Hippocampal Volume and First Major Depressive Episode After Cancer Diagnosis in Breast Cancer Survivors
Abstract
OBJECTIVE: Patients experiencing their first major depressive episode after receiving a diagnosis of cancer are frequently seen in clinical oncology settings; however, little is known about the neurobiological basis of the first episode. In previous studies, a smaller hippocampus than in healthy comparison subjects has been observed in patients with a history of recurrent and prolonged major depressive episodes. The purpose of the present study was to investigate whether there is an association between hippocampal volume and a first major depressive episode after cancer diagnosis in cancer survivors. METHOD: The subjects were 68 female cancer survivors who had undergone breast cancer surgery 3 or more years earlier (mean interval=4.3 years, SD=0.9). The hippocampal volume and delayed recall function of the 17 cancer survivors who had their first major depressive episode after receiving their cancer diagnosis and the 51 with no history of major depressive episode at any time during their lives were measured by magnetic resonance imaging and the Wechsler Memory Scale—Revised, respectively. RESULTS: The mean duration of the major depressive episode after cancer diagnosis was 11.9 weeks (SD=14.2). There were no significant differences in left or right hippocampal volume or in delayed recall function between the cancer survivors with and without a major depressive episode after cancer diagnosis. CONCLUSIONS: First major depressive episodes after cancer diagnosis in female cancer survivors do not appear to be associated with hippocampal volume. However, a longitudinal study with healthy comparison subjects is needed to draw a definite conclusion.
Experiencing cancer is a stressful life event. Even after the successful treatment of cancer, patients face many long-term problems, such as fear of recurrence (1, 2). Previous studies have reported that 1%–54% of cancer patients experience a major depressive episode (3, 4), but major depressive episodes after cancer diagnosis have generally been assumed to be reactive depression, with a short duration (2–4). Although a past history of a major depressive episode before cancer diagnosis has been reported to be a risk factor for a major depressive episode after a cancer diagnosis, patients who experience their first major depressive episode after receiving a cancer diagnosis are frequently encountered in clinical oncology settings (1, 3, 4). Little is known, however, about the neurobiological basis of first depressive episodes after cancer diagnosis in cancer patients.
Studies using high-resolution magnetic resonance imaging (MRI) have reported an 8%–15% smaller hippocampal gray matter volume in female patients with a history of recurrent major depressive episodes than in matched comparison subjects (5, 6), and other studies have found 11%–19% smaller hippocampal volumes in a group of male and female patients with recurrent major depressive episodes (7, 8). Yet another study reported the alteration of temporal gray matter, including that of the hippocampus, in patients with chronic treatment-resistant major depressive episodes (9, 10). However, several investigators have not found any reductions in hippocampal volume in depressed patients (11–13), including some reports that assessed the combined volumes of the amygdala-hippocampal complex as a single measurement (14–17). Most of the studies that found a reduced hippocampal volume were performed in older patients (5, 6, 18), and many of the reports described patients with recurrent (5–8) and/or treatment-resistant major depressive episodes (9, 10). Although one study that reported a smaller hippocampal volume did not find a significant association between the number of episodes and the volume (18), it has been reported that the hippocampal volume of patients with recurrent major depressive episodes is inversely correlated with the cumulative duration of the depressive episodes (5, 6, 19) and with the number of years that have passed since illness onset (8). In addition, prolonged stressor exposure and excessive glucocorticoid treatment have been reported to damage the hippocampal neurons of animals (20). Thus, the small hippocampal volume observed in patients with recurrent major depressive episodes may be a consequence of prolonged and severe recurrent major depressive episodes (21), but no direct evidence of causality was obtained in a longitudinal study.
The hippocampus has been found to play a role in the negative-feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis (22), which responds to stress. A smaller hippocampus has been reported to alter the response of the HPA axis to stress in animals (23), and dysregulation of the HPA axis has been reported in patients with a major depressive episode (24). These findings suggest that a smaller hippocampus may play a role in the development of a major depressive episode after a stressful life event; furthermore, early-life stressors, which are a risk factor for a major depressive episode (25), have been associated with a smaller hippocampus (26). Thus, a smaller hippocampus may predispose an individual to the development of a major depressive episode after a stressful life event. To our knowledge, two cross-sectional studies have demonstrated no significant association between hippo-campal volume and the first major depressive episode in a group of men and women (8, 27). However, one of the two studies reported a smaller hippocampus in a male subgroup, and the possibility that a smaller hippocampus may predispose an individual to the development of a major depressive episode could not be discounted.
To our knowledge, no previous study has investigated whether an association exists between hippocampal volume and a first major depressive episode after a cancer diagnosis in cancer patients. Although some of the factors associated with major depressive episodes after cancer diagnosis are thought to be the same as those in depressed patients without cancer (28), first major depressive episodes after cancer diagnosis also exhibit unique characteristics, such as the impact of having a life-threatening illness and a relatively late onset. Thus, first major depressive episodes after cancer diagnosis may have a different etiology and pathophysiology from those of chronic major depressive episodes, including the recurrent, treatment-resistant, or early-onset episodes examined in previous studies on the relation between hippocampal volume and depressive episodes. The purpose of this study was to compare the hippocampal volumes of cancer patients who experienced their first major depressive episode after receiving their cancer diagnosis and those of cancer patients who had no history of such episodes at any time in their life. A secondary purpose was to investigate whether an association exists between delayed recall function as a surrogate marker of hippocampal function and a depressive episode after cancer diagnosis.
Method
Subjects
This study was approved by the institutional review board and the ethics committee of the National Cancer Center in Tokyo. The study was performed after obtaining the patients’ written informed consent.
The subjects were recruited between February 1998 and April 1999 during follow-up visits to the Division of Breast Surgery, National Cancer Center Hospital East. We selected all patients in the records for breast cancer surgery who had survived more than 3 years since surgery so that the interview itself would not be distressing (29, 30). The aim of this protocol study was to investigate the associations between brain morphology and major depressive episodes and distressing cancer-related recollections in cancer survivors. Previously, we reported a significant association between distressing cancer-related recollections and hippocampal volume (31) in cancer survivors; this previous study population partially overlapped with that of the present study. The inclusion criteria were 1) female gender, 2) age between 18 and 55 years, and 3) no history of major depressive episode before cancer. Only women were selected to minimize sex-based brain differences. Exclusion criteria were 1) double cancer or clear evidence of residual or recurrent cancer during regular medical checkups conducted by an oncologist (S.I.), 2) a history of any neurological disorder or traumatic brain injury accompanied by periods of unconsciousness, 3) a history of substance abuse or dependence, 4) a family history of early dementia among first- or second-degree relatives, 5) any physical symptoms that interfered with daily life, as assessed by performance status defined by the Eastern Cooperative Oncology Groups (32), 6) psychotropic medication within the previous month, and 7) cognitive impairment defined as a score of less than 24 on the Mini-Mental State Examination (33, 34).
Of the 394 patients who had survived more than 3 years after surgery, 187 patients met the criteria, and 137 could be contacted at the clinic. Fifty-three of the 137 refused to participate in the study (26 were too busy to participate, 10 were not interested, seven refused to undergo the MRI examination, three lived too far away to come to the hospital, one was averse to the hospital, and six refused for unknown reasons). Eighty-four subjects were interviewed with a semistructured interview, including the Structured Clinical Interview for DSM-IV Axis I Disorders, clinician version (35), that was conducted by a trained psychiatrist (T.N.). After the interview, nine subjects who had a history of a major depressive episode before the cancer were excluded from the analyses. Seven subjects were excluded because of an MRI acquisition error. The remaining 68 study participants were currently not being treated for breast cancer except by adjuvant tamoxifen therapy (N=29). There were no significant differences in age, number of days after surgery, or pathological stage between the 68 subjects who participated in the study and the 100 eligible subjects who did not.
Whether the subjects had a history of major depressive episode, but not its intensity, was determined by semistructured interview. Two psychiatrists both assessed the same nine subjects, and the kappa value for history of major depressive episodes after cancer diagnosis was 1.0.
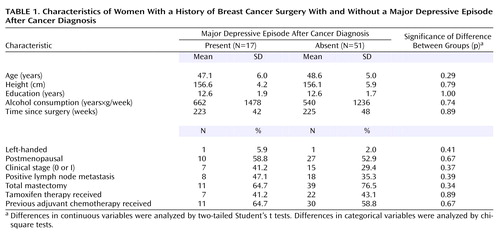
Of the 68 study participants, 17 (25%) met the criteria for major depressive episode after cancer diagnosis. The mean duration of the episode was 11.9 weeks (median=4.0, SD=14.2, range=2–50), and the mean number of major depressive episodes after the diagnosis was 1.1 (median=1.0, SD=0.3, range=1–2). Only two (12%) of the 17 subjects had two episodes after cancer diagnosis; the other 15 (88%) had just one episode. None of the subjects had experienced a major depressive episode with psychotic features, and none of the subjects had a history of any other axis I psychiatric disorder. The 17 subjects had been in remission for a mean of 215 weeks (median=229, SD=66, range=66–334) since the episode. None of the subjects met the criteria for a current major depressive episode at the time of the investigation. Of the 51 subjects who had never experienced a major depressive episode, one subject had a history of posttraumatic stress disorder. As shown in Table 1, statistical analyses indicated no significant differences in the backgrounds of the groups that did or did not experience a major depressive episode after cancer diagnosis. None of the subjects in the present study had taken any psychotropic medication for at least one month before the MRI examination. Furthermore, none of the subjects had ever taken antidepressants or mood stabilizers. Nine subjects (53%) in the first major depressive episode group had a history of taking anxiolytics or hypnotics (administered for 4 weeks or longer in two subjects and for less than 4 weeks in seven subjects in the past), and 15 subjects (29%) in the group that did not experience a depressive episode had a history of taking anxiolytics or hypnotics (administered for 4 weeks or longer in three subjects and for less than 4 weeks in 12 subjects in the past).
MRI Acquisition and Volumetric Measurements
The details concerning MRI acquisition and hippocampal volume measurements were described in our previous study (31). Briefly, the images were generated on a 1.5-T MRI unit (Signa scanner, GE Medical Systems, Milwaukee) with three-dimensional spoiled gradient-recalled acquisition of 1.5-mm on contiguous sections perpendicular to the anterior-posterior commissure plane under the following conditions: field of view=230 mm, matrix=256×256 pixels, TR=25 msec, TE=5 msec, flip angle=45°. The images were analyzed by ANALYZE-PC software (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn.). The boundaries of the hippocampus were similar to those reported previously (31) and were traced manually with a pen-tablet pointing device. Measurements were mainly made in the coronal orientation, and other perspectives were used to aid anatomic localization. A sagittal view was used to confirm the anterior end of the hippocampal head. The details concerning amygdala volume measurements were described in previous studies (36). The semiautomatic volumetric procedures for measuring whole brain volume and intracranial volume were similar to those reported previously (31, 37). Raters were blind to the subjects’ characteristics. The intraclass correlation coefficients for intrarater reliability based on 30 subjects and interrater reliability based on 10 subjects were 0.97 and 0.91, respectively, for the hippocampus and 0.94 and 0.82, respectively, for the amygdala. The coefficients based on 10 subjects were 0.99 and 0.99, respectively, for whole brain volume and 0.99 and 0.99, respectively, for intracranial volume.
Memory Measures
We used the delayed recall index and percent retention (delayed recall score/immediate recall score×100) from the Wechsler Memory Scale—Revised (38, 39) as surrogate markers of hippocampal function. We also obtained the Wechsler Memory Scale—Revised indexes of attention/concentration, immediate visual memory, and immediate verbal memory for each participant.
Data Analyses
In previous studies (5–7), patients’ hippocampal volume differed from that of comparison subjects by 8%–19%, and based on our pilot study, we estimated that the difference and standard deviation would be 8% and 9%, respectively. Assuming a lifetime prevalence of major depressive episode after cancer diagnosis in cancer survivors of 20%, 63 subjects were needed (13 cancer survivors with a major depressive episode and 50 without) for a two-tailed alpha of 0.05 and a beta of 0.20. The groups with and without a major depressive episode after cancer diagnosis in this study consisted of 17 and 51 subjects, respectively.
Hippocampal volume, amygdala volume, and whole brain volume were analyzed by one-way analysis of variance (ANOVA) and analysis of covariance (ANCOVA), with age, alcohol consumption, handedness, and intracranial volume used to control for differences between the two groups. Repeated-measures ANCOVA with side as the repeated-measures (within-group) factor and age, alcohol consumption, handedness, and intracranial volume as covariates was used to compare left and right hippocampal volume in the subjects with and without a major depressive episode after cancer diagnosis. In a subanalysis to minimize the influence of age, we also performed a paired t test to compare regional brain volumes between the 17 subjects with a first major depressive episode and a one-to-one age- and education-matched subject group from among the 51 subjects without a major depressive episode.
The comparisons of the subjects with and without a major depressive episode after cancer diagnosis also included analysis of the delayed recall index, percentage retention, attention/concentration index, immediate visual index, and immediate verbal memory index by ANOVA and by ANCOVA, with years of education and alcohol consumption as covariates.
As an additional analysis to investigate the possibility of a smaller hippocampus already being present in the subjects who had a major depressive episode after cancer diagnosis, we analyzed the difference in the number of subjects who had a major depressive episode after cancer diagnosis between those with a small hippocampus and those with a large hippocampus with the chi-square test. We used median hippocampal volumes on the left and right to divide the subjects into those with low and high left and right hippocampal volumes.
We also examined associations between medical factors, such as type of mastectomy, interval since cancer surgery, clinical stage, history of adjuvant chemotherapy and tamoxifen therapy, and hippocampal volume with the two-tailed Student’s t test and Pearson’s correlation test to exclude associations between medical factors and hippocampal volume because all subjects were cancer survivors and no healthy comparison subjects were examined. In addition, we stratified all the subjects into four groups according to their age and surveyed the means and standard deviations of the hippocampal volumes in each group to examine whether a nonlinear association exists between age and hippocampal volume, since a nonlinear association between age and brain volume has been previously reported (40).
The alpha level of significance in most statistical analyses was p<0.05. Since multiple comparisons were made in the analyses of memory function, the p value was set at 0.01. All analyses were performed by using SPSS software version 11 (SPSS, Inc., Chicago).
Results
Hippocampal Volume
The mean left and right hippocampal volumes of the subjects who experienced a major depressive episode after their cancer diagnosis (N=17) and of the subjects who never experienced a major depressive episode (N=51) are shown in Figure 1. No statistically significant differences in the volumes of the left or right hippocampus were seen between the two groups based on the results of ANOVAs, and ANCOVAs also did not produce any significant differences (left: F=0.66, df=1, 62, p=0.42; right: F=1.17, df=1, 62, p=0.28). A repeated-measures ANCOVA showed no significant interaction between side and the first major depressive episode after cancer diagnosis (F=0.01, df=1, 62, p=0.92) and no significant main effect of side (F=1.08, df=1, 62, p=0.30). A subanalysis using a paired t test between the 17 cancer survivors who experienced a first major depressive episode after cancer diagnosis and their age- and education-matched comparison subjects revealed no significant difference in the left or right hippocampal volume (left hippocampus: t=0.32, df=16, p=0.75; right hippocampus: t=0.51, df=16, p=0.62).
As an additional analysis, we used the median left hippocampal volume (3,096.6 mm3) to divide the subjects into those with low and high left hippocampal volume. Eight of the subjects with a small left hippocampus (N=34) had a major depressive episode after cancer diagnosis, as opposed to nine of the subjects with a large left hippocampus (N=34), but the difference was not significant (χ2=0.08, df=1, p=0.78). The median value for the right side (3,276.0 mm3) was then used as the dividing line to separate subjects with a low and a high right hippocampal volume. Ten of the subjects with a small right hippocampus (N=34) had a major depressive episode after cancer diagnosis versus seven of the subjects with a large right hippocampus (N=34), but this difference also was not significant (χ2=0.71, df=1, p=0.40).
Memory Functioning
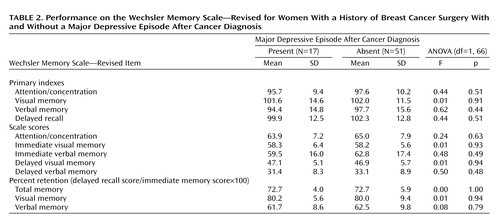
The memory measures of subjects who experienced a major depressive episode after cancer diagnosis and of those who did not are shown in Table 2. No significant differences in the delayed recall index, the percent retention of visual and verbal memory scores, attention/concentration index, immediate visual memory index, or immediate verbal memory index were observed between the two groups. ANCOVAs showed no significant differences in the delayed recall index (F=0.56, df=1, 64, p=0.46); the percent retention of total (F=0.00, df=1, 63, p=0.98), visual (F=0.03, df=1, 63, p=0.85), or verbal memory scores (F=0.20, df=1, 63, p=0.66); or the attention/concentration index (F=0.46, df=1, 64, p=0.50), immediate visual memory index (F=0.03, df=1, 64, p=0.87), or immediate verbal memory index (F=0.72, df=1, 64, p=0.40) between the two groups.
Hippocampal Volume and Medical Factors
As additional analyses, we examined possible associations between medical factors and hippocampal volume. No significant correlation between hippocampal volume and the interval since cancer surgery was seen (r=0.116, N=68, p=0.35), and no significant difference in the hippocampal volumes between subjects with a clinical stage of 0 or I (N=22) and those with a clinical stage of II or III (N=46) (t=0.39, df=66, p=0.70), between subjects with (N=41) and without (N=27) a history of adjuvant chemotherapy (t=0.63, df=66, p=0.53), between subjects who had (N=29) and who had not (N=39) received tamoxifen therapy (t=0.59, df=66, p=0.56), or between subjects who had undergone a total mastectomy (N=50) and those who had undergone a partial mastectomy (N=18) (t=0.93, df=66, p=0.36) were seen. Although a previous study reported a nonlinear association between age and brain volume, no obvious differences in the mean hippocampal volumes of the four age groups were observed in the present study. A linear regression analysis also revealed no significant association between age and hippocampal volume (data not shown).
Amygdala Volumes
The mean left amygdala volume was 1,357 mm3 (SD=169) in the subjects with a major depressive episode and 1,380 mm3 (SD=162) in the subjects with no depressive episodes. The mean right amygdala volume was 1,464 mm3 (SD=147) in the depressive episode group and 1,468 mm3 (SD=166) in the group without depressive episodes. No significant differences in the left or right amygdala volumes between subjects with and without a first major depressive episode after cancer diagnosis were revealed by an ANOVA (left: F=0.24, df=1, 66, p=0.63; right: F=0.01, df=1, 66, p=0.92) or an ANCOVA (left: F=1.12, df=1, 62, p=0.29; right: F=0.28, df=1, 62, p=0.60). A subanalysis using a paired t test between the 17 cancer survivors with a first major depressive episode after cancer diagnosis and their age- and education-matched comparison subjects revealed no significant differences in the left or right amygdala volumes (left amygdala: t=0.32, df=16, p=0.75; right amygdala: t=0.51, df=16, p=0.62).
Discussion
To our knowledge, this is the first study to investigate whether an association exists between hippocampal volume and a first major depressive episode after cancer diagnosis in cancer survivors. The results showed no significant differences in the left or right hippocampal volumes of cancer survivors who experienced a first major depressive episode after cancer diagnosis and those who had no history of major depressive episodes. The major depressive episodes after cancer diagnosis were of relatively short duration and were nonpsychotic, and almost all of the cancer survivors with a major depressive episode experienced only one episode. No significant differences in sociodemographic or medical factors were seen between the two groups in the present study. These results suggest the absence of a significant association between hippocampal volume and a first major depressive episode after cancer diagnosis in cancer survivors.
In a previous study investigating the possible association between hippocampal volume and a first major depressive episode, the mean duration of the depressive episode was 37 weeks—that is, three times longer than the duration of the depressive episode seen in the present study (27). No significant associations between the total or gray hippocampal volume and the first major depressive episode among male and female subjects as a whole or among the female subgroup were seen; however, a significantly smaller hippocampus was observed only among the male subgroup that experienced a first major depressive episode (27). In another recent study, no significant association between gray hippocampal volume and the first major depressive episode among a combined group of male and female patients was seen (8). The results of the present study, indicating that a significant association between hippocampal volume and the occurrence of a first major depressive episode of short duration does not exist among female cancer survivors, are consistent with the findings of these previous studies (8, 27). Thus, the hippocampal volume does not appear to be significantly associated with the occurrence of a first major depressive episode of short duration among female patients.
Sheline and colleagues (5, 6, 19) demonstrated a smaller hippocampus in patients with recurrent major depressive episodes, but the cumulative duration of the depressive episodes in their study (mean duration: 151 weeks, SD=147) was longer than that in the present study. They also demonstrated an inverse correlation between the hippocampal volume and the cumulative duration of the recurrent episodes (5, 6, 19). No significant reduction in the mean hippocampal volume of the first major depressive episode group may have been observed in the present study because the duration of the first episode was shorter (mean duration=11.6 weeks). Moreover, since the durations of the episodes in the present study were short, with a narrow range (SD=14.2 weeks), a significant correlation between the duration of the depressive episodes and the hippocampal volume—if such a correlation did indeed exist—may have been undetectable.
The first major depressive episodes in the cancer patients in this study were uniquely characterized by the existence of an obvious stressor (the diagnosis of a life-threatening illness) and a relatively late onset. Previous studies reporting a smaller hippocampus in patients with depressive episodes did not describe the preceding physical illness or the stressor encountered before the onset of the episode (5–10, 18). Differences in the natures of the stressors may be related to the differences in the results of the previous studies and ours. In addition, the first major depressive episodes after cancer diagnosis in the present study remitted without the use of antidepressants, had a relatively short duration, had no psychiatric comorbidities, and had a relatively late onset. On the other hand, the depressive episodes in the previous studies describing a reduced hippocampal volume were chronic, recurrent (6–8, 19), treatment resistant (9, 10), and/or had, in part, an early onset (8). Patients experiencing such episodes often exhibited psychiatric comorbidities, suggesting a possible genetic predisposition toward depression, compared to the subjects in the present study (41). Thus, the results of the present study suggest that nonchronic, late-onset, nonrefractory first major depressive episodes with no psychiatric comorbidities after cancer diagnosis may not be associated with a smaller hippocampus.
When the subjects in the present study were divided into two groups according to the median hippocampal volume value, no significant difference was found between the proportion of subjects with the first major depressive episode in those with a larger hippocampus and those with a smaller hippocampus. We speculate that a smaller hippocampus might not be a cause of susceptibility to a first major depressive episode after cancer diagnosis, although further study regarding this point is needed.
The results of the present study showed no significant differences in memory function, including delayed recall function, between the cancer survivors who experienced a first major depressive episode after cancer diagnosis and those who had never experienced a major depressive episode. In previous studies, a decrease in memory function was not prominent among patients who experienced a first major depressive episode (42, 43). However, a recent report by MacQueen et al. (8, 44) demonstrated a significant alteration of memory function in patients who had experienced a first major depressive episode when memory function was examined with a task that has been recognized as a method of examining hippocampus-dependent memory. Although no significant difference in delayed memory function was found between subjects who had or had not experienced a depressive episode in the present study, more specific measurements may demonstrate an alteration in hippocampal function in cancer survivors who experience a first major depressive episode after cancer diagnosis.
The amygdala has been implicated in major depressive episodes (45). However, the results of previous studies measuring amygdala volume in patients with major depressive episodes have been inconsistent (46–48). A previous study reported a significantly larger amygdala volume, as measured with MRI, in patients who had experienced a first major depressive episode (47). The difference between the results of this previous study and ours may be attributed to differences in the age and sex of the subjects, the duration of the depressive episodes, the depressed state (current versus past episode), and the methods used to measure amygdala volume. Further study is needed to determine whether amygdala volume is related to the occurrence of a first major depressive episode.
The present study had the following limitations:
| 1. | Because of its cross-sectional design, it could not confirm any causality between hippocampal volume and a first major depressive episode after cancer diagnosis | ||||
| 2. | Since the results were obtained from only one institution, the subjects were not representative of all cancer survivors | ||||
| 3. | All of the subjects were cancer survivors, and no healthy comparison subjects who had not experienced cancer were examined. Although the present study did not show any significant associations between hippocampal volume and medical factors, we could not exclude the possibility of the cancer experience itself having unknown effects on hippocampal volume or the presence of cancer-associated factors diluting the difference between the two groups. In the present study, some of the subjects had received tamoxifen. Previous studies have shown that tamoxifen produces mood-stabilizing effects through the inhibition of protein kinase C (49, 50) | ||||
| 4. | In the present study, we did not examine the presence of important depression-associated factors, such as a family history of major depressive episodes (51) | ||||
| 5. | Because the number of subjects in this study was determined so that an 8% difference in hippocampal volume could be detected between the two groups, we were unable to detect differences smaller than 8% | ||||
| 6. | Although the present study showed no significant differences in total hippocampal volume, since we did not separately measure the volume of the gray or white matter of the hippocampus, we could not exclude the possibility of a significant difference in gray or white matter volumes | ||||
In conclusion, no significant differences in left or right hippocampal volumes were found between female cancer survivors who experienced a nonchronic first major depressive episode of relatively short duration and their respective comparison subjects, who had never experienced a major depressive episode. However, a longitudinal study involving healthy comparison subjects and a functional neuroimaging study are needed to elucidate possible associations between major depressive episodes after cancer diagnosis and hippocampal volume.
 |
 |
Received April 1, 2003; revision received Dec. 10, 2003; accepted Dec. 14, 2003. From the Psycho-Oncology Division, National Cancer Center Research Institute East; and the Psychiatry Division, the Division of Breast Surgery, and the Department of Radiology, National Cancer Center Hospital East, Chiba, Japan. Address reprint requests to Dr. Uchitomi, Psycho-Oncology Division, National Cancer Center Research Institute East, 6-5-1, Kashiwanoha, Kashiwa, Chiba 277-8577, Japan; [email protected] (e-mail). Supported in part by three grants from the Japanese Ministry of Health, Labor, and Welfare (one each from Grant-in-Aid for Cancer Research, the Second-Term Comprehensive 10-Year Strategy for Cancer Control, and Research on Brain Science), a grant from the Japan Society for the Promotion of Science (a Grant-in-Aid for Scientific Research [B] KAKENHI 13470100), and a grant from the Japanese Ministry of Education, Culture, Science, and Technology (Target-Oriented Brain Science Program). Drs. Inagaki, Matsuoka, Sugahara, and Nakano are recipients of Research Resident Fellowships from the Foundation for the Promotion of Cancer Research. The authors thank Morihiro Sugishita, Ph.D., Hiroshi Matsuda, M.D., Ph.D., Takeshi Uema, M.D., Nobumasa Kato, M.D., Ph.D., and Etsuro Mori, M.D., Ph.D., for their advice and Ms. Yuko Kojima and Ms. Nobue Taguchi for research assistance.
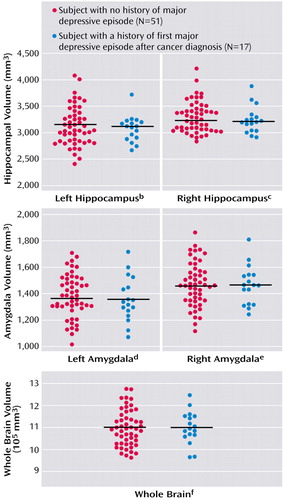
Figure 1. Hippocampal Volume of Female Survivors of Breast Cancer Surgery With and Without a Major Depressive Episode After Cancer Diagnosisa
aHorizontal bars indicate mean volumes.
bNonsignificant difference between groups (ANOVA: F=0.28, df=1, 66, p=0.60). The mean volumes of the subjects without and with a major depressive episode was 3,123 mm3 (SD=370) and 3,071 mm3 (SD=246), respectively.
cNonsignificant difference between groups (ANOVA: F=0.51, df=1, 66, p=0.48). The mean volumes of the subjects without and with a major depressive episode was 3,290 mm3 (SD=292) and 3,233 mm3 (SD=253), respectively.
dNonsignificant difference between groups (ANOVA: F=0.24, df=1, 66, p=0.63). The mean volumes of the subjects without and with a major depressive episode was 1,380 mm3 (SD=162) and 1,357 mm3 (SD=170), respectively.
eNonsignificant difference between groups (ANOVA: F=0.01, df=1, 66, p=0.92). The mean volumes of the subjects without and with a major depressive episode was 1,468 mm3 (SD=166) and 1,464 mm3 (SD=147), respectively.
fNonsignificant difference between groups (ANOVA: F=0.01, df=1, 66, p=0.93). The mean volumes of the subjects without and with a major depressive episode were 1,096,117 mm3 (SD=82,001) and 1,098,165 mm3 (SD=74,143), respectively.
1. Uchitomi Y, Mikami I, Kugaya A, Akizuki N, Nagai K, Nishiwaki Y, Akechi T, Okamura H: Depression after successful treatment for nonsmall cell lung carcinoma. Cancer 2000; 89:1172–1179Crossref, Medline, Google Scholar
2. Kornblith AB: Psychosocial adaptation of cancer survivors, in Psycho-oncology. Edited by Holland JC. New York, Oxford University Press, 1998, pp 223–254Google Scholar
3. Massie MJ, Popkin MK: Depressive disorders. Ibid, pp 518–540Google Scholar
4. Chochinov HM: Depression in cancer patients. Lancet Oncol 2001; 2:499–505Crossref, Medline, Google Scholar
5. Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW: Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93:3908–3913Crossref, Medline, Google Scholar
6. Sheline YI, Sanghavi M, Mintun MA, Gado MH: Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034–5043Crossref, Medline, Google Scholar
7. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS: Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157:115–117Link, Google Scholar
8. MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT: Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100:1387–1392Crossref, Medline, Google Scholar
9. Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM: Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression: controlled magnetic resonance imaging study. Br J Psychiatry 1998; 172:527–532Crossref, Medline, Google Scholar
10. Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP: Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry 2002; 180:434–440Crossref, Medline, Google Scholar
11. Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA: Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000; 47:1087–1090Crossref, Medline, Google Scholar
12. Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ: Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry 2001; 50:960–964Crossref, Medline, Google Scholar
13. Von Gunten A, Fox NC, Cipolotti L, Ron MA: A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci 2000; 12:493–498Crossref, Medline, Google Scholar
14. Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH Jr, Krishnan KR: Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 1993; 47:163–173Crossref, Medline, Google Scholar
15. Pantel J, Schroder J, Schad LR, Friedlinger M, Knopp MV, Schmitt R, Geissler M, Bluml S, Essig M, Sauer H: Quantitative magnetic resonance imaging and neuropsychological functions in dementia of the Alzheimer type. Psychol Med 1997; 27:221–229Crossref, Medline, Google Scholar
16. Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, Figiel GS, Spritzer CE: Quantitative cerebral anatomy in depression: a controlled magnetic resonance imaging study. Arch Gen Psychiatry 1993; 50:7–16Crossref, Medline, Google Scholar
17. Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S: Hippocampal/amygdala volumes in geriatric depression. Psychol Med 1999; 29:629–638Crossref, Medline, Google Scholar
18. Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR: Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 48:301–309Crossref, Medline, Google Scholar
19. Sheline YI, Gado MH, Kraemer HV: Untreated depression and hippocampal volume loss. Am J Psychiatry 2003; 160:1516–1518Link, Google Scholar
20. McEwen BS: Stress and hippocampal plasticity. Annu Rev Neurosci 1999; 22:105–122Crossref, Medline, Google Scholar
21. Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57:925–935Crossref, Medline, Google Scholar
22. Herman JP, Cullinan WE: Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997; 20:78–84Crossref, Medline, Google Scholar
23. Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF: Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry 2001; 58:1145–1151Crossref, Medline, Google Scholar
24. Whiteford HA, Peabody CA, Csernansky JG, Warner MD, Berger PA: Elevated baseline and postdexamethasone cortisol levels: a reflection of severity or endogeneity? J Affect Disord 1987; 12:199–202Crossref, Medline, Google Scholar
25. Wise LA, Zierler S, Krieger N, Harlow BL: Adult onset of major depressive disorder in relation to early life violent victimisation: a case-control study. Lancet 2001; 358:881–887Crossref, Medline, Google Scholar
26. Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D: Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000; 57:1115–1122Crossref, Medline, Google Scholar
27. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller H-J: Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002; 159:1112–1118Link, Google Scholar
28. Akechi T, Okuyama T, Imoto S, Yamawaki S, Uchitomi Y: Biomedical and psychosocial determinants of psychiatric morbidity among postoperative ambulatory breast cancer patients. Breast Cancer Res Treat 2001; 65:195–202Crossref, Medline, Google Scholar
29. Uchitomi Y, Mikami I, Nagai K, Nishiwaki Y, Akechi T, Okamura H: Depression and psychological distress in patients during the year after curative resection of non-small-cell lung cancer. J Clin Oncol 2003; 21:69–77Crossref, Medline, Google Scholar
30. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
31. Nakano T, Wenner M, Inagaki M, Kugaya A, Akechi T, Matsuoka Y, Sugahara Y, Imoto S, Murakami K, Uchitomi Y: Relationship between distressing cancer-related recollections and hippocampal volume in cancer survivors. Am J Psychiatry 2002; 159:2087–2093Link, Google Scholar
32. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655Crossref, Medline, Google Scholar
33. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
34. Mori E, Mitani Y, Yamadori A: Usefulness of a Japanese version of the Mini-Mental State in neurological patients. Shinkeishinrigaku 1985; 1:82–90Google Scholar
35. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Washington, DC, American Psychiatric Press, 1997Google Scholar
36. Matsuoka Y, Yamawaki S, Inagaki M, Akechi T, Uchitomi Y: A volumetric study of amygdala in cancer survivors with intrusive recollections. Biol Psychiatry 2003; 54:736–743Crossref, Medline, Google Scholar
37. Mori E, Hirono N, Yamashita H, Imamura T, Ikejiri Y, Ikeda M, Kitagaki H, Shimomura T, Yoneda Y: Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s disease. Am J Psychiatry 1997; 154:18–24Link, Google Scholar
38. Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp, 1987Google Scholar
39. Sugishita M: [Wechsler Memory Scale—Revised.] Tokyo, Nihonbunkakagakusya, 2001 (Japanese)Google Scholar
40. Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J: Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58:461–465Crossref, Medline, Google Scholar
41. Kendler KS, Gardner CO, Prescott CA: Clinical characteristics of major depression that predict risk of depression in relatives. Arch Gen Psychiatry 1999; 56:322–327Crossref, Medline, Google Scholar
42. Basso MR, Bornstein RA: Relative memory deficits in recurrent versus first-episode major depression on a word-list learning task. Neuropsychology 1999; 13:557–563Crossref, Medline, Google Scholar
43. Kessing LV: Cognitive impairment in the euthymic phase of affective disorder. Psychol Med 1998; 28:1027–1038Crossref, Medline, Google Scholar
44. MacQueen GM, Galway TM, Hay J, Young LT, Joffe RT: Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychol Med 2002; 32:251–258Crossref, Medline, Google Scholar
45. Drevets WC: Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 2001; 11:240–249Crossref, Medline, Google Scholar
46. Sheline YI, Gado MH, Price JL: Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 1998; 9:2023–2028Crossref, Medline, Google Scholar
47. Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ: Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 2002; 51:708–714Crossref, Medline, Google Scholar
48. Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J: Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000; 30:117–125Crossref, Medline, Google Scholar
49. Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK: A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry 2000; 57:95–97Crossref, Medline, Google Scholar
50. Manji HK, Moore GJ, Chen G: Bipolar disorder: leads from the molecular and cellular mechanisms of action of mood stabilizers. Br J Psychiatry Suppl 2001; 41:S107-S119Google Scholar
51. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Crossref, Medline, Google Scholar



