar
31. Fiore MC: Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care 2000; 45:1196–1199Medline, Google Scholar
32. Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB: Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994; 105:524–533Crossref, Medline, Google Scholar
33. Cepeda-Benito A: Meta-analytical review of the efficacy of nicotine chewing gum in smoking treatment programs. J Consult Clin Psychol 1993; 61:822–830Crossref, Medline, Google Scholar
34. Kaufmann V, Jepson C, Rukstalis M, Perkins K, Audrain-McGovern J, Lerman C: Subjective effects of an initial dose of nicotine nasal spray predict treatment outcome. Psychopharmacology (Berl) 2004; 172:271–276Crossref, Medline, Google Scholar
35. Transdermal Nicotine Study Group: Transdermal nicotine for smoking cessation: six-month results from two multicenter controlled trials. JAMA 1991; 266:3133–3138Crossref, Medline, Google Scholar
36. Fiore MC, Smith SS, Jorenby DE, Baker TB: The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA 1994; 271:1940–1947Crossref, Medline, Google Scholar
37. Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, Hays JT, Lewis SF, Baker TB: Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995; 274:1347–1352Crossref, Medline, Google Scholar
38. Pierce JP, Gilpin EA: Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA 2002; 288:1260–1264Crossref, Medline, Google Scholar
39. Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR: High-dose nicotine patch therapy: percentage of replacement and smoking cessation. JAMA 1995; 274:1353–1358Crossref, Medline, Google Scholar
40. Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, Corlando A, Gustavsson G, Sawe U, Giuntini C: Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J 1996; 9:643–651Crossref, Medline, Google Scholar
41. Silagy C, Lancaster T, Stead L, Mant D, Fowler G: Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2001;(3):CD000146Google Scholar
42. Bolin LJ, Antonuccio DO, Follette WC, Krumpe P: Transdermal nicotine: the long and short of it. Psychol Addict Behav 1999; 13:152–156Crossref, Google Scholar
43. Tonnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, Rijcken B, Sawe U: Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Eur Respir J 1999; 13:238–246Crossref, Medline, Google Scholar
44. Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, Wiseman D: Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995; 90:31–42Crossref, Medline, Google Scholar
45. Pomerleau OF, Pomerleau CS, Marks JL, Snedecor SM, Mehringer AM, Namenek Brouwer RJ, Saules KK: Prolonged nicotine patch use in quitters with past abstinence-induced depressed mood. J Subst Abuse Treat 2003; 24:13–18Crossref, Medline, Google Scholar
46. Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Sulser F: Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995; 56:395–401Medline, Google Scholar
47. Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM: Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology 1994; 11:133–141Crossref, Medline, Google Scholar
48. Sanchez C, Hyttel J: Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 1999; 19:467–489Crossref, Medline, Google Scholar
49. Slemmer JE, Martin BR, Damaj MI: Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 2000; 295:321–327Medline, Google Scholar
50. Dalsgareth OJ, Hansen NC, Soes-Petersen U, Evald T, Hoegholm A, Barber J, Vestbo J: A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res 2004; 6:55–61Medline, Google Scholar
51. Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM: A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997; 337:1195–1202Crossref, Medline, Google Scholar
52. Tonnesen P, Tonstad S, Hjalmarson A, Lebargy F, Van Spiegel PI, Hider A, Sweet R, Townsend J: A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med 2003; 254:184–192Crossref, Medline, Google Scholar
53. Gold PB, Rubey RN, Harvey RT: Naturalistic, self-assignment comparative trial of bupropion SR, a nicotine patch, or both for smoking cessation treatment in primary care. Am J Addict 2002; 11:315–331Crossref, Medline, Google Scholar
54. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB: A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340:685–691Crossref, Medline, Google Scholar
55. Collins BN, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, Pinto A, Hawk L, Niaura R, Epstein LH, Lerman C: Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine Tob Res 2004; 6:27–37Crossref, Medline, Google Scholar
56. Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, Sachs DP, Rigotti NA, Niaura R, Hays JT, Hurt RD: Effects of gender on relapse prevention in smokers treated with bupropion SR. Am J Prev Med 2002; 22:234–239Crossref, Medline, Google Scholar
57. Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS: Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002; 288:468–474Crossref, Medline, Google Scholar
58. Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, Hurt RD: Bupropion for smoking cessation: predictors of successful outcome. Chest 2001; 119:1357–1364Crossref, Medline, Google Scholar
59. Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, Sachs DP, Wolter TD, Buist AS, Johnston JA, White JD: Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: a randomized, controlled trial. Ann Intern Med 2001; 135:423–433Crossref, Medline, Google Scholar
60. Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L: Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend 2002; 67:219–223Crossref, Medline, Google Scholar
61. Cunningham JA, Ferrence R, Cohen J, Adlaf EM: Interest in self-help materials among a general population sample of smokers. Addict Behav 2003; 28:811–816Crossref, Medline, Google Scholar
62. Lerman C, Niaura R, Collins BN, Wileyto P, Audrain-McGovern J, Pinto A, Hawk L, Epstein LH: Effects of bupropion on depression symptoms in a smoking cessation clinical trial. Psychol Addict Behav 2004; 18:362–366Crossref, Medline, Google Scholar
63. Wewers ME, Dhatt R, Tejwani GA: Naltrexone administration affects ad libitum smoking behavior. Psychopharmacology (Berl) 1998; 140:185–190Crossref, Medline, Google Scholar
64. Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, Niaura RS, Sirota AD: Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 1999; 142:139–143Crossref, Medline, Google Scholar
65. Sutherland G, Stapleton JA, Russell MA, Feyerabend C: Naltrexone, smoking behaviour and cigarette withdrawal. Psychopharmacology (Berl) 1995; 120:418–425Crossref, Medline, Google Scholar
66. Covey LS, Glassman AH, Stetner F: Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis 1999; 18:31–40Crossref, Medline, Google Scholar
67. David S, Lancaster T, Stead LF: Opioid antagonists for smoking cessation. Cochrane Database Syst Rev 2001;(3):CD003086Google Scholar
68. Ahmadi J, Ashkani H, Ahmadi M, Ahmadi N: Twenty-four week maintenance treatment of cigarette smoking with nicotine gum, clonidine and naltrexone. J Subst Abuse Treat 2003; 24:251–255Crossref, Medline, Google Scholar
69. Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD: A randomized trial of naltrexone for smoking cessation. Addiction 1999; 94:1227–1237Crossref, Medline, Google Scholar
70. Krishnan-Sarin S, Meandzija B, O’Malley S: Naltrexone and nicotine patch in smoking cessation: a preliminary study. Nicotine Tob Res 2003; 5:851–857Crossref, Medline, Google Scholar
71. Niaura R, Spring B, Borrelli B, Hedeker D, Goldstein MG, Keuthen N, DePue J, Kristeller J, Ockene J, Prochazka A, Chiles JA, Abrams DB: Multicenter trial of fluoxetine as an adjunct to behavioral smoking cessation treatment. J Consult Clin Psychol 2002; 70:887–896Crossref, Medline, Google Scholar
72. Spring B, Wurtman J, Wurtman R, el-Khoury A, Goldberg H, McDermott J, Pingitore R: Efficacies of dexfenfluramine and fluoxetine in preventing weight gain after smoking cessation. Am J Clin Nutr 1995; 62:1181–1187Crossref, Medline, Google Scholar
73. Blondal T, Gudmundsson LJ, Tomasson K, Jonsdottir D, Hilmarsdottir H, Kristjansson F, Nilsson F, Bjornsdottir US: The effects of fluoxetine combined with nicotine inhalers in smoking cessation—a randomized trial. Addiction 1999; 94:1007–1015Crossref, Medline, Google Scholar
74. Norregaard J, Tonnesen P, Petersen L: Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Prev Med 1993; 22:261–271Crossref, Medline, Google Scholar
75. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB: Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA 1994; 271:589–594Crossref, Medline, Google Scholar
76. Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, Benowitz N: Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med 2004; 140:426–433Crossref, Medline, Google Scholar
77. Evans WE, Relling MV: Pharmacogenomics: translating functional genomics into rational therapeutics. Science 1999; 286:487–491Crossref, Medline, Google Scholar
78. Poolsup N, Li Wan Po A, Knight TL: Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther 2000; 25:197–220Crossref, Medline, Google Scholar
79. Lerman C, Niaura R: Applying genetic approaches to the treatment of nicotine dependence. Oncogene 2002; 21:7412–7420Crossref, Medline, Google Scholar
80. Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, Griffiths SE, Walton RT: Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics 2004; 14:83–90Crossref, Medline, Google Scholar
81. Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E, Murphy M, Griffiths S, Walton R: Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ 2004; 328:989–990Crossref, Medline, Google Scholar
82. Balfour DJ: Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Curr Drug Targets CNS Neurol Disord 2002; 1:413–421Crossref, Medline, Google Scholar
83. Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH: The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J 2004; 4:184–192Crossref, Medline, Google Scholar
84. Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L: Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA 1998; 95:9608–9613Crossref, Medline, Google Scholar
85. Crowley JJ, Oslin DW, Patkar AA, Gottheil E, DeMaria PA Jr, O’Brien CP, Berrettini WH, Grice DE: A genetic association study of the mu opioid receptor and severe opioid dependence. Psychiatr Genet 2003; 13:169–173Crossref, Medline, Google Scholar
86. Oslin D, Berrettini WH, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP: A functional polymorphism in the mu opioid receptor gene is associated with therapeutic response in alcohol-dependent patients treated with naltrexone. Neuropsychopharmacology 2003; 28:1546–1552Crossref, Medline, Google Scholar
87. Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A, Kucharski S, Krishnan S, Niaura R, Epstein LH: Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol 2003; 22:541–548Crossref, Medline, Google Scholar
88. Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Murdter TE, Roots I, Brockmoller J: Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics 2003; 13:619–626Crossref, Medline, Google Scholar
89. Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF: Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology 2003; 45:122–132Crossref, Medline, Google Scholar
90. Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, Boyd NR, Shields PG: Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol 1999; 18:14–20Crossref, Medline, Google Scholar
91. Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D: The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 1996; 6:73–79Crossref, Medline, Google Scholar
92. Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, Sirota LA, Marcus SE, Greenberg BD, Lucas FR IV, Benjamin J, Murphy DL, Hamer DH: A genetic association for cigarette smoking behavior. Health Psychol 1999; 18:7–13Crossref, Medline, Google Scholar
93. Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, Amos CI, Wan Y, Cinciripini P, Hong WK, Wu X: Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 1998; 90:358–363Crossref, Medline, Google Scholar
94. Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ: Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991; 48:648–654Crossref, Medline, Google Scholar
95. Ritchie T, Noble EP: [3H]Naloxone binding in the human brain: alcoholism and the Taq1 A D2 dopamine receptor polymorphism. Brain Res 1996; 718:193–197Crossref, Medline, Google Scholar
96. Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA: D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997; 7:479–484Crossref, Medline, Google Scholar
97. Anokhin AP, Todorov AA, Madden PA, Grant JD, Heath AC: Brain event-related potentials, dopamine D2 receptor gene polymorphism, and smoking. Genet Epidemiol 1999; 17(suppl 1):S37-S42Google Scholar
98. Blum K, Braverman ER, Dinardo MJ, Wood RC, Sheridan PJ: Prolonged P300 latency in a neuropsychiatric population with the D2 dopamine receptor A1 allele. Pharmacogenetics 1994; 4:313–322Crossref, Medline, Google Scholar
99. Noble EP: The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000; 1:309–333Crossref, Medline, Google Scholar
100. Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S: The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 2001; 1:152–156Crossref, Medline, Google Scholar
101. Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR: Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000; 22:133–139Crossref, Medline, Google Scholar
102. David SP, Niaura R, Papandonatos GD, Shadel WG, Burkholder GJ, Britt DM, Day A, Stumpff J, Hutchison K, Murphy M, Johnstone E, Griffiths SE, Walton RT: Does the DRD2-Taq1 A polymorphism influence treatment response to bupropion hydrochloride for reduction of the nicotine withdrawal syndrome? Nicotine Tob Res 2003; 5:935–942Crossref, Medline, Google Scholar
103. Cinciripini P, Wetter D, Tomlinson G, Tsoh J, De Moor C, Cinciripini L, Minna J: The effects of the DRD2 polymorphism on smoking cessation and negative affect: evidence for a pharmacogenetic effect on mood. Nicotine Tob Res 2004; 6:229–239Crossref, Medline, Google Scholar
104. Froom P, Melamed S, Benbassat J: Smoking cessation and weight gain. J Fam Pract 1998; 46:460–464Medline, Google Scholar
105. Hall SM, Ginsberg D, Jones RT: Smoking cessation and weight gain. J Consult Clin Psychol 1986; 54:342–346Crossref, Medline, Google Scholar
106. Lerman C, Berrettini W, Pinto A, Patterson F, Crystal-Mansour S, Wileyto EP, Restine SL, Leonard DG, Shields PG, Epstein LH: Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl) 2004; 174:571–577Medline, Google Scholar
107. Gauderman J: Sample size requirements for matched case-control studies of gene-environment interactions. Stat Med 2002; 21:35–50Crossref, Medline, Google Scholar
108. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH: Smoking and mental illness: a population-based prevalence study. JAMA 2000; 284:2602–2610Google Scholar
109. De Leon J, Diaz F, Rogers T, Browne D, Dinsmore L: Initiation of daily smoking and nicotine dependence in schizophrenia and mood disorders. Schizophr Res 2002; 56:47–54Crossref, Medline, Google Scholar
110. Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C, Gault J, Lee MJ, Logel J, Olincy A, Ross RG, Stevens K, Sullivan B, Vianzon R, Virnich DE, Waldo M, Walton K, Freedman R: Smoking and mental illness. Pharmacol Biochem Behav 2001; 70:561–570Crossref, Medline, Google Scholar
111. George TP, Vessicchio JC, Termine A, Bregartner AF, Rounsaville BJ, Kosten TR: A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry 2002; 52:53–61Crossref, Medline, Google Scholar
112. George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, Rounsaville BJ, Kosten TR: Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000; 157:1835–1842Link, Google Scholar
113. Shields AE, Blumenthal D, Weiss KB, Comstock CB, Currivan D, Lerman C: Barriers to translating emerging genetic research on smoking into clinical practice: perspectives of primary care physicians. J Gen Intern Med 2005; 20:131–138Crossref, Medline, Google Scholar
114. Shields AE, Lerman C, Sullivan P: Translating emerging research on the genetics of smoking into clinical practice: ethical and social considerations. Nicotine Tob Res 2004; 6:675–688Crossref, Medline, Google Scholar



