Impact of Sleep Deprivation and Subsequent Recovery Sleep on Cortisol in Unmedicated Depressed Patients
Abstract
OBJECTIVE: One night of sleep deprivation induces a transient improvement in about 60% of depressed patients. Since depression is associated with abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis, the authors measured cortisol secretion before, during, and after therapeutic sleep deprivation for 1 night. METHOD: Fifteen unmedicated depressed inpatients participated in a combined polysomnographic and endocrine study. Blood was sampled at 30-minute intervals during 3 consecutive nights before, during, and after sleep deprivation. Saliva samples were collected at 30-minute intervals during the daytime before and after the sleep deprivation night. RESULTS: During the night of sleep deprivation, cortisol levels were significantly higher than at baseline. During the daytime, cortisol levels during the first half of the day were higher than at baseline in the patients who responded to sleep deprivation but not in the nonresponders. During recovery sleep, cortisol secretion returned to baseline values. CONCLUSIONS: This study demonstrated a significant stimulatory effect of 1 night of sleep deprivation on the HPA axis in unmedicated depressed patients. The results suggest that the short-term effects of antidepressant treatments on the HPA axis may differ from their long-term effects. A higher cortisol level after sleep deprivation might transiently improve negative feedback to the hypothalamus or interact with other neurotransmitter systems, thus mediating or contributing to the clinical response. The fast return to baseline values coincides with the short clinical effect.
One night of sleep deprivation induces a rapid, albeit transient, improvement of mood in about 60% to 70% of patients with major depressive disorder (1, 2). The clinical usefulness of the procedure is limited by the fact that more than 80% of unmedicated depressed patients who respond positively to a night of sleep deprivation experience a relapse into depression after the next night of sleep (1) or even after a brief nap (3).
The mechanism by which sleep deprivation exerts its antidepressant effect is still unknown. Presently, there is no commonly accepted hypothesis concerning the mechanism of action of sleep deprivation nor an explanation for the observation that subsequent sleep after sleep deprivation leads to relapse.
One of the most robust biological abnormalities in depression is an altered regulation of corticotropin (ACTH) and cortisol secretory activity in the majority of patients (for overview, see reference 4). It has been suggested that changes in the corticotropin-releasing factor (CRF) systems are a crucial aspect in the pathophysiology of major depression (5) (for an overview, see reference 6). In addition, it has been suggested that antidepressants act through normalization of the hypothalamic-pituitary-adrenal (HPA) axis dysregulation (7) (for a review, see reference 8). This hypothesis is confounded by the fact that many antidepressants have a direct stimulatory effect on the activity of the HPA axis independent of psychopathological state (9–12). Therefore, it is of interest whether therapeutic sleep deprivation—especially if successful—interferes with the activity of the HPA axis in depressed patients.
Up to now, the studies on the effect of sleep deprivation on HPA axis activity were mainly performed with healthy subjects. Whereas earlier studies showed little effect of sleep deprivation on the HPA axis (13, 14), later investigations demonstrated a significant elevation of cortisol after sleep deprivation (15). Vgontzas and co-workers (16) reported a significant reduction of cortisol after sleep deprivation in healthy humans during the postdeprivation nighttime period. These authors proposed that a reduction of CRF and cortisol secretion might be the mechanism through which sleep deprivation relieves depression temporarily. However, in their study cortisol was not measured during sleep deprivation but in the postdeprivation night. Patients with major depression who improve after sleep deprivation usually have a relapse after the postdeprivation night. To our knowledge, up to now there have been no studies of depressed patients in which HPA activity was measured not only during a night of sleep deprivation or the day after but also during subsequent sleep.
It was therefore the primary objective of this study to determine precisely the impact of total sleep deprivation on cortisol secretion in patients with major depressive disorder during the night of sleep deprivation and the ensuing 24 hours in comparison to baseline conditions before sleep deprivation. The hypothesis was that the HPA axis is dampened by sleep deprivation in patients who respond to sleep deprivation but quickly returns to baseline levels during the recovery night.
Method
The study protocol was approved by the ethical committee of the University of Freiburg. Written informed consent was obtained from all subjects before study entry, after the procedure had been fully explained. We included 19 depressed inpatients, age 19 to 58 years, who were diagnosed as having major depression according to the Structured Clinical Interview for DSM-III-R (17) and who scored at least 18 points on the Hamilton Depression Rating Scale (18). The exclusion criteria were intake of any psychotropic drugs during and at least 1 week before the study, substance abuse, suicidality, history of endocrine disorders, pregnancy, postpartum depression, lactation, and any sleep disorder other than depression-related insomnia. The drug-free interval was longer than 4 weeks for all but one patient, who stopped doxepin intake 7 days before the study. Four patients dropped out, one because of problems with the blood withdrawal procedure and two because of exclusion criteria that had not been recognized at the time of inclusion. One patient withdrew her consent during the study, before the sleep deprivation night. Another patient felt worse on the morning after the sleep deprivation night, and her participation was terminated prematurely. However, her endocrine data were included in the analysis. The remaining patients (nine female and six male patients) had a mean age of 34.1 years (SD=13.4). The mean score on the 21-item Hamilton depression scale at study entry was 24.0 (SD=5.8), and the mean body mass index was 22.6 kg/m2 (SD=3.7). Ten of the 15 patients reported diurnal variations of mood, all with improvement in the evening.
Each patient was given a physical and neurological examination, routine serum chemistry and hematology studies, an ECG and EEG, and a urine analysis to measure intake of illicit drugs or benzodiazepines.
Procedures
The study was carried out in the sleep laboratory of the Department of Psychiatry and Psychotherapy of the University of Freiburg. On nights 1 and 2, the patients were allowed to adapt to the laboratory conditions, and baseline polysomnographic sleep measures were obtained.
During nights 3, 4, and 5, blood was sampled at 30-minute intervals from 9:30 p.m. until 8.30 a.m. the next morning. The samples were collected through an indwelling catheter placed in a forearm vein 3 hours before the beginning of blood sampling on each of the 3 days. Between samplings the catheter was perfused by isotonic saline at a low speed (25 ml/hour). Between 11:00 p.m. and 7:00 a.m. the samples were taken from an adjoining room through polyethylene tubing. The total amount of blood collected per night was 110 ml.
From the evening of day 3 until the morning after the last night, the subjects stayed in the research ward. During the 40 hours, the experimental conditions were standardized as follows: standardized meals at 8:00 a.m., 12:30 p.m., and 6:00 p.m. on both days, no major physical activities, and a semisupine position in bed or a sitting position in a large armchair, which allowed reading or watching television. The subjects were strictly advised not to sleep and were therefore constantly observed by a team of three research fellows (including J.J.).
On days 4 and 5, saliva was collected at 30-minute intervals from 8:00 a.m. until 10:00 p.m. by using small cotton swabs that the subjects chewed for 30–60 seconds (for details of this method, see reference 19).
Serum and saliva cortisol levels were measured by radioimmunoassay using commercial kits with intra- and interassay coefficients of variations of less than 10%. All measurements were made in duplicate. The serum and saliva samples were stored at –80°C.
Sleep recordings were performed during all 4 nights from “lights out” (11:00 p.m.) to “lights on” (7:00 a.m.) according to standard criteria (the method of polysomnography in our laboratory is described in reference 20).
Depression Ratings
The 21-item Hamilton depression scale was administered at the beginning of the investigation (18). Since the 21-item version is not suitable for repeated measurements in a sleep deprivation experiment, an abbreviated version with six items was used to measure depressive mood on the days before and after the sleep deprivation night as well as on the day after the recovery night at 9:00 a.m. and 6:00 p.m. The six-item Hamilton depression scale has a maximum score of 22 points and includes the following items: depressed mood, guilt feelings, decreased work ability and interest in usual activities, psychomotor retardation, anxiety, and physical symptoms (21). Antidepressant response to sleep deprivation was defined as at least a 30% improvement in the average of the 9:00 a.m. to 6:00 p.m. scores on the six-item Hamilton depression scale on the day after sleep deprivation compared with the day before sleep deprivation.
Statistical Analysis
For descriptive purposes, means and standard deviations were calculated. To compare cortisol levels during and after sleep deprivation with baseline values, the area under the curve was calculated as the sum of all concentration values within the selected time interval (the first and the last value divided by 2) multiplied by the sampling interval (0.5 hours). Areas under the curve were calculated for the interval from 11:00 p.m. to 7:00 a.m., which corresponds to the registration time of polysomnography in nights 3 and 5, and for first and the second halves of the night, i.e., from 11:00 p.m. to 3:00 a.m. and from 3:00 a.m. to 7:00 a.m., respectively. Areas under the curve for saliva cortisol secretion during the daytime were calculated for the whole period between 8:00 a.m. and 10:00 p.m. as well as for the first half and the second half of the day, i.e., from 8:00 a.m. to 3:00 p.m. and from 3:00 p.m. to 10:00 p.m., respectively. Statistical analysis was performed by analysis of variance (ANOVA) for repeated measurements, and group (responders versus nonresponders to sleep deprivation) was used as a factor. In the case of statistically obvious differences (p<0.10), two-tailed Student’s t tests for dependent samples were calculated for comparisons of the 3 consecutive nights and of the days before and after sleep deprivation; p<0.05 was considered to be significant.
Results
Antidepressant Effects of Sleep Deprivation
Eight out of the 15 patients were classified as responders to sleep deprivation (mean age=37.5 years, SD=13.1), showing an at least 30% improvement of the averaged values on the six-item Hamilton depression scale on the day after the sleep deprivation night. Seven were classified as nonresponders (mean age=30.3, SD=13.7). Four of the eight responders did not maintain this 30% improvement on the day after the recovery night.
Cortisol Secretion
During the night of sleep deprivation mean serum cortisol secretion was higher than at baseline (Figure 1), whereas during the recovery night the mean cortisol concentrations were comparable to those for the baseline night. ANOVAs detected significant effects of night on the areas under the curve for the whole period between 11:00 p.m. and 7:00 a.m. and for the first half of the night (11:00 p.m. to 3:00 a.m.) but not for the second half of the night (3:00 a.m. to 7:00 a.m.) (Table 1). There were no significant group or interaction effects, i.e., no evidence for a difference in endocrine response between responders and nonresponders to sleep deprivation. There was no impact of gender on the activation response. Further statistical evaluation by t tests revealed that during sleep deprivation the mean area under the curve for serum cortisol was significantly higher than during the night before and during the night after sleep deprivation (Table 1). During the recovery night, the mean area under the curve was slightly but not significantly lower than during the baseline night.
The mean curves for saliva cortisol concentrations (Table 2) were similar during the day after sleep deprivation and the day before. There was a slight, nonsignificant tendency toward higher concentrations during the first half of the day after sleep deprivation than during the comparable period of the day before sleep deprivation, but this did not extend to the second half of the day. ANOVA did not detect a significant impact of day or group (Table 2). There was a nearly significant interaction effect (p=0.10) for the first half of the day. Individual t tests demonstrated that the responders to sleep deprivation had significantly higher cortisol concentrations between 8:00 a.m. and 3:00 p.m. on the day after sleep deprivation than on the day before, whereas no parallel difference was present in the nonresponders (Figure 2, Table 2).
Discussion
This study demonstrates that 1 night of therapeutic sleep deprivation slightly but significantly increases nocturnal cortisol secretion in patients with a major depressive episode. In the group as a whole, sleep deprivation did not enhance integrated cortisol levels during the daytime to levels higher than those on the day before. The responders to sleep deprivation, however, exhibited a significant rise of cortisol during the first half of the day, whereas nonresponders did not.
Regardless of the fact that our results were obtained in depressed patients, who might differ from healthy humans with respect to some functional aspects of the endocrine system, the stimulatory effect of sleep deprivation on the HPA axis in general is in line with earlier findings in healthy humans. These also demonstrated significant increases of cortisol secretion in response to total sleep deprivation for 1 night (15, 22–24). The magnitude of cortisol elevation in the depressed patients was similar to that found in healthy subjects (23, 24, and our own unpublished data). As in our responders to sleep deprivation, in healthy subjects the cortisol elevation persisted the next day (23, 24), whereas in the study by Leproult and co-workers (15) a significant increase of cortisol was observed only in the evening after sleep deprivation.
Our results are not consistent with earlier findings on the impact of sleep deprivation on the HPA axis in depressed patients. The results in many of these studies, however, are not comparable with those in the present investigation because of methodological factors. Some investigators took one blood sample after sleep deprivation (25, 26). In some studies, patients were not free of medication (26, 27). Parry and co-workers (28) investigated the effects of sleep deprivation on cortisol in subjects with premenstrual dysphoric disorder and in normal comparison subjects and did not find quantitative effects on cortisol secretion. However, they performed only partial sleep deprivation, in the first or second half of the night. Goetze and Toelle (29), who investigated depressed patients receiving medication, measured the 24-hour rhythms of free urinary cortisol over 5 days, including 1 night of sleep deprivation, and found enhanced cortisol levels in response to sleep deprivation. A similar finding was reported by Bouhuys and co-workers (30), who obtained urine samples every 3 hours over a 24-hour period after 1 night’s sleep deprivation in unmedicated patients and found an increase of cortisol, which, however, was not related to clinical response. In a second study by Baumgartner et al. (31), cortisol was measured during a night of sleep deprivation. An increase in cortisol was found in responders but not in nonresponders to sleep deprivation. A similar result, yet in a very small group of depressed patients, was also reported by Gerner et al. (32). We believe that our study is the first to compare cortisol concentrations from frequent sampling in 3 consecutive nights before, during, and after sleep deprivation in unmedicated depressed patients. In terms of a general activation of HPA activity following sleep deprivation, our results agree with the findings of Goetze and Toelle (29) and Bouhuys et al. (30).
We did not find that cortisol secretion during recovery sleep was significantly lower than during the baseline night. Therefore, we could not confirm the hypothesis of Vgontzas et al. (16), who reported a significant reduction of cortisol in healthy subjects during recovery sleep after sleep deprivation, in comparison to a baseline night before sleep deprivation, and who proposed that this decrease might be the mechanism through which sleep deprivation relieves depression temporarily.
Since sleep deprivation improves mood in depressed patients, it is noteworthy that the procedure activates the HPA axis over the short term, since remission of depressive symptoms is associated with a normalization of HPA axis dysfunction (8, 33–35). The data from our study suggest that the short-term effects of antidepressant treatments on HPA activity may differ from, and may even be in the opposite direction from, their medium- or long-term effects. The same issue was also addressed by Kling et al. (36), who measured HPA activity after electroconvulsive therapy (ECT) and found a significant increase of HPA activity in response to ECT, which also confirmed earlier findings by Aperia et al. (37) and Rudorfer et al. (38). One might assume that initial activation of HPA activity leads to a transient enhancement of negative feedback on the axis, leading to reduced CRF secretion. This assumption is supported by the findings of Posener et al. (39), who demonstrated that, contrary to an earlier hypothesis, cortisol feedback in the HPA axis is not impaired in patients with major depression.
The trend observed in this and earlier studies that initial HPA activation is associated with therapeutic response to sleep deprivation is still preliminary and should be confirmed in large groups of subjects. At least there was definitely no trend in the opposite direction, i.e., no reduction of cortisol secretion in patients who showed an improvement of mood after sleep deprivation. It remains speculative whether HPA effects are related to the mechanism of the therapeutic action of sleep deprivation. The fact that a nocturnal elevation of cortisol was present in both nonresponders and responders to sleep deprivation does not support a relationship. However, the responders showed a more sustained elevation, persisting during the first half of the next day. Some other findings support the view that an increase of HPA activity might be relevant for the therapeutic effect. It has been demonstrated that hydrocortisone infusion but not placebo or CRF infusion has a short-term antidepressant effect, as does sleep deprivation (40). Also, an earlier investigation by Goodwin et al. (41) demonstrated short-term antidepressant effects of cortisol infusion in depressed patients, which was the opposite of what the authors expected. Another important issue is the functional coupling of cortisol and psychostimulant-like effects. Earlier studies indicated an increase of dopamine, noradrenaline, and serotonin after sleep deprivation, i.e., neurobiological effects similar to those following the intake of psychostimulants, such as amphetamines (see reference 42 for overview). The rewarding effects of psychostimulants, however, are dependent on the HPA axis (43). A cortisol increase following sleep deprivation might therefore mediate psychostimulant-like actions of increased aminergic neurotransmitter release. This view agrees with that of DeBattista and co-workers (40), who stated that the antidepressant effects of hydrocortisone might be mediated by an interaction with neurotransmitters, such as dopamine.
In conclusion, this study demonstrates that—much as in healthy humans—sleep deprivation significantly activates the HPA axis in unmedicated depressed patients.
 |
 |
Presented at the 2nd International Congress on Hormones, Brain and Neuropsychopharmacology, Rhodes, Greece, July 15–19, 2000; published partly as an abstract in Neuropsychopharmacology (2000; 23[suppl 2]:S82–S83). Received March 3, 2003; revision received Oct. 20, 2003; accepted Oct. 24, 2003. From the Department of Psychiatry and Psychotherapy, University Hospital of Freiburg, Freiburg, Germany; the Department of Psychiatry, Medical Clinic, University Hospital of Luebeck, Luebeck, Germany; and the Department of Psychology, University of Dusseldorf, Dusseldorf, Germany. Address reprint requests to Dr. Voderholzer, Abteilung für Psychiatrie und Psychotherapie, Klinikum der Albert-Ludwigs-Universität, Hauptstrasse 5, 79104 Freiburg, Germany; [email protected] (e-mail). Supported by grant VO 542/2-1 from Deutsche Forschungsgemeinschaft (DFG). The authors thank Gerda Deeb and Claudine Heinrich for analysis of polysomnographic recordings, Mrs. Frühauf for measurements of serum and saliva cortisol, and Sabine Ecker and Petra Hasselbach for statistical analysis.
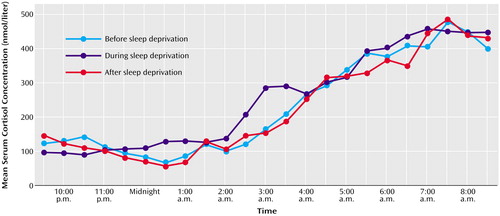
Figure 1. Nocturnal Serum Cortisol Concentrations Before, During, and After 1 Night of Sleep Deprivation in 15 Drug-Free Depressed Patientsa
aFor clarity, standard deviations are not included. The average standard deviations for the cortisol concentrations were 141 nmol/liter for the baseline night and 142 nmol/liter for the sleep deprivation night.
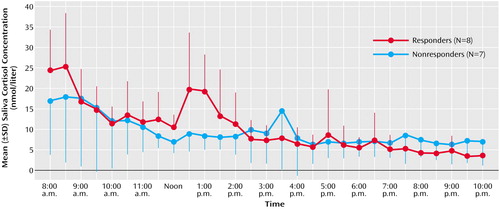
Figure 2. Daytime Saliva Cortisol Concentrations After 1 Night of Sleep Deprivation in Drug-Free Depressed Patients Whose Symptoms Did or Did Not Responda
aResponse was defined as an improvement from baseline of at least 30% in the average of the scores between 9:00 a.m. and 6:00 p.m. on a six-item abbreviation (21) of the Hamilton Depression Rating Scale.
1. Wu JC, Bunney WE: The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry 1990; 147:14–21Link, Google Scholar
2. Wirz-Justice A, Van den Hoofdakker RH: Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry 1999; 46:445–453Crossref, Medline, Google Scholar
3. Riemann D, Wiegand M, Lauer CH, Berger M: Naps after total sleep deprivation in depressed patients: are they depressogenic? Psychiatry Res 1993; 94:109–120Crossref, Google Scholar
4. Plotsky PM, Owens MJ, Nemeroff CB: Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 1998; 21:293–307Crossref, Medline, Google Scholar
5. Heim C, Owens MJ, Plotsky PM, Nemeroff CB: Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull 1997; 33:185–192Medline, Google Scholar
6. Arborelius L, Owens MJ: The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160:1–12Crossref, Medline, Google Scholar
7. Barden N, Reul JMHM, Holsboer F: Do antidepressants stabilize mood through actions on the hypothalamic-pituitary adrenocortical system? Trends Neurosci 1995; 18:6–11Crossref, Medline, Google Scholar
8. Holsboer F, Barden N: Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev 1996; 17:187–205Crossref, Medline, Google Scholar
9. Asnis GM, Halbreich U, Rabinovich H, Ryan ND, Sachar EJ, Nelson B, Puig-Antich J, Novacenko H: The cortisol response to desipramine in endogenous depressives and normal controls: preliminary findings. Psychiatry Res 1985; 14:225–233Crossref, Medline, Google Scholar
10. Jarrett DB, Miewald JM, Kupfer DJ: Acute changes in sleep-related hormone secretion of depressed patients following oral imipramine. Biol Psychiatry 1988; 24:541–554Crossref, Medline, Google Scholar
11. Golden RN, Ekstrom D, Brown TM, Ruegg R, Dwight LE, Haggerty JJ Jr, Garbutt JC, Pedersen CA, Mason GA, Browne J, Carson SW: Neuroendocrine effects of intravenous clomipramine in depressed patients and healthy subjects. Am J Psychiatry 1992; 149:1168–1175Link, Google Scholar
12. Bhagwagar Z, Hafizi S, Cowen PJ: Acute citalopram administration produces correlated increases in plasma and salivary cortisol. Psychopharmacology (Berl) 2002; 163:118–120Crossref, Medline, Google Scholar
13. Akerstedt T, Palmblad J, de la Torre B, Marana R, Gillberg M: Adrenocortical and gonadal steroids during sleep deprivation. Sleep 1980; 3:23–30Crossref, Medline, Google Scholar
14. Kant GJ, Genser SG, Torne DR, Pfalser JL, Mougey EH: Effects of 72 hours sleep deprivation on urinary cortisol and indices of metabolism. Sleep 1984; 7:142–146Crossref, Medline, Google Scholar
15. Leproult R, Copinschi G, Buxton O, van Cauter E: Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997; 20:865–870Medline, Google Scholar
16. Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP: Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 1999; 51:205–215Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
18. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
19. Kirschbaum C, Hellhammer DH: Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994; 19:313–333Crossref, Medline, Google Scholar
20. Voderholzer U, Hornyak M, Thiel B, Huwig-Poppe C, Kiemen A, König A, Backhaus J, Riemann D, Berger M, Hohagen F: Impact of experimentally induced serotonin deficiency by tryptophan depletion on sleep EEG in healthy subjects. Neuropsychopharmacology 1998; 18:112–124Crossref, Medline, Google Scholar
21. Bech P, Gram LF, Dein E, Jacobson O, Vitger J, Bohuys TG: Quantitative ratings of depressive states. Acta Psychiatr Scand 1975; 51:161–170Crossref, Medline, Google Scholar
22. Von Treuer K, Norman TR, Armstrong SM: Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res 1996; 20:7–14Crossref, Medline, Google Scholar
23. Chapotot F, Buguet A, Gronfier C, Brandenberger G: Hypothalamo-pituitary-adrenal axis activity is related to the level of central arousal: effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology 2001; 73:312–321Crossref, Medline, Google Scholar
24. Ho AH, Gronfier C, Czeisler CA: Effects of prolonged sleep deprivation on cortisol secretion in humans (abstract). Sleep 2001; 24(suppl):A251Google Scholar
25. Baumgartner A, Gräf KJ, Kürten I, Meinhold H: Thyrotropin (TSH) and thyroid hormone concentrations during partial sleep deprivation in patients with major depressive disorder. J Psychiatr Res 1990; 24:281–292Crossref, Medline, Google Scholar
26. Ebert D, Kaschka WP, Loew T, Beck G: Cortisol and beta-endorphin responses to sleep deprivation in major depression—the hyperarousal theories of sleep deprivation. Neuropsychobiology 1994; 29:64–68Crossref, Medline, Google Scholar
27. Yamaguchi N, Maeda K, Kuromaru S: The effects of sleep deprivation on the circadian rhythm of plasma cortisol levels in depressive patients. Folia Psychiatr Neurol Jpn 1978; 32:479–487Medline, Google Scholar
28. Parry BL, Javeed S, Laughlin GA, Hauger R, Clopton P: Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry 2000; 48:920–931Crossref, Medline, Google Scholar
29. Goetze U, Toelle R: Circadian rhythm of free urinary cortisol, temperature, and heart rate in endogenous depressives and under antidepressant therapy. Neuropsychobiology 1987; 18:175–184Crossref, Medline, Google Scholar
30. Bouhuys AL, Fientge F, Van den Hoofdakker RH: Effects of total sleep deprivation on urinary cortisol, self-rated arousal and mood in depressed patients. Psychiatry Res 1990; 34:149–162Crossref, Medline, Google Scholar
31. Baumgartner A, Gräf KJ, Kürten I, Meinhold H, Scholz P: Neuroendocrinological investigations during sleep deprivation in depression, I: early morning levels of thyrotropin, TH, cortisol, prolactin, LH, FSH, estradiol, and testosterone. Biol Psychiatry 1990; 28:556–568Crossref, Medline, Google Scholar
32. Gerner RH, Post RM, Gillin JC, Bunney WE: Biological and behavioral effects of one night’s sleep deprivation in depressed patients and normals. J Psychiatr Res 1979; 15:21–40Crossref, Medline, Google Scholar
33. Holsboer F, Liebl R, Hofschuster E: Repeated dexamethasone suppression test during depressive illness: normalization of test result compared with clinical improvement. J Affect Disord 1982; 4:93–101Crossref, Medline, Google Scholar
34. Greden JF, Gardner R, King D, Grunhaus L, Carroll BJ, Kronfol Z: Dexamethasone suppression test in antidepressant treatment of melancholia. Arch Gen Psychiatry 1983; 40:493–500Crossref, Medline, Google Scholar
35. Heuser IJE, Schweiger U, Gotthardt U, Schmider J, Lammers C-H, Dettling M, Yassouridis A, Holsboer F: Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry 1996; 153:93–99Link, Google Scholar
36. Kling MA, Geracioti TD, Licinio J, Michelson D, Oldfield EH, Gold PW: Effects of electroconvulsive therapy on the CRH-ACTH-cortisol system in melancholic depression: preliminary findings. Psychopharmacol Bull 1994; 30:489–494Medline, Google Scholar
37. Aperia B, Thorén M, Zettergren M, Wetterberg L: Plasma pattern of adrenocorticotropin and cortisol during electroconvulsive therapy in patients with major depressive illness. Acta Psychiatr Scand 1984; 70:361–369Crossref, Medline, Google Scholar
38. Rudorfer MV, Risby ED, Osman OT, Gold PW, Potter WZ: Hypothalamic-pituitary-adrenal axis and monoamine transmitter activity in depression: a pilot study of central and peripheral effects of electroconvulsive therapy. Biol Psychiatry 1991; 29:253–264Crossref, Medline, Google Scholar
39. Posener JA, DeBattista C, Williams GH, Schatzberg AF: Cortisol feedback during the HPA quiescent period in patients with major depression. Am J Psychiatry 2001; 158:2083–2085Link, Google Scholar
40. DeBattista C, Posener JA, Kalehzan BM, Schatzberg AF: Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. Am J Psychiatry 2000; 157:1334–1337Link, Google Scholar
41. Goodwin GM, Muir WJ, Seckl JR, Bennie J, Carroll S, Dick H, Fink G: The effects of cortisol infusion upon hormone secretion from the anterior pituitary and subjective mood in depressive illness and in controls. J Affect Disord 1992; 26:73–83Crossref, Medline, Google Scholar
42. Ebert D, Berger M: Neurobiological similarities in antidepressant sleep deprivation and psychostimulant use: a psychostimulant theory of antidepressant sleep deprivation. Psychopharmacology (Berl) 1998; 140:1–10Crossref, Medline, Google Scholar
43. Marinelli M, Oliveira C, Moal M, Piazza P: Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 1997; 16:156–161Crossref, Medline, Google Scholar



