Secondary Mania in Older Adults
Although mania is commonly associated with bipolar disorder, it can have many etiologies (1). Thus, “primary mania” results from bipolar disorder, whereas “secondary mania” results from pharmacological, metabolic, or neurologic causes (1, 2). Older adults are at risk for secondary mania because of increased medical comorbidities and neurological changes. In one retrospective study of 50 patients with mania who were older than 65 years, it was the first manic episode for 28% of the patients and 71% had a comorbid neurological disorder (3).
The etiology of mania is important because although acute symptomatic treatment of both primary and secondary mania may be similar, appropriate treatment of secondary mania includes addressing the cause (1). We present here two case histories of secondary mania in older adults, discuss their presentations and differential diagnosis in turn, and discuss treatment.
Case 1
Past History
Ms. A, a 67-year-old married African American woman with no previous psychiatric history, was seen for an acute manic episode with psychotic symptoms. She had been in her usual state of health until 3 days before admission, when she developed an abnormally elated mood accompanied by delusions and racing thoughts.
The patient’s medical history was remarkable for a history of well-controlled hypertension, a resection of a left parieto-occipital meningioma, and a three-vessel coronary bypass graft for angina 4 years earlier. She reported two episodes of transient slurred speech, one just before the meningioma resection and the other 4 months before she was seen for the manic episode. Her usual medications included extended-release nifedipine (60 mg/day), ticlopidine (250 mg t.i.d.), and benazepril (20 mg/day).
Present Illness
Approximately 3 weeks before her hospital admission, Ms. A reported that she had contracted an upper respiratory tract infection with a cough and had begun treatment with intermittent doses of pseudoephedrine, 60 mg, plus hydrocodone, 5 mg. Her infectious symptoms were not improving, so clarithromycin was added. After 7 days of clarithromycin therapy, Ms. A arose from her bed earlier than usual and announced to her daughter, “I feel free! I feel alive!” These statements were accompanied by an elated mood and an unprecedented interest in the Bible. The family reported this was a marked change, as Ms. A was typically rather quiet and reserved.
Over the next 2 days, Ms. A became increasingly hyperverbal and would quote scripture while she ran through the house with her hands up in the air. She began frantically writing a lengthy and disorganized missive to God in which she apologized for past sins and transgressions. Her manic symptoms persisted and escalated to include emotional effusiveness, overfamiliarity, sleeplessness, and the development of delusions and auditory hallucinations. She asserted that she was receiving messages from Bob Hope through the television, appeared suspicious of family members when they expressed concern, and was unwilling to undergo an outpatient magnetic resonance imaging (MRI) scan because she thought she was under arrest. She described hearing voices of individuals from previous “waking” dreams. There was no history of confusion, disorientation, stereotyped motor activity, or changes in level of consciousness.
Ms. A was first admitted to the neurology service for evaluation and management, and she was subsequently transferred to the inpatient psychiatry service. There were no remarkable physical or neurological findings. During the mental status examination Ms. A was noted to be an attentive, cooperative woman who appeared her stated age and had a lively, engaging demeanor and normal motor activity. Her speech was mildly pressured and tangential, requiring redirection to guide her back to the question. She described herself as “better than ever—excellent,” and her affect was congruent with her mood. She described ideas of reference in that characters on television shows were making special references to her recent religious enlightenment. She denied further auditory hallucinations or paranoid delusions. Notably, her cognitive abilities were not impaired, and she scored 28 out of 30 on the Mini-Mental State Examination (MMSE) (4), although she exhibited some errors on confrontation naming, e.g., “tie” for a “tassel.”
Ms. A’s serum chemistry, cerebrospinal fluid and cultures, urinalysis, blood cultures, and chest X-ray were unremarkable. The hematology profile was notable for a mild normocytic anemia with a hematocrit of 33.0 ml/dl. Her erythrocyte sedimentation rate was slightly elevated at 48 mm/hour. Her level of thyroid-stimulating hormone was low at 0.23 μU/ml (normal=0.39–0.45) with a normal free T4 level. A brain computed tomography (CT) scan showed evidence only of her past meningioma resection. A brain MRI revealed encephalomalacia related to the previous surgery as well as abnormal hyperintensities in the brain stem, periventricular matter, and deep white matter. These hyperintensities most likely represented chronic ischemic changes. An electroencephalogram (EEG) showed slow sharp waves in the area of the surgical resection but no epileptiform activity or other abnormal patterns.
Low-dose haloperidol was administered from the beginning of Ms. A’s stay in the psychiatry unit and was tapered off by the time of discharge. Throughout her hospital stay, her manic and psychotic symptoms gradually dissipated. At the time of discharge, Ms. A was taking clonazepam, 0.5 mg b.i.d., which was tapered and discontinued after discharge. Her family members reported that her disposition at the time of discharge was somewhat brighter than her baseline but not remarkably so.
Discussion
Although bipolar disorder can have a late onset in persons over the age of 50 without a previous psychiatric history or a family history of bipolar disorder (5–8), new-onset mania in older adults is most commonly secondary (1, 2). Mania in older adults tends to be more debilitating than in younger adults, as evidenced by the lower scores on the Global Assessment Scale scores of older manic patients (9). In addition, patients whose first manic episode is after the age of 58 exhibit increased cognitive impairment, which is partially reversible (9, 10). Thus, it is important to rule out delirium and dementia. Mania is typically characterized by an abnormally elevated or irritable mood lasting at least 1 week. It may be accompanied by one or more of the following: grandiosity, decreased need for sleep, increased talkativeness, flight of ideas, distractibility, increased goal-directed activity, psychomotor agitation, and excess involvement in potentially harmful activities, all of which lead to a marked decrease in the level of functioning. These features distinguish themselves from delirium, in which the cardinal feature is a waxing and waning alteration in consciousness accompanied by a change in cognition (e.g., disorientation or memory or language disturbances). The symptoms of delirium may be accompanied by changes in affect, such as anxiety and fear. Differentiation between the two is accomplished by longitudinal observation as well as monitoring for affective changes. This patient had manic symptoms without any waxing or waning of consciousness or other evidence of delirium, which suggests a diagnosis of mania.
The cognitive dysfunction that often accompanies mania in older adults (11) may suggest a diagnosis of dementia. However, there are differences in the presentations and premorbid histories of dementia and mania. Agitation and psychosis in dementia are typically phenomena that occur later in the course of illness, rather than in the initial presentation (12, 13). Agitation without psychosis occurs in dementia and may be manifest as “sundowning,” which is commonly defined as increased agitation and restlessness beginning in the late afternoon and extending to early evening (14).
Most important, dementia would likely be preceded by changes in cognitive abilities in the absence of affective symptoms. The cognitive changes of dementia usually occur over years, in contrast to those of mania, which are abrupt and accompanied by affective symptoms. After a period of agitation, the cognitive abilities of the demented patient may improve slightly but will still be markedly impaired, given the likely advanced stage of dementia. The nondemented manic patient would tend to recover mostly from the cognitive impairment (15). Comprehensive neuropsychological testing of Ms. A was not performed because of her relatively normal score on the MMSE. It is interesting that Ms. A did not exhibit the pronounced cognitive deficits described by Young (11). Had she exhibited such deficits, dementia would have been ruled out on the basis of her premorbid history.
Because Ms. A had a history of a meningioma resection, it is possible that her mania was related to seizure activity. Mania may occur in epileptic patients during interictal periods and can last up to 8 weeks (16). This finding highlights the fact that mania in epilepsy need not be associated with the disturbances of a seizure but perhaps the brain insult itself. For Ms. A, seizure would be a reasonable avenue to pursue because there were several factors that could have given rise to epileptic foci. Her meningioma resection could provide such a focus, although its occipital location makes it unlikely that a related seizure would give rise to her behavioral changes. Epileptic foci can originate from stroke—a very real possibility in this older patient with a history of vascular disease, cardiac bypass surgery, and two possible transient ischemic attacks. Neuroimaging studies, however, did not reveal any evidence of stroke, nor did the EEG reveal any epileptiform activity.
Although Ms. A and her family reported no history of falls, she was taking nifedipine, ticlopidine, and benazepril. All of these agents are capable of causing hypotensive episodes. If Ms. A had an episode of orthostasis, she may have fallen and sustained a head injury. Indeed, in a 1-year follow-up study of 66 subjects with closed-head injuries, 9% experienced manic episodes, and many of them had basal temporal lesions (17). Damage to the hypothalamus has been associated with lasting, rapidly fluctuating moods (18). Mania after head injury has mostly been described in case reports after closed-head injuries and postsurgical intervention for subdural hematomas. In one case series, the average onset of mania after head injury was 2.8 years, with a range of 0–12 years, and irritable euphoria and assaultive behavior were common symptoms (19). Some researchers have found a preponderance of right-sided lesions following mania related to head injury (20), but there have been isolated case reports of mania following left hemispheric lesions (21). The CT showed no evidence of contusion or skull fracture for Ms. A.
It was important to rule out endocrine disorders in this older female patient, as endocrine abnormalities, such as thyroid disorders, should be considered in older patients with acute mental status changes. Classically, hypothyroidism is associated with mental slowing and depression, but it can lead to florid psychosis (22), as can hyperthyroidism (23, 24). It is interesting that the degree of hypothyroidism appears to be unrelated to the degree of psychiatric symptoms in patients who are psychotic because of hypothyroidism (25).
Antibiotics can cause mania in older adults, which raised the possibility that clarithromycin was the source of Ms. A’s mania. For this reason, her clarithromycin was stopped at admission. There are several reports of secondary mania apparently induced by clarithromycin (26–30). This could be a side effect of this class of medications (albeit an infrequent one), as other macrolides have been reported to be associated with mania (27). Older adults may be more vulnerable to such effects not only because they are more likely to receive antibiotics but also because slower P450 microenzyme metabolism could result in higher plasma levels of the drug. For example, older adults metabolize clarithromycin more slowly than do younger adults (31, 32). The mechanism behind antibiotic-induced mania is unclear but could be related to γ-aminobutyric acid (GABA) antagonism. There is evidence that ciprofloxacin is a GABA antagonist (33). Clarithromycin may have led to CNS disinhibition brought about by GABA antagonism, but we know of no documentation of GABA antagonism by clarithromycin.
The short-term treatment of Ms. A required only low-dose haloperidol and clonazepam. As her symptoms subsided, both of these medications were tapered and discontinued. Because the presumptive etiological agent, clarithromycin, was removed, Ms. A did not require continuing therapy with a mood stabilizer.
Case 2
Past History
Mr. B, a 60-year-old man with no past psychiatric history, was involuntarily admitted after being seen in a clinic with insomnia, increased energy level, pressured speech, tangential thinking, and grandiose delusions. He had been married and divorced twice, with no children, and was living alone in his own home. He was employed as a freelance sports journalist. Although he claimed to have unusually close relationships with several women, there was no evidence so support this claim; he did not meet criteria for a diagnosis of erotomania. On a recent business trip he spent several hundred dollars on clothing to “catch the ladies’ eyes” and had his eyebrow and tongue pierced as he thought this would make him more attractive.
Mr. B had been diagnosed with hypertension but was untreated. He acknowledged episodes of depression in the past, but none had required hospitalization. He denied abuse of alcohol or illicit substances in the past, and the only remarkable aspect of his family psychiatric history was that his brother was diagnosed with panic disorder.
Present Illness
Mr. B was admitted to the inpatient psychiatry service, where he continued to display manic symptoms for approximately 4 days while his medication doses were being titrated, all the while requesting a “decongestant for the brain.” His speech was pressured with some clanging, and his affect was superficial, dysphoric, and tearful at times. Mr. B felt he possessed special powers; he claimed that he was a “sounder,” which he described as a person who can see into the future, and that he had the ability to “run the United Nations.” He stated that his powers “make quantum leaps look like picnics.” His rapid thought processes led him to feel that the rest of the world was slow, to the point that he felt telephones dialed too slowly. At times Mr. B experienced auditory hallucinations of music and television commercials.
The results of serum chemistries, a complete blood count, liver function tests, and thyroid function tests were all within normal limits. The results of a fluorescent treponema antibody absorption test and a urine drug screen were negative.
During a workup for his manic episode, a CT scan revealed a right-sided heterogenous, partially cystic, and calcified mass in the medial aspect of the right temporal lobe. Differential diagnosis included a giant aneurysm dermoid/epidermoid lesion, a glioma, and a nerve sheath tumor, such as a meningioma or an atypical schwannoma. An MRI with gadolinium performed 2 days later revealed a well-circumscribed extra-axial mass 3.4 cm (anterior-posterior) by 3.0 cm (transverse) by 3.0 cm (craniocaudal), which extended into the right foramen ovale, medial to the right temporal lobe (Figure 1). The neurosurgery service was consulted and opted to debulk the tumor in approximately 2 months.
Mr. B’s drug doses were titrated to 20 mg/day of olanzapine and 1500 mg b.i.d. of divalproex sodium. By the ninth day of his hospitalization he insisted on being discharged to his own home with outpatient follow-up. His mental status examination was markedly improved with euthymic mood, no abnormal movements, and logical and goal-directed thought processes, without psychosis or thoughts of harming himself or others.
Follow-Up
Mr. B underwent a right pterional craniotomy, and the mass was resected. The psychiatry consultation service followed him closely during his hospital stay. Postoperatively his recovery was complicated by pneumonia and some dysphagia. He was treated with several antibiotics and transferred to the rehabilitation medicine service, where his mental status continued to improve.
Discussion
Older patients with new-onset mania include individuals who have a history of depression as well as those without any past psychiatric history (34). It was possible that Mr. B’s clinical picture was one of first-episode mania in bipolar disorder. However, as in case 1, it was important to rule out other contributing factors.
For a 60-year-old man with impulsive behaviors (piercing his eyebrow and tongue), one should consider the possibility of substance abuse even though the patient may deny it, as this patient did. Although substance abuse is often associated with younger adults, it must be ruled out in older adults with mania (5). Although we know of no specific data regarding the incidence of substance-induced manic syndromes among older adults, older adults are likely more sensitive to the effects of illicit substances, such as amphetamines, methamphetamine, and cocaine. We did not find any evidence of substance abuse in this patient.
Although Mr. B had a history of depressed mood, he was not taking an antidepressant. If he had been, it would have been important to bear in mind that several psychotropic medications can cause mania. Indeed, some researchers have found that older adults are more likely to have initial manic episodes from antidepressant therapy than are younger adults (35). Tricyclic antidepressants have long been recognized as a risk factor for secondary mania (36). The mechanism underlying this association is unknown but could be related to noradrenergic activity. Venlafaxine, which is a norepinephrine reuptake inhibitor at higher doses (37), has been associated with mania (38). However, some selective serotonin reuptake inhibitors, such as paroxetine (39) and fluoxetine (40), have been associated with manic episodes in younger adults yet have relatively little norepinephrine reuptake inhibition (37). Thus, mania may be induced by receptor activity that is not related to antidepressant action.
Paradoxically, several atypical antipsychotics (olanzapine, risperidone, quetiapine, and ziprasidone), which are approved for treatment of bipolar disorder and/or mania, have been associated with mania (41). In a critical review of 33 reported cases, Rachid et al. (41) concluded that there was “strong evidence” to support a causal link. They discussed the hypothesis that secondary mania induced by atypical antipsychotics may reflect potent blockade of serotonin 5-HT2A but not dopamine D2 receptors. This pattern of receptor activity could presumably lead to frontal disinhibition.
In a review of 50 consecutive psychiatric admissions for mania of people over the age of 65, Tohen et al. (3) found that 12 of 14 cases of first-episode mania were related to a neurological disorder or infection, most commonly stroke. Fujikawa et al. (44) also suggested that most cases of secondary mania in older adults result from stroke. However, the incidence of poststroke mania is low and has been estimated at 1% of all strokes (43). We considered the possibility that Mr. B had had a stroke in light of his untreated hypertension, although in the absence of any focal deficits this would be unlikely. Indeed, there was no evidence of stroke on the CT or MRI.
In the course of the neurological workup for Mr. B, the right-sided mass was discovered. Older adults with new-onset mania are more than twice as likely to have an underlying neurological disorder as are older bipolar patients who have had many manic episodes (3, 44). Although mania is not a common presentation of cerebral tumors, of six patients who developed mania either before or after the removal of a tumor, five had tumors that were frontal or temporal in location and often in the right hemisphere (45). This patient’s right-sided tumor is in keeping with these observations.
As Mendez (46) discussed, a variety of brain lesions have been reported as correlates of mania. Bilateral orbitofrontal and right temporoparietal (47, 48), right basal and medial temporal lobe (49), basal ganglia (50), thalamic (51), and right frontotemporal (52) lesions have all been associated with mania. A young patient with bilateral dorsomedial thalamic lesions exhibited a secondary mania, and a single photon emission computed tomography (SPECT) study revealed hypoperfusion of the bilateral prefrontal regions (53). Subcortical arteriosclerotic encephalopathy (Binswanger’s disease) led to first-episode mania in a 65-year-old man (54). Subcortical hyperintensities have been reported in geriatric patients with mania (11). Jorge et al. (17) found that temporal basal polar lesions were a commonality underlying secondary mania after traumatic brain injury. Jorge et al. reported that this association was significant even after they accounted for lesions in other areas of the brain.
The exact mechanism by which brain insult leads to mania is unclear, although there is evidence of associations between right-sided lesions and mania (46, 55). Fenn and George (56) reported an instance in which a left-sided temporal infarct preceded the first episode of mania in a 78-year-old man. Several researchers have argued that right orbitofrontal damage is the sine qua non of secondary mania (45, 48, 57). Case reports of mania associated with other lesions are consistent with the argument for right orbitofrontal damage, in that there could be disruption of the pathways between limbic or prefrontal areas and other deeper structures, such as the basal ganglia, thalamus, and hypothalamus (50, 58).
Treatment Considerations
The symptomatic treatment of secondary mania in older adults is relatively similar to the treatment of primary mania, but proper treatment demands a determination of the etiology of secondary mania. Here we shall discuss treatment options for behavioral management of acute mania in older adults. Regardless of the agent used, secondary mania typically does not require prophylaxis, as does primary mania.
For acute agitation associated with secondary mania, benzodiazepines and antipsychotics are reasonable choices. Benzodiazepines may be used in the treatment of acute agitation associated with secondary mania, but one must use them cautiously in older adults. Aging tends to slow the oxidative metabolic pathways in the liver, so benzodiazepines that are metabolized through conjugated processes, which are not impaired, are preferred. Thus, a shorter-acting benzodiazepine that is metabolized conjugatively, such as lorazepam, would be a suitable choice.
Atypical antipsychotics lessen many of the complications of typical antipsychotics, but they can cause sedation. Although the Food and Drug Administration (FDA) does not differentiate between primary and secondary mania, it seems reasonable to use atypical antipsychotics while bearing in mind the recent FDA warning regarding death and atypical antipsychotics in older adults. The consensus guidelines on the use of antipsychotics for older adults suggest that a preferred treatment of mania is an atypical antipsychotic and a mood stabilizer (52). Further, the consensus guidelines indicate that the preferred medications could be chosen from risperidone, quetiapine, and olanzapine and, in some instances, aripiprazole as well (52). The major factors influencing selection are the presence of complicating medical conditions, such as constipation, diabetes, etc.
Mood stabilizers, such as divalproex sodium or lithium, are viable treatment options but tend to have more side effects for older adults. Unfortunately, both medications can cause sedation and nausea. Lithium can be especially problematic in older patients because they are more likely to take nonsteroidal antiinflammatory drugs as well, which would reduce the renal clearance of lithium. Moreover, lithium can lead to hypothyroidism. Older adults are often more sensitive to side effects of medications than are younger adults, so doses should be lowered accordingly. Mania associated with structural central nervous system disease may respond better to valproate or carbamazepine (59). To our knowledge, the newer anticonvulsant agents topiramate and lamotrigine have not been studied in this particular patient population (59), and lamotrigine is less desirable because of its protracted titration period. Overall, unless the patient has hepatic failure, divalproex is a reasonable choice for treatment when a mood stabilizer is needed.
Conclusions
Secondary mania in older adults is a serious medical condition that requires a comprehensive differential diagnosis. Older adults are more susceptible to disorders that can lead to secondary mania, so a thorough past psychiatric history is essential. Late-onset bipolar disorder is possible, but it is not the most likely etiology in older adults (3). New-onset mania in older adults calls for neuroimaging studies to rule out tumor and stroke as causes. Pharmacological treatment of the acute condition is largely the same as for primary mania but with doses lower than those for younger adults because of older adults’ slower metabolism and sensitivity to side effects (9). Because secondary mania generally does not require prophylactic treatment, it is questionable whether treatment with divalproex, lithium, or carbamazepine is necessary. Fortunately, the majority of deficits that accompany secondary mania in older adults resolve if the etiology is determined and treated.
Received Feb. 4, 2005; revision received May 23, 2005; accepted May 27, 2005. From the Psychiatry Service, Palo Alto VA Health Care System, Palo Alto, Calif.; and the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine. Address correspondence and reprint requests to Dr. Brooks, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305; [email protected] (e-mail). The authors thank Stephanie Woodard for her assistance with the manuscript.
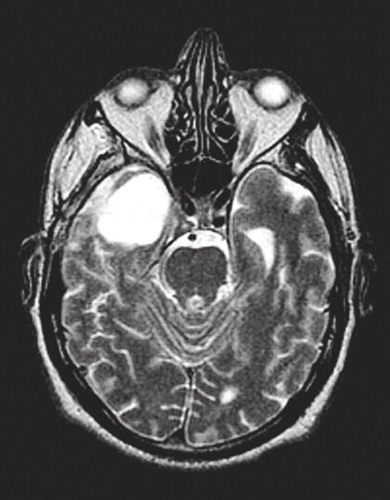
Figure 1. MRI of the Brain Showing a Right-Sided Mass in a 60-Year-Old Man With Secondary Mania
1.. Krauthammer C, Klerman GL: Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry 1978; 35:1333–1339Crossref, Medline, Google Scholar
2.. Hoblyn JC, Brooks JO: Herbal supplements in older adults: consider interactions and adverse events that may result from supplement use. Geriatrics 2005; 60:18, 22–23Google Scholar
3.. Tohen M, Shulman KI, Satlin A: First-episode mania in late life. Am J Psychiatry 1994; 151:130–132Link, Google Scholar
4.. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
5.. Depp CA, Jin H, Mohamed S, Kaskow J, Moore DJ, Jeste DV: Bipolar disorder in middle-aged and elderly adults: is age of onset important? J Nerv Ment Dis 2004; 192:796–799Crossref, Medline, Google Scholar
6.. Depp CA, Jeste DV: Bipolar disorder in older adults: a critical review. Bipolar Disord 2004; 6:343–367Crossref, Medline, Google Scholar
7.. Almeida OP, Fenner S: Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr 2002; 14:311–322Crossref, Medline, Google Scholar
8.. Moorhead SR, Young AH: Evidence for a late onset bipolar-I disorder sub-group from 50 years. J Affect Disord 2003; 73:271–277Crossref, Medline, Google Scholar
9.. Young RC, Klerman GL: Mania in late life: focus on age at onset. Am J Psychiatry 1992; 149:867–876Link, Google Scholar
10.. Mirchandani IC, Young RC: Management of mania in the elderly: an update. Ann Clin Psychiatry 1993; 5:67–77Crossref, Medline, Google Scholar
11.. Young RC: Geriatric mania. Clin Geriatr Med 1992; 8:387–399Crossref, Medline, Google Scholar
12.. Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, Habeych M, DeKosky ST: Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2003; 15:346–353Crossref, Medline, Google Scholar
13.. Schneider LS, Dagerman KS: Psychosis of Alzheimer’s disease: clinical characteristics and history. J Psychiatr Res 2004; 38:105–111Crossref, Medline, Google Scholar
14.. Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A: Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry 2001; 158:704–711Link, Google Scholar
15.. Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M: Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270Link, Google Scholar
16.. Kudo T, Ishida S, Kubota H, Yagi K: Manic episode in epilepsy and bipolar I disorder: a comparative analysis of 13 patients. Epilepsia 2001; 42:1036–1042Crossref, Medline, Google Scholar
17.. Jorge RE, Robinson RG, Starkstein SE, Arndt SV, Forrester AW, Geisler FH: Secondary mania following traumatic brain injury. Am J Psychiatry 1993; 150:916–921Link, Google Scholar
18.. Hoheisel HP, Walch R: Uber manisch-depressive und verwandte Verstimmungszustande nach Hirnverletztung. Arch Psychiatr Nervenkr 1952; 188:1–25Crossref, Google Scholar
19.. Shukla S, Cook BL, Mukherjee S, Godwin C, Miller MG: Mania following head trauma. Am J Psychiatry 1987; 144:93–96Link, Google Scholar
20.. Shulman KI, Herrmann N: Bipolar disorder in old age. Can Fam Physician 1999; 45:1229–1237Medline, Google Scholar
21.. Lim LC: Mania following left hemisphere injury. Singapore Med J 1996; 37:448–450Medline, Google Scholar
22.. Heinrich TW, Grahm G: Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003; 5:260–266Crossref, Medline, Google Scholar
23.. Nath J, Sagar R: Late-onset bipolar disorder due to hyperthyroidism. Acta Psychiatr Scand 2001; 104:72–73Crossref, Medline, Google Scholar
24.. Irwin R, Ellis PM, Delahunt J: Psychosis following acute alteration of thyroid status. Aust NZ J Psychiatry 1997; 31:762–764Crossref, Medline, Google Scholar
25.. Jain VK: Affective disturbance in hypothyroidism. Br J Psychiatry 1971; 119:279–280Crossref, Medline, Google Scholar
26.. Abouesh A, Hobbs WR: Clarithromycin-induced mania (letter). Am J Psychiatry 1998; 155:1626Medline, Google Scholar
27.. Abouesh A, Stone C, Hobbs WR: Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol 2002; 22:71–81Crossref, Medline, Google Scholar
28.. Cone LA, Sneider RA, Nazemi R, Dietrich EJ: Mania due to clarithromycin therapy in a patient who was not infected with human immunodeficiency virus. Clin Infect Dis 1996; 22:595–596Crossref, Medline, Google Scholar
29.. Nightingale SD, Koster FT, Mertz GJ, Loss SD: Clarithromycin-induced mania in two patients with AIDS. Clin Infect Dis 1995; 20:1563–1564Crossref, Medline, Google Scholar
30.. Ortiz-Dominguez A, Berlanga C, Gutierrez-Mora D: A case of clarithromycin-induced manic episode (antibiomania). Int J Neuropsychopharmacol 2004; 7:99–100Crossref, Medline, Google Scholar
31.. Chu SY, Wilson DS, Guay DR, Craft C: Clarithromycin pharmacokinetics in healthy young and elderly volunteers. J Clin Pharmacol 1992; 32:1045–1049Crossref, Medline, Google Scholar
32.. Fraschini F, Scaglione F, Demartini G: Clarithromycin clinical pharmacokinetics. Clin Pharmacokinet 1993; 25:189–204Crossref, Medline, Google Scholar
33.. Green MA, Halliwell RF: Selective antagonism of the GABA(A) receptor by ciprofloxacin and biphenylacetic acid. Br J Pharmacol 1997; 122:584–590Crossref, Medline, Google Scholar
34.. Van Gerpen MW, Johnson JE, Winstead DK: Mania in the geriatric patient population: a review of the literature. Am J Geriatr Psychiatry 1999; 7:188–202Crossref, Medline, Google Scholar
35.. Jain H, Young RC: Antidepressants and late life mania, in 1998 Annual Meeting of the Society for Neuroscience Abstracts. Washington, DC, Society for Neuroscience, 1988Google Scholar
36.. Young RC, Jain H, Kiosses DN, Meyers BS: Antidepressant-associated mania in late life. Int J Geriatr Psychiatry 2003; 18:421–424Crossref, Medline, Google Scholar
37.. Stahl SM: Essential Psychopharmacology, 2nd ed. Cambridge, UK, Cambridge University Press, 2000Google Scholar
38.. Yuksel FV, Basterzi AD, Goka E: Venlafaxine-induced mania. Can J Psychiatry 2004; 49:786–787Medline, Google Scholar
39.. Morishita S, Arita S: Induction of mania in depression by paroxetine. Hum Psychopharmacol 2003; 18:565–568Crossref, Medline, Google Scholar
40.. Nakra BR, Szwabo P, Grossberg GT: Mania induced by fluoxetine (letter). Am J Psychiatry 1989; 146:1515–1516Medline, Google Scholar
41.. Rachid F, Bertschy G, Bondolfi G, Aubry JM: Possible induction of mania or hypomania by atypical antipsychotics: an updated review of reported cases. J Clin Psychiatry 2004; 65:1537–1545Crossref, Medline, Google Scholar
42.. Tohen M, Castillo J, Pope HG, Herbstein J: Concomitant use of valproate and carbamazepine in bipolar and schizoaffective disorders. J Clin Psychopharmacol 1994; 14:67–70Crossref, Medline, Google Scholar
43.. Starkstein SE, Boston JD, Robinson RG: Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis 1988; 176:87–100Crossref, Medline, Google Scholar
44.. Fujikawa T, Yamawaki S, Touhouda Y: Silent cerebral infarctions in patients with late-onset mania. Stroke 1995; 26:946–949Crossref, Medline, Google Scholar
45.. Robinson RG, Starkstein SE: Neuropsychiatric aspects of cerebrovascular disorders, in Textbook of Neuropsychiatry. Edited by Hales RE, Yudofsky SC. Washington, DC, American Psychiatric Press, 1997, pp 607–633Google Scholar
46.. Mendez MF: Mania in neurologic disorders. Curr Psychiatry Rep 2000; 2:440–445Crossref, Medline, Google Scholar
47.. Bakchine S, Lacomblez L, Benoit N, Parisot D, Chain F, Lhermitte F: Manic-like state after bilateral orbitofrontal and right temporoparietal injury: efficacy of clonidine. Neurology 1989; 39:777–781Crossref, Medline, Google Scholar
48.. Starkstein SE, Robinson RG: Mechanism of disinhibition after brain lesions. J Nerv Ment Dis 1997; 185:108–114Crossref, Medline, Google Scholar
49.. Bornke C, Postert T, Przuntek H, Buttner T: Acute mania due to a right hemisphere infarction. Eur J Neurol 1998; 5:407–409Crossref, Google Scholar
50.. Tureki G, Mari JDJ, Porto JAD: Bipolar disorder following a left basal ganglia stroke (letter). Br J Psychiatry 1993; 163:690Crossref, Medline, Google Scholar
51.. Robinson RG: Mood disorders secondary to stroke. Semin Clin Neuropsychiatry 1997; 2:244–251Medline, Google Scholar
52.. Gafoor R, O’Keane V: Three case reports of secondary mania: evidence supporting a right frontotemporal locus. Eur Psychiatry 2003; 18:32–33Crossref, Medline, Google Scholar
53.. Benke T, Kurzthaler I, Schmidauer Ch, Moncayo R, Donnemiller E: Mania caused by a diencephalic lesion. Neuropsychologia 2002; 40:245–252Crossref, Medline, Google Scholar
54.. Hain C, Peter K: [Initial manifestation of a manic syndrome in advanced age in subcortical arteriosclerotic encephalopathy (Binswanger disease)]. Psychiatr Prax 1999; 26:305–307 (German)Medline, Google Scholar
55.. Shulman KI: Disinhibition syndromes, secondary mania and bipolar disorder in old age. J Affect Disord 1997; 46:175–182Crossref, Medline, Google Scholar
56.. Fenn D, George K: Post-stroke mania late in life involving the left hemisphere. Aust NZ J Psychiatry 1999; 33:598–600Crossref, Medline, Google Scholar
57.. Robinson RG, Boston JD, Starkstein SE, Price TR: Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry 1988; 145:172–178Link, Google Scholar
58.. Cummings JL: Organic psychoses: delusional disorders and secondary mania. Psychiatr Clin North Am 1986; 9:293–311Crossref, Medline, Google Scholar
59.. Evans DL: Bipolar disorder: diagnostic challenges and treatment considerations. J Clin Psychiatry 2000; 61(suppl 13):26–31Google Scholar



