Efficacy and Tolerability of Citalopram in the Treatment of Late-Life Anxiety Disorders: Results From an 8-Week Randomized, Placebo-Controlled Trial
Abstract
OBJECTIVE: Anxiety disorders are highly prevalent in elderly persons. However, to date, the efficacy of selective serotonin reuptake inhibitors (SSRIs) for the treatment of anxiety disorders in this age group has not been established. METHOD: Thirty-four participants age 60 and older with a DSM-IV anxiety disorder (mainly generalized anxiety disorder) and a Hamilton Anxiety Rating Scale score of 17 or higher were randomly assigned under double-blind conditions to either citalopram or placebo. Response was defined as a score of 1 (very much improved) or 2 (much improved) on the Clinical Global Improvement scale or a 50% reduction in the Hamilton anxiety scale score. Response and side effects with citalopram and placebo were compared by using chi-square tests and linear modeling. RESULTS: Eleven (65%) of the 17 citalopram-treated participants responded by 8 weeks, versus four (24%) of the 17 placebo-treated participants. The most common and problematic side effect in the citalopram group was sedation. CONCLUSIONS: The authors believe this to be the first prospective controlled study to test the efficacy of an SSRI in the management of anxiety disorders among the elderly. These results support the efficacy of citalopram in late-life anxiety disorders. They need to be replicated in a larger study group.
Epidemiologic studies of community-living elderly have shown that some anxiety disorders are common. The prevalence of generalized anxiety disorder among the elderly is 3.7%–7.4% (1–4), which is on par with prevalence estimates of that disorder in young adults. Furthermore, this disorder in elderly persons is associated with impairments in quality of life, increased health care utilization, and poorer functional recovery after disabling medical events (such as stroke) (5, 6).
Despite these data, there is evidence that these anxiety disorders in elderly persons are undertreated (7) and that treatment rates have not improved over time, as has been seen with late-life depression (8). This lack of treatment may be secondary to the scant pharmacotherapy research on elderly persons with anxiety disorders. In the past 20 years, of the medicines available in the United States only benzodiazepines and buspirone have been studied in prospective, controlled trials, to our knowledge (9, 10). As benzodiazepines are known to cause cognitive and psychomotor impairments in the elderly, leading to falls and fractures, they are suboptimal for older persons (11–17). Similarly, buspirone is not often used clinically, in part because of the high comorbidity of major depressive disorder with anxiety. Selective serotonin reuptake inhibitors (SSRIs) are clearly efficacious for anxiety disorders in younger adults (18, 19); however, we know of no published studies of SSRI treatment for late-life anxiety disorders, other than a small, open-label study with fluvoxamine (20) and our previous evaluations of comorbid anxiety in late-life depression (21, 22). As these investigations suggested that SSRIs may be helpful in improving anxiety symptoms in elderly persons, the present study was conducted to determine the efficacy of citalopram, a commonly used SSRI, for the treatment of elderly persons with acute anxiety disorders. We hypothesized that citalopram would be superior to placebo in bringing about a clinically significant reduction in symptom burden.
Method
Adults age 60 years and older were recruited for a double-blind, randomized, placebo-controlled 8-week study of citalopram. Participants were recruited from the community through advertisements and referrals and from a primary care site in Pittsburgh. Primary care recruitment was done by two methods: elicitation of clinical referrals from primary care practitioners and systematic screening of patients at the practice by a sampling procedure previously described (23). Participants who screened positive for anxiety symptoms were invited to meet with study personnel. The study was approved by the University of Pittsburgh’s institutional review board, and all participants gave written informed consent before participation. The participants underwent assessments with the Structured Clinical Interview for DSM-IV, administered by raters (E.J.L., B.T.) trained for reliability. All participants were required to meet the criteria for a DSM-IV anxiety disorder, and each was given a principal diagnosis of generalized anxiety disorder (N=30), panic disorder (N=3), or posttraumatic stress disorder (PTSD) (N=1). All participants scored 17 or higher on the Hamilton Anxiety Rating Scale (24), administered by using a structured instrument to optimize reliability (25). Those meeting the criteria for a current major depressive episode were excluded, as such subjects were recruited for other studies by our group. Other reasons for exclusion were dementia, history of psychosis, unstable medical illness, and active alcohol or substance abuse. The participants were also assessed at baseline with the Hamilton Depression Rating Scale (26), the Mini-Mental State Examination (27), and scales for instrumental and physical activities of daily living (28).
Medication use was determined by subject report. To improve recruitment and retention of a representative clinical study group, any participant taking a benzodiazepine before the start of the study was allowed to continue taking an equipotent dose of lorazepam (maximum, 2 mg/day) as long as the dose was kept constant; no other psychotropic medication was allowed for at least 2 weeks before the beginning of the study and during the study. This decision was based on routine clinical practice, in that elderly persons are the leading consumers of benzodiazepines (29, 30) and these medications are clinically noted to be difficult to withdraw until after patients are successfully treated with another medication.
The participants were seen weekly during the first 4 weeks of the trial and biweekly thereafter. Supportive management was offered during the trial, as described previously (31), but no formal psychotherapy was provided. The subjects were randomly assigned to citalopram or placebo on the basis of a computer program that was developed at our institution and uses stratified permuted block randomization. Medication was provided in a double-blind fashion such that the citalopram dose was started at 10 mg/day and increased after 1 week to 20 mg/day. A further increase to 30 mg was made after 4 weeks if the participant did not achieve response by that time. Side effects were measured by using the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale, a 48-item scale that was developed to measure somatic and psychic side effects of antidepressant treatment (32). It has four subscales: neurologic, psychic, autonomic, and other. The scale is scored at baseline (before the start of medication) and at all follow-up visits. Side effects were also measured on the basis of the participants’ reports in response to the question “Have you had any side effects to the study medication since the last visit?” The primary outcome measures were the Hamilton anxiety scale and the Clinical Global Improvement scale (CGI), both administered by independent evaluators blind to treatment condition. Scores on the Hamilton anxiety scale were obtained at all visits. CGI improvement scores were obtained only at weeks 4 and 8. A participant was declared a responder on the basis of a 50% reduction in Hamilton anxiety score or a CGI rating of 1 or 2.
The rates of response and side effects were compared in the citalopram and placebo groups by using two-tailed chi-square tests and linear modeling. Because a greater proportion of men were randomly assigned to the placebo group, a Cochran-Mantel-Haenszel test (33) was performed to test group differences while stratifying by gender. Following an intent-to-treat principle, for participants who dropped out we used data from the last available visit to determine response. The two groups were compared in terms of change in Hamilton anxiety score by using a mixed-effect repeated-measures linear model (intercept and time were random components). The same model was used to compare the groups in terms of side effects as assessed with the UKU Side Effect Rating Scale.
Results
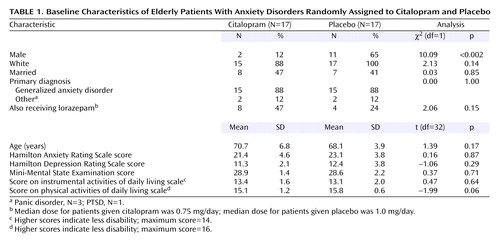
We screened or assessed a total of 791 elderly persons from a primary care practice or the community. From this group, 47 persons signed consent forms and were extensively evaluated for anxiety disorders (17 responded to advertisements or word of mouth, 21 were referred, and nine were primary practice patients). Of these 47 participants, 10 refused randomization and three were excluded (one had spontaneous improvement of anxiety before randomization, one did not meet the diagnostic criteria for any anxiety disorder other than specific phobia, and one was in a major depressive episode). Thus, 34 participants were randomly assigned, stratified by diagnosis, 17 each to citalopram and placebo. Comorbidity was common: of the 30 subjects with a primary diagnosis of generalized anxiety disorder, 17 had at least one current or past comorbid disorder (current or past specific phobia, N=8; past major depressive disorder, N=6; current panic disorder, N=5; current or past social phobia, N=3; current or past obsessive-compulsive disorder, N=2; current depressive disorder not otherwise specified, N=1; current hypochondriasis, N=1; current dysthymic disorder, N=1; current PTSD, N=1). Of the three subjects with a primary diagnosis of panic disorder, all had additional disorders (current or past social phobia, N=3; current or past specific phobia, N=2; current dysthymic disorder, N=2; past PTSD, N=1). The subject with PTSD also had specific phobia and past major depressive disorder. There were no differences in demographic characteristics or baseline clinical measures except for sex, with more men randomly assigned to placebo (Table 1).
Most of the participants—29 (85%) of 34—completed the 8-week study. Five dropped out before week 8 (three taking citalopram and two taking placebo), only one because of side effects (one patient receiving citalopram experienced intolerable sedation after one dose). Eleven of the 17 citalopram-treated participants responded, for a response rate of 65%, with a 95% confidence interval (CI) of 42%–87%, compared to a response rate of 24% (95% CI=3%–44%) for the placebo group (four of 17 participants). These rates were found by using either response criterion: 50% reduction in Hamilton anxiety score or CGI rating of 1 or 2 (i.e., all responders met both response criteria). The probability of response was significantly greater for the participants assigned to citalopram (χ2=5.86, df=1, p<0.02), for a relative risk of response of 2.57 for citalopram (95% CI=1.05–6.27). Further, the difference in response rates remained significant when we controlled for gender (Cochran-Mantel-Haenszel test: χ2=4.22, df=1, p<0.04; Breslow-Day test for homogeneity of the odds ratios: χ2=0.46, df=1, p=0.50, which demonstrated that the assumptions for the statistical test were met). When we evaluated response in terms of reduction in Hamilton anxiety score to 10 or less, we found that eight of the 17 citalopram-treated subjects, versus three of the 17 who received placebo, attained this level of remission of symptoms (χ2=3.36, df=1, p=0.07). Similarly, a mixed-effect linear model of Hamilton anxiety scale scores over the 8 weeks of treatment demonstrated a significant time effect (F=34.90, df=1, 30, p<0.0001), indicating a general decrease of anxiety over time, and a treatment-by-week interaction (F=3.79, df=1, 145, p=0.05), indicating greater improvement in anxiety in the citalopram group than in the placebo group (Figure 1); this improvement showed an effect size (Cohen’s d) of 0.79 (95% CI=0.01–1.46) (34).
Because most participants had generalized anxiety disorder, we repeated the same analysis for only those with generalized anxiety disorder. We again found a significant difference in response rate between citalopram (10 of 15, or 67%) and placebo (four of 15, or 27%) (χ2=4.82, df=1, p<0.03).
The rates of reported side effects were similar in the two groups. Twelve of the 17 citalopram-treated participants complained of at least one side effect, versus nine of the 17 placebo-treated participants. The most common side effects in both groups were dry mouth, nausea, and fatigue (N=3 each for the citalopram group and N=3, N=2, and N=2, respectively, for the placebo group). We also assessed side effects with the UKU Side Effect Rating Scale. A mixed-effect linear model of the total scores over time showed a nearly significant group-by-week interaction (F=2.99, df=1, 144, p<0.09), suggesting a decrease in side effects in the citalopram group and no change over time in the placebo group (Figure 2). An evaluation of the four subscales of the UKU Side Effect Rating Scale by mixed-effect linear models did not show any significant differences between the citalopram and placebo groups (data not shown).
As 12 (35%) of the 34 subjects received fixed doses of coprescribed lorazepam, we compared the response rates of those receiving and not receiving lorazepam. We found no differences in response attributable to this medication (data not shown). Of these 12 subjects, two had been switched to lorazepam from alprazolam, rather than continuing previous treatment; both of these subjects were randomly assigned to citalopram, and both exited the study early as nonresponders.
Discussion
To our knowledge, this is the first report of a controlled prospective study of an SSRI for treating anxiety disorders in elderly persons. Our data suggest that citalopram is efficacious in the management of acute anxiety disorders in this age group, as the participants had a clinically and statistically significant improvement in anxiety symptoms over 8 weeks of treatment. The improvement in symptoms cannot be attributed to coprescription of a benzodiazepine, which was kept at a steady and low dose during the study in those who were already taking benzodiazepines.
These data are consistent with findings by Katz et al. (35), who found in a retrospective analysis of industry data that extended-release venlafaxine was as efficacious and well tolerated in elderly as in younger persons. In the present study, citalopram was well tolerated, with side effects similar to those for placebo. The reduction in overall side effects (as measured by the UKU Side Effect Rating Scale) during treatment with citalopram may reflect improvement in anxiety-related somatization, as has been seen in the treatment of late-life depression with comorbid anxiety (21).
Three limitations should be noted. First, our small study group and the preponderance of recruitment through advertisements and referrals may limit the generalizability of the results; for example, the confidence interval of the effect size is wide. Second, our randomization failed to produce equal gender proportions in the citalopram and placebo groups, and while we did statistically control for this, these results may nevertheless reflect lower treatment responsiveness of anxiety disorder symptoms in men than in women. Finally, while our study group had some diagnostic heterogeneity, the vast majority of participants had generalized anxiety disorder, reflective of the high prevalence of this diagnosis in elderly persons, and all participants met the symptomatic threshold (based on Hamilton anxiety scale score) for moderate to severe symptoms. Because of these limitations, we recommend that these results be replicated in a setting representative of anxious nondepressed elderly persons (such as in primary care) with a larger study group so that effect sizes can be more precisely estimated and subgroup analyses can determine the generalizability of treatment response (36). Notwithstanding these limitations, this study suggests that, as in younger people, SSRIs are efficacious and well tolerated in the treatment of anxiety disorders in elderly persons.
 |
Received Dec. 30, 2003; revision received Feb. 19, 2004; accepted March 4, 2004. From the Intervention Research Center for Late-Life Mood Disorders, Department of Psychiatry, University of Pittsburgh School of Medicine and VA Pittsburgh Healthcare System. Address correspondence and reprint requests to Dr. Lenze, Western Psychiatric Institute and Clinic, Rm. E1124, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by an investigator-initiated grant from Forest Pharmaceuticals and by NIMH grants K23 MH-64196, P30 MH-52247, and MH-65416.
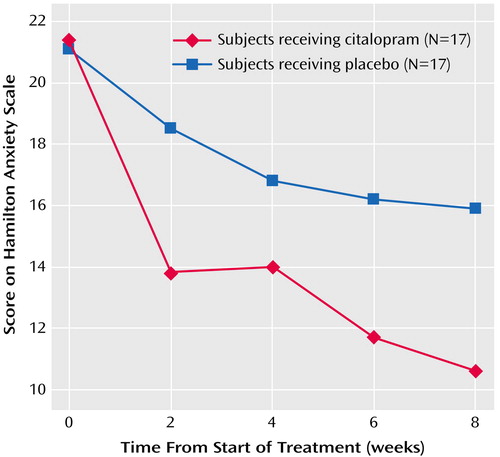
Figure 1. Changes in Hamilton Anxiety Rating Scale Scores for Elderly Patients With Anxiety Disorders Who Received Citalopram or Placeboa
aScore improvement was greater in the citalopram group than the placebo group (group-by-time interaction: p=0.05, mixed-effect linear model).
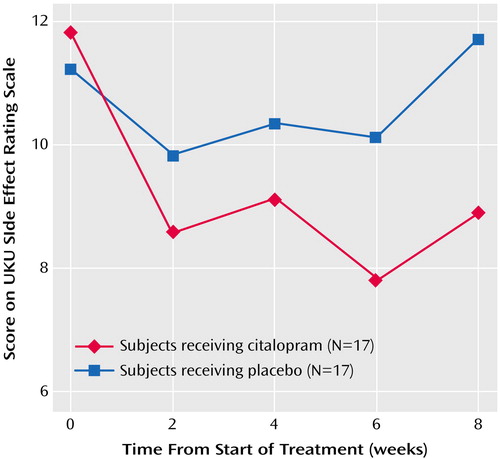
Figure 2. Changes in Scores on the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale for Elderly Patients With Anxiety Disorders Who Received Citalopram or Placeboa
aNonsignificant difference between groups in change in score over time (group-by-time interaction: p=0.09, mixed-effect linear model).
1. Lindesay J, Briggs K, Murphy E: The Guy’s/Age Concern survey: prevalence rates of cognitive impairment, depression and anxiety in an urban elderly community. Br J Psychiatry 1989; 155:317–329Crossref, Medline, Google Scholar
2. Manela M, Katona C, Livingston G: How common are the anxiety disorders in old age? Int J Geriatr Psychiatry 1996; 11:65–70Crossref, Google Scholar
3. Beekman A, Bremmer MA, Deeg D, Van Balkom A, Smit J, DeBeurs E, Vandyck R, van Tilburg W: Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry 1998; 13:717–726Crossref, Medline, Google Scholar
4. Uhlenhuth EH, Balter MB, Mellinger GD, Cisin IH, Clinthorne J: Symptom checklist syndromes in the general population: correlations with psychotherapeutic drug use. Arch Gen Psychiatry 1983; 40:1167–1173Crossref, Medline, Google Scholar
5. de Beurs E, Beekman ATF, van Balkom AJLM, Deeg DJH, Van Dyck R, van Tilburg W: Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med 1999; 29:583–593Crossref, Medline, Google Scholar
6. Astrom M: Generalized anxiety disorder in stroke patients: a 3-year longitudinal study. Stroke 1996; 27:270–275Crossref, Medline, Google Scholar
7. Harman JS, Rollman BL, Hanusa BH, Lenze EJ, Shear MK: Physician office visits of adults for anxiety disorders in the United States: 1985–1998. J Gen Intern Med 2002; 17:165–172Crossref, Medline, Google Scholar
8. Harman JS, Mulsant BH, Kelleher KJ, Schulberg HC, Kupfer DJ, Reynolds CF III: Narrowing the gap in treatment of depression. Int J Psychiatry Med 2001; 31:239–253Crossref, Medline, Google Scholar
9. Koepke HH, Gold RL, Linden ME, Lion JR, Rickels K: Multicenter controlled study of oxazepam in anxious elderly outpatients. Psychosomatics 1982; 23:641–645Crossref, Medline, Google Scholar
10. Bohm C, Robinson DS, Gammans RE, Shrotriya RC, Alms DR, Leroy A, Placchi M: Buspirone therapy in anxious elderly patients: a controlled clinical trial. J Clin Psychopharmacol 1990; 10(3 suppl):47S-51SGoogle Scholar
11. Ray WA, Griffin MR, Downey W: Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA 1989; 262:3303–3307Crossref, Medline, Google Scholar
12. Salzman C, Fisher J, Nobel K, Glassman R, Wolfson A, Kelley M: Cognitive improvement following benzodiazepine discontinuation in elderly nursing home residents. Int J Geriatr Psychiatry 1992; 7:89–93Crossref, Google Scholar
13. Herings RMC, Stricker BHC, deBoer A, Bakker A, Sturmans F: Benzodiazepines and the risk of falling leading to femur fractures. Arch Intern Med 1995; 155:1801–1807Crossref, Medline, Google Scholar
14. Liu BA, Topper AK, Reeves RA, Gryfe C, Maki BE: Falls among older people: relationship to medication use and orthostatic hypotension. J Am Geriatr Soc 1995; 43:1141–1145Crossref, Medline, Google Scholar
15. Hemmelgarn B, Suissa S, Huang A, Boivin J-F, Pinard G: Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA 1997; 278:27–31Crossref, Medline, Google Scholar
16. Hanlon JT, Horner RD, Schmader KE, Fillenbaum GG, Lewis IK, Wall WE, Landerman LR, Pieper CF, Blazer DG, Cohen HJ: Benzodiazepine use and cognitive function among community-dwelling elderly. Clin Pharmacol Ther 1998; 64:684–692Crossref, Medline, Google Scholar
17. Pomara N, Tun H, DaSilva D, Hernando R, Deptula D, Greenblatt DJ: The acute and chronic performance effects of alprazolam and lorazepam in the elderly: relationship to duration of treatment and self-rated sedation. Psychopharmacol Bull 1998; 34:139–153Medline, Google Scholar
18. Davidson J, Bose A, Su G: Escitalopram in the treatment of generalized anxiety disorder, in Proceedings of the XII World Congress of Psychiatry. New York, World Psychiatric Association, 2002Google Scholar
19. Casacalenda N, Boulenger JP: Pharmacologic treatments effective in both generalized anxiety disorder and major depressive disorder: clinical and theoretical implications. Can J Psychiatry 1998; 43:722–730Crossref, Medline, Google Scholar
20. Wylie ME, Miller MD, Shear MK, Little JT, Mulsant BH, Pollock BG, Reynolds CF III: Fluvoxamine pharmacotherapy of anxiety disorders in later life: preliminary open-trial data. J Geriatr Psychiatry Neurol 2000; 13:43–48Crossref, Medline, Google Scholar
21. Lenze EJ, Mulsant BH, Dew MA, Shear MK, Houck P, Pollock BG, Reynolds CF III: Good treatment outcomes in late-life depression with comorbid anxiety. J Affect Disord 2003; 77:247–254Crossref, Medline, Google Scholar
22. Mulsant BH, Reynolds CF III, Shear MK, Sweet RA, Miller M: Comorbid anxiety disorders in late-life depression. Anxiety 1996; 2:242–247Crossref, Medline, Google Scholar
23. Mulsant BH, Alexopoulos GS, Reynolds CF III, Katz IR, Abrams R, Oslin D, Schulberg HC: Pharmacologic treatment of depression in older primary care patients: the PROSPECT algorithm. Int J Geriatr Psychiatry 2001; 16:585–592Crossref, Medline, Google Scholar
24. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
25. Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM: Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety 2001; 13:166–178Crossref, Medline, Google Scholar
26. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
27. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
28. Fillenbaum GG: Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
29. Gleason PP, Schulz R, Smith NL, Newsom JT, Kroboth PD, Kroboth FJ, Psaty BM: Correlates and prevalence of benzodiazepine use in community-dwelling elderly. J Gen Intern Med 1998; 13:243–250Crossref, Medline, Google Scholar
30. van Balkom AJ, Beekman AT, de Beurs E, Deeg DJ, Van Dyck R, van Tilburg W: Comorbidity of the anxiety disorders in a community-based older population in the Netherlands. Acta Psychiatr Scand 2000; 101:37–45Crossref, Medline, Google Scholar
31. Miller MD, Frank E, Reynolds CF III: The art of clinical management in pharmacologic trials with depressed elderly patients: lessons from the Pittsburgh Study of Maintenance Therapies in Late-Life Depression. Am J Geriatr Psychiatry 1999; 7:228–234Crossref, Medline, Google Scholar
32. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K: The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 334:1–100Crossref, Medline, Google Scholar
33. Mantel N, Haenszel W: Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748Medline, Google Scholar
34. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
35. Katz IR, Reynolds CF III, Alexopoulos GS, Hackett D: Venlafaxine ER as a treatment for generalized anxiety disorder in older adults: pooled analysis of five randomized placebo-controlled clinical trials. J Am Geriatr Soc 2002; 50:18–25Crossref, Medline, Google Scholar
36. Kraemer HC, Wilson T, Fairburn CG, Agras WS: Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002; 59:877–883Crossref, Medline, Google Scholar



