Characteristics of Depressed Preschoolers With and Without Anhedonia: Evidence for a Melancholic Depressive Subtype in Young Children
Abstract
OBJECTIVE: This study investigated whether a melancholic subtype similar to that established in depressed adults can be identified in depressed preschool children. METHOD: A final group total of 156 preschool children between the ages of 3.0 and 5.6 years and their caregivers underwent a comprehensive psychiatric assessment that included a structured psychiatric interview modified for young children. The clinical characteristics of four study groups (N=156) were compared: depressed preschoolers with anhedonia, depressed preschoolers without anhedonia (“hedonic”), a psychiatric comparison group with DSM-IV attention deficit hyperactivity disorder and/or oppositional defiant disorder, and a healthy comparison group. RESULTS: Fifty-four depressed preschoolers were identified, and 57% of this depressed group was anhedonic, a symptom deemed to be highly developmentally and clinically significant when arising in the preschool period. The anhedonic depressed subgroup identified was characterized by greater depression severity, alterations in stress cortisol reactivity, increased family history of major depressive disorder, and increased frequency of psychomotor retardation as well as other melancholic symptoms, such as a lack of brightening in response to joyful events. CONCLUSIONS: The clinical characteristics of this depressed subgroup are consistent with those described in melancholic depressed adults and suggest that a melancholic depressed subtype can be manifest in children as young as age 3.
DSM formally established the symptom of anhedonia, in addition to sad mood, as a cardinal identifying feature of major depressive disorder in DSM-III-R. Anhedonia as a specific symptom was deemed key to differentiating between “endogenous/psychotic” and “neurotic/reactive” depression, a binary distinction emphasized in investigations of depressive disorders during the 1970s and 1980s (1). Klein (2) suggested that anhedonia was a specific marker of an “endogenomorphic” depression characterized by melancholia and better response to tricyclic antidepressants. Clark et al. (3) provided independent validation for a melancholic depressed subtype characterized by anhedonia and found that this group displayed greater symptom severity and better treatment response than depressed patients with “normal” hedonic tone. The endogenous versus nonendogenous distinction was supported by the finding that, as would be expected, nonendogenous depression had a greater temporal relationship with threatening life events than the endogenous subtype (4, 5). Lemke et al. (6) demonstrated a relationship between anhedonia and psychomotor retardation in adults with major depressive disorder, providing further support for the construct. Consistent with these findings, Zimmerman et al. (7) observed anhedonia in 90% of adults with “melancholic” depression. Evidence for a biological basis for the symptom of anhedonia was suggested by data demonstrating that depressed anhedonic individuals had altered responses to dextroamphetamine, suggesting an underlying dysfunction in the brain reward system (8). In keeping with this, Kendler et al. (9) provided evidence for a genetic basis for the symptom of anhedonia.
Subsequent research, including longitudinal outcome data, suggested that the distinction between “endogenous/psychotic” and “reactive/neurotic” depressive subtypes had questionable validity and limited clinical utility (10). However, a melancholic major depressive disorder subtype was retained in DSM-IV. As defined by DSM-IV, this subtype is characterized by anhedonia, a depressed mood that does not improve even in response to joyful events or good things happening, psychomotor retardation, early or middle insomnia, weight loss, and inappropriate guilt. Other investigations have suggested that individuals with melancholic major depressive disorder are more likely to display dexamethasone nonsuppression, a robust and well-replicated biological finding associated with major depressive disorder (11, 12). In keeping with earlier data suggesting better biological treatment response (to tricyclic antidepressants and ECT) and decreased likelihood of placebo response among a melancholic major depressive disorder subtype, more recent investigations have confirmed that this subtype also shows a better response to fluoxetine (2, 12–14).
There has been surprisingly little investigation of a melancholic depressive subtype in populations of depressed children and adolescents. Ryan et al. (15) found some evidence for “endogenous” and “nonendogenous” subtypes in a sample of depressed 6–18-year-old children based on principal-component analysis. The validity of these distinct subtypes was further supported by significant differences in symptom frequencies between these groups (15).
We (16) have recently shown that DSM-IV major depressive disorder can be identified in preschool children when the diagnostic assessment is modified to account for age-adjusted symptom manifestations. The validity of this clinically significant preschool depressive syndrome has been supported by numerous factors, including a unique symptom constellation, a family history of related disorders, social impairment, and severity and stability of depressive symptoms (16, 17).
Significant alterations in hypothalamic-pituitary-adrenal axis reactivity in response to stress, consistent with those established in depressed adults, have been found in these depressed preschoolers compared to both psychiatric and healthy comparison groups (18). Furthermore, a pattern of neuropsychological deficits similar to those seen in depressed adults was also found in this group of depressed preschoolers (19). These findings lend further support to the validity and clinical significance of this syndrome.
When depressed preschoolers were compared to both psychiatric and healthy comparison groups, anhedonia emerged as the most specific symptom of depression, while sadness or irritability emerged as the most sensitive symptom (20). Notably, when the symptom of anhedonia was observed, the odds of having the diagnosis of depression was infinitely high (mathematically incalculable) since this symptom was found only in depressed preschoolers and not in either comparison group (20). These findings suggest that anhedonia does not occur normatively in the preschool period, and thus when it occurs, it is significant and specific for a clinical depressive syndrome.
Independent of the clear and established importance of anhedonia in depressed adults and its apparent specificity to depression in preschoolers, any manifestation of anhedonia in a young child was hypothesized to be developmentally and clinically significant on a theoretical basis as well. This is because the experience and pursuit of pleasure and joy are a central part of normative early childhood life experience. Barring adverse circumstances, early childhood is an inherently joyful time of life, with pleasure seeking and exploration among the primary developmental challenges. Based on the primacy of positive hedonic tone to the young child, the age-appropriate manifestation of anhedonia, conceptualized as the lack of pleasure or interest in activities and play, was hypothesized to be clinically significant when it was observed. Joy is one of seven primary emotions evident at birth in human infants and hypothesized by Darwin as early as the late 19th century to be an innate emotion (21). The primacy of the experience of pleasure or lack of pleasure and distress, also referred to as “hedonic tone,” has been integral to theories of human development, ranging from psychoanalysis to ethology.
Based on the characteristics and importance of the melancholic subtype in depressed adults and the specificity of the symptom of anhedonia in depressed preschool children, we hypothesized that depressed preschool children who displayed anhedonia would be a more severely disturbed subgroup, similar to the melancholic depressed subtype described in DSM-IV. We also hypothesized that depressed preschoolers with anhedonia would display greater stress cortisol reactivity, a greater family history of major depressive disorder, and higher frequencies of symptoms characteristic of melancholically depressed adults. This is the first investigation to our knowledge of a melancholic depressive subtype in the youngest group of clinically depressed children identified to date, those from 3 to 5 years of age.
Method
Study Group
A total of 174 preschoolers between the ages of 3.0 and 5.6 years were assessed in the Early Emotional Development Program at Washington University School of Medicine as part of a study of the nosology of preschool depression. Three groups of preschoolers were recruited from mental health and primary care clinics for study participation: 1) those with symptoms of depression; 2) those with symptoms of “disruptive” psychiatric disorders, in particular, attention deficit hyperactivity disorder (ADHD) and/or oppositional defiant disorder; and 3) those without symptoms of psychiatric disorders (“healthy”). One hundred fifty-six children fell into one of three study groups of interest for these analyses. Comparison groups with nonaffective psychiatric disorders and those without disorders were included to determine the specificity of findings to affective disorders. Children were recruited from community pediatrician’s offices with a checklist designed to screen for early-onset behavior problems (the Preschool Feelings Checklist, Luby et al., 2004, found at the web site of the Journal of the American Academy of Child and Adolescent Psychiatry) and by consecutive case ascertainment from a specialty mental health clinic exclusively serving young children. Excluded were children with chronic medical illnesses and/or neurological problems and those with pervasive developmental disorders and/or language and cognitive delays that would have prohibited their ability to understand the study questions (for details of the recruitment procedures, see Luby et al. [16]). After complete description of the study and procedures, written informed consent was obtained from the children’s guardians. Assent was not obtained because of the young age of the study subjects.
Assessments
Preschoolers and their primary caregivers underwent a comprehensive 2–3 hour assessment during which a structured diagnostic interview, a version of the Diagnostic Interview Schedule for Children (22) that was modified for young children, was administered to caregivers about the child. To develop this interview, the applicant and colleagues collaborated with the authors of the Diagnostic Interview Schedule for Children—Young Child to create this modified version for the parents of young children, the Diagnostic Interview Schedule for Children—Young Child (23). Several items from the Diagnostic Interview Schedule for Children were modified in the Diagnostic Interview Schedule for Children to account for their age-appropriate developmental manifestations. This was deemed necessary at face value since some items, as they were described in the Diagnostic Interview Schedule for Children, did not apply to the life experiences of preschool children. The most obvious were the items that applied to school behavior across all modules. Because preschoolers are not in academic school settings, all items that addressed schoolwork were modified to address “activities and play” (e.g., difficulty focusing on “activities and play” rather than schoolwork). Along these lines, for the assessment of concentration, “decisions” were described as “choices.” A more subtle modification was that the term “sad or depressed” was changed to “sad or unhappy” to better express how parents tend to view the negative mood state of a young child. Items about anhedonia assessed whether the child was having “no fun.” These items were unchanged from the standard Diagnostic Interview Schedule for Children. Furthermore, because preschool children are less verbally competent than older children, items that addressed preoccupation with death and suicidality were modified to account for the possibility that these symptoms might be manifested as persistent themes in play (in addition to the possibility that they might be verbally expressed). All remaining major depressive disorder items on the Diagnostic Interview Schedule for Children were unchanged. All diagnostic modules with known relevance to young children were modified along these lines and used in this study (e.g., schizophrenia and substance abuse modules were not administered).
The parents were interviewed with the Family Interview for Genetic Studies (24) to assess the history of mental disorders in the child’s first- and second-degree relatives. The Family Interview for Genetic Studies assesses from the parent informant information about the presence of diagnosed psychiatric disorders in relatives on both sides of the family. The Family Interview for Genetic Studies also employs standard probes to assess the presence of symptoms of axis I disorders. Children were videotaped and coded responding to structured emotionally evocative tasks from the Laboratory Temperament Assessment Battery (25). All assessments were conducted, and all coding was completed by raters who were blind to the diagnostic status of the child.
Salivary Cortisol Collection Methods
Salivary cortisol was collected several times at specifically designated intervals during the assessment by having the child place a sterile dental cotton roll in his or her mouth. To avoid any potential contamination of the assay, no salivary stimulant was used. The first cortisol sample was obtained from all preschool subjects upon entry into the assessment (with the parent present), then 60 minutes later, after an emotionally evocative stressful event (approximately 30 minutes after the stressor) that included an extended separation from the parent. “Stressful” events were structured play tasks (from the Laboratory Temperament Assessment Battery, as described) designed to produce transient and mild frustration in the child (such as not being able to unlock a transparent box with a desirable toy inside). Separation from the parent was also deemed “mildly stressful.” To control for non-stress-related elevations of cortisol, assessments were conducted either at 9:00 a.m. (50% of the group) or at 1:00 p.m. Time of the day (assessment time) was then considered a potential confounding variable in all analyses.
The first saliva sample was taken at least 1 hour after a meal, and children were provided with a snack of water and crackers no later than 30 minutes before the prestress cortisol sample. Before salivary cortisol collection, ear temperature was taken to verify afebrile status. Information pertinent to conditions known to alter salivary cortisol values was obtained (e.g., recent tooth loss or fever, use of inhalers), and data were not used if contaminants were present.
Analysis
For the purpose of this investigation, we compared two subgroups of depressed preschoolers: those with symptoms of anhedonia, a hypothesized “melancholic” subgroup, and those without anhedonia, who will be referred to as “hedonic.” The presence or absence of anhedonia (and therefore subgroup status) was based solely on parent report on items pertinent to anhedonia on the Diagnostic Interview Schedule for Children—Young Child. These two groups were compared to each other and to both nondepressed comparison groups (psychiatric and healthy) on all demographic factors, including age, gender, family income and education, marital status, and ethnicity. The frequency rates of various depressive symptoms and family history of major depressive disorder were compared between the groups with the chi-square statistic.
To derive a formula for calculating weighted depression severity scores, principal-component analysis was performed over all symptoms of depression from the major depressive disorder module from the Diagnostic Interview Schedule for Children—Young Child for the entire study group (26). A one-factor solution was found and used for the calculation of weighted depression severity scores. Factor analytically derived severity scores were compared between four diagnostic groups by using Kruskal-Wallis one-way analysis of variance (ANOVA) and post hoc U tests since homogeneity of variance was not fulfilled. In addition to factor analytically derived severity scores, severity scores based on the total number of symptoms were also derived. These were compared between the anhedonic and hedonically depressed subgroups by using t tests (two-tailed).
Hierarchical cluster analysis was performed to further explore the symptom structure and as a check on the naturalness of the hypothesized subtypes (27). Cluster analyses with the average linkage between groups based on all symptoms of depression from the Diagnostic Interview Schedule for Children—Young Child were performed. Severity scores among these clusters were then compared by using ANOVA and post hoc t tests.
To investigate differences in cortisol “stress reactivity” between the study groups of interest, cortisol change variables were calculated. The percent change between the first (baseline) and second (after the experimentally induced “stressor”) cortisol values (change of 1% to 2%) was calculated as a method of representing “reactivity.” Change of 1% to 2% was (cortisol 2 – cortisol 1/cortisol 1100). This transformation allowed for standardization of the baseline and stress response values to facilitate between subjects’ comparisons of change values. Cortisol change scores by percent were then compared between the two nondepressed comparison groups and the two depressed subgroups. Differences in cortisol percent change scores between these groups have been previously published with different analytic methods (18).
Results
There were no statistically significant differences between the two groups of depressed preschoolers on any demographic variable with the exception of income (Table 1). Fifty-four depressed preschoolers were in our total group of 156 preschool children included in these analyses. Fifty-seven percent (N=31) of depressed preschoolers were anhedonic, and the remaining 43% (N=23) were hedonic. The anhedonic group contained a higher frequency of girls than boys (the anhedonic depressed preschool group was composed of 64% girls, while the hedonic depressed group had 52% girls); however, these differences were not statistically significant. Differences were found between all four study groups in the areas of income (Kruskal-Wallis χ2=7.91, df=3, p<0.05) and stressful life events (χ2=9.84, df=3, p<0.05). Post hoc analyses yielded significant differences in family income between hedonic depressed and healthy comparison groups, with the healthy group having significantly higher incomes (Mann-Whitney U=422.50, z=–2.54, p<0.05). Differences were also found for stressful life events between the four groups (χ2=9.84, df=3, p<0.05). Post hoc analyses revealed differences between the hedonic depressed and healthy comparison groups (U=325.50, z=–2.61, p<0.01) and the hedonic depressed and psychiatric comparison groups with ADHD and/or oppositional defiant disorder (U=248.00, z=–2.72, p<0.01) with the hedonic depressed group experiencing more stressful life events than both comparison groups.
Symptom Severity Scores
Principal-component analysis, which uses all symptoms of depression over the entire study group, resulted in a one-factor solution that explained 30% of the total variance. This factor revealed high loadings for “typical” depressive and vegetative symptoms. The relative symptom weights derived from this one-factor solution were useful for deriving individual depression severity scores. We have previously demonstrated a statistically significant hierarchy in these factor analytically derived severity scores in the expected direction between the healthy, psychiatric, and depressed study groups, as well as a high Cronbach’s alpha for these depressive symptoms (17).
When the factor analytically derived weighted depression severity scores were compared among the four study groups of interest, a significant difference among groups was found (χ2=109.67, df=3, p<0.0001). Post hoc group comparisons revealed that both anhedonic and hedonic depressed preschoolers each had significantly higher depression severity scores than both the psychiatric and normal comparison subjects (U ranging from 0.0 to 32.0, df= 67–84, all p<0.0001). Comparisons of each depressed group to individual comparison groups were as follows: anhedonic depressed versus psychiatric groups: U=9.0, p<0.0001; anhedonic depressed versus healthy groups: U=0.0, p<0.0001; hedonic versus psychiatric groups: U=32.0, p<0.0001; hedonic versus healthy groups: U=3.0, p<0.0001. When the two depressed groups were compared, the anhedonic group had significantly greater severity scores than the hedonic depressed group (U=146.0, p<0.001) (Figure 1). Notably, the psychiatric comparison group also had significantly greater severity scores than the healthy comparison group (U=736.0, p<0.001).
A similar statistically significant hierarchy among the four study groups was also found when they were compared on a similar (unweighted) variable representing the sum of all depression symptoms. Each depressed subgroup (anhedonic and hedonic) had a significantly higher sum symptom score than the healthy comparison subjects (anhedonic versus healthy and hedonic versus healthy, both U=2.5, p<0.0001) and the psychiatric comparison subjects (anhedonic versus psychiatric: U=14.5, p<0.0001; hedonic versus psychiatric comparison subjects: U=27.0, p<0.0001). When the two depressed groups were compared to each other, the anhedonic group had significantly more symptoms of depression than the depressed hedonic preschoolers (U=218.5, p<0.02).
In addition, hierarchical cluster analysis yielded two distinct clusters (subgroups of subjects) at the last joining stage. The smaller cluster (N=15) consisted exclusively of children with anhedonia, while the second subgroup was a “mixed group.” Comparison of the smaller cluster (N=15) with the mixed group cluster revealed that this purely anhedonic group had significantly higher factor scores than the mixed group (t=11.83, df=171, p<0.001).
Family History of Depression
In a comparison of all four study groups on a measure of family history of psychiatric disorders according to the Family Interview for Genetic Studies, a near-significant difference between groups was found for numbers of family members with a history of major depressive disorder (χ2=7.54, df=3, p=0.057). When we compared the two depressed subgroups by family history of major depressive disorder, the depressed anhedonic group had a significantly greater family history of major depressive disorder than the depressed hedonic group (U=195.0, z=–2.59, df=50, p<0.01). When this group was compared with both the psychiatric and healthy groups, significant and nearly significant differences were also found (psychiatric: U=501.5, z=–1.95, df=73, p=0.05; healthy: U=576.0, z=–2.28, df=82, p<0.05). These differences were not evident when similar comparisons were made to the depressed group as a whole (combining the anhedonic and hedonic depressives) (χ2=1.77, df=2, p=0.41). However, significant differences in family history of affective disorders in general were found between the depressed group as a whole and the two comparison groups (16).
Neurovegetative Signs and Symptoms
The frequency of all DSM-IV symptoms of depression as well as additional symptoms hypothesized to be manifestations of depression in preschoolers were compared between the two groups. Depressed preschoolers with anhedonia had significantly greater frequencies of appearing “slowed down” and/or “restless” (t=2.18, df=38.72, p<0.05) and having decreased energy at near-significant levels (t=1.80, df=52, p=0.08) on the Diagnostic Interview Schedule for Children—Young Child than the depressed hedonic group. These symptoms have been shown to occur with significantly greater frequency in depressed preschoolers as a whole compared to both psychiatric and normal comparison groups (16).
Significant differences were also found between the hedonic and anhedonic depressed preschoolers on an item titled “unreactive” that addressed whether there was improvement (even temporary) in mood in response to joyful events or good things happening. This item parallels the DSM-IV melancholic criterion A that was described as “lack of reactivity to usually pleasurable stimuli (does not feel much better, even temporarily, when something good happens).” The anhedonic depressives were significantly more likely than the hedonic depressives to be unreactive or to have no improvement in mood in response to joyful events (t=2.74, df=52, p<0.01). The anhedonic depressed group also displayed more “unreactivity” to joyful events when compared to both healthy (t=3.58, df=85, p=0.001) and psychiatric (t=2.91, df=76, p<0.01) groups.
Stress Cortisol Reactivity
A Kruskal-Wallis test comparing the scores for percent change in cortisol (from baseline to after the separation stressor) between the four diagnostic groups revealed near-significant differences between the two depressed and the psychiatric and healthy groups (χ2=6.95, df=3, p<0.08). Since results were less than significant in the four-group comparison, exploratory post hoc U tests were conducted. Significant differences were found between the depressed anhedonic group compared to the healthy (U=319.0, z=–2.35, p<0.05) and psychiatric groups (U=246.0, z=–2.15, p<0.05) (Figure 1). Because the exploratory analyses suggested that main sources of differences arose from the most severely depressed anhedonic—not the hedonic—group, another three-group Kruskal-Wallis test of these cortisol percent change scores was conducted. This analysis included only the most severely ill depressed anhedonic group and the psychiatric and healthy groups, revealing significant differences between these three groups (χ2=6.32, df=2, p<0.05) (Figure 2). Post hoc U tests revealed significant differences between the depressed anhedonic group and the healthy (U=319.0, z=–2.35, p<0.05) and psychiatric (U=246.0, z=–2.15, p<0.05) groups. We also explored whether there were differences between the depressed hedonic and anhedonic groups. Although no statistically significant differences were found, it was notable that the anhedonic group displayed greater percent change scores in cortisol than the hedonic depressed preschoolers.
The finding that the experimental psychosocial stressors used in this study served effectively as physiological stressors to the preschool subjects has been previously established (A.H. Heffelfinger, unpublished data, 2003). The behavioral results from the Laboratory Temperament Assessment Battery (the structured measure that enacts emotionally evocative events to produce the stress paradigm, as previously described) will be presented elsewhere.
Discussion
Our findings provide evidence for a melancholic major depressive disorder subtype in children between 3 and 5 years of age. Similar to melancholic depressed adults, this subtype is characterized by anhedonia, lack of reactivity or brightening in response to joyful events, psychomotor retardation, a significantly greater family history of major depressive disorder, and a greater severity of depressive symptoms than in hedonically depressed preschoolers.
Furthermore, anhedonic depressed preschoolers showed alterations of cortisol in response to stress when compared to both normal and psychiatric groups. They also had greater elevations than hedonic depressed preschoolers, although these differences did not reach statistical significance. However, given the small group sizes, the possibility that negative findings are the result of a type II error is raised, suggesting that investigations in larger groups should be undertaken. These findings are consistent with greater dexamethasone nonsuppression that was first described in melancholically depressed adults almost 30 years ago (28). A detailed presentation and discussion of cortisol findings and their relevance to the validity of major depressive disorder in preschool children have been presented elsewhere (18).
A clear psychosocial precipitant of depression has also been described as a characteristic feature of a nonmelancholic (e.g., reactive/neurotic) depression in adults (29–31). Consistent with this, only hedonic—and not anhedonic depressed preschoolers—had significantly more stressful life events. Similarly, lower family income was found only in the hedonic group, not in the healthy comparison group.
When comparing groups of depressed prepubertal children and adolescents, some investigators have suggested that the symptom of anhedonia, the proposed marker of a melancholic subtype, may occur less frequently in younger depressed children (15, 32). In contrast, Luby et al. (16) found a relatively high frequency (57%) of this symptom in a group of depressed preschoolers when this symptom was assessed by using age-appropriate questions.
Validation for a clinical depressive syndrome in preschool children as early as age 3 has been previously provided (16, 17, 20). These findings of significantly higher severity scores, a greater family history of major depressive disorder, and a higher frequency of neurovegetative signs among depressed anhedonic than among depressed hedonic preschoolers suggest that this symptom may be a marker of a biologically based melancholic major depressive disorder subtype in young children. As such, these data provide further evidence for a valid melancholic subtype of major depressive disorder in preschool children between the ages of 3 and 5.
These findings are consistent with the early findings and interpretation of Klein (2) on the significance of anhedonia in adult major depressive disorder and suggest that, as with adults, anhedonia is a marker of a more severe, biologically and genetically based melancholic depressive subtype in preschool children. Based on the known normative developmental challenges of the preschool period, it stands to reason that when anhedonia occurs in a preschool child, it is a marker of serious psychopathology. Findings from this study suggest that future studies that employ larger groups of depressed preschool children are warranted to further investigate the validity and characteristics of a melancholic subtype.
 |
Received June 11, 2003; revision received Sept. 16, 2003; accepted Dec. 4, 2003. From the Department of Psychiatry, Washington University School of Medicine. Address reprint requests to Dr. Luby, Department of Psychiatry, Washington University School of Medicine, 660 S. Euclid Ave., Box 8134, St. Louis, MO 63110; [email protected] (e-mail). Supported by NIMH grant K08-MH-01462-01 and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award to Dr. Luby.

Figure 1Weighted (Factor Analytically Derived) Depression Severity Scores for Depressed Preschoolers With and Without Anhedonia, Psychiatric Comparison Preschoolers, and Healthy Comparison Preschoolers
aSignificant difference in a four-way comparison of all groups (p<0.0001, post hoc U test).
bSignificant difference between depressed anhedonic and hedonic groups (U=146.0, p<0.001) and psychiatric and healthy comparison groups (U=736.0, p<0.001).
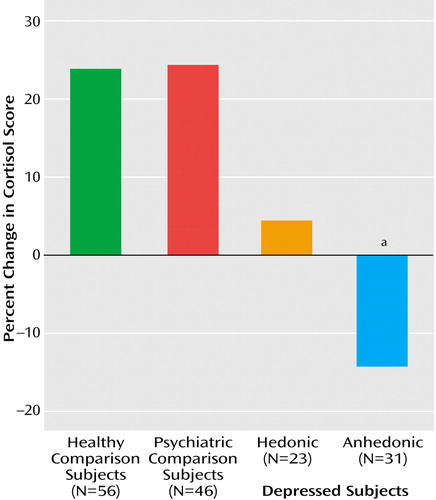
Figure 2. Change in Cortisol Scores for Depressed Preschoolers With and Without Anhedonia, Psychiatric Comparison Preschoolers, and Healthy Comparison Preschoolers
aSignificant difference between depressed anhedonic group and the two comparison groups (anhedonic versus healthy: U=319.0, z=–2.35, p=0.05; anhedonic versus psychiatric: U=246.0, z=–2.15, p<0.05). Adapted from the Archives of General Psychiatry 2003; 60:1248. Copyright © American Medical Association. All rights reserved.
1. Nelson JC, Charney DS: The symptoms of major depressive illness. Am J Psychiatry 1981; 138:1–13Link, Google Scholar
2. Klein DF: Endogenomorphic depression. Arch Gen Psychiatry 1974; 31:447–454Crossref, Medline, Google Scholar
3. Clark DC, Fawcett J, Salazar-Gruesco E, Fawcett E: Seven-month clinical outcome of anhedonic and normally hedonic depressed inpatients. Am J Psychiatry 1984; 141:1216–1220Link, Google Scholar
4. Brown GW, Harris TO, Hepworth C: Life events and endogenous depression: a puzzle reexamined. Arch Gen Psychiatry 1994; 51:525–534Crossref, Medline, Google Scholar
5. Frank E, Anderson B, Reynolds CF III, Ritenour A, Kupfer DJ: Life events and the research diagnostic criteria endogenous subtype: a confirmation of the distinction using the Bedford College methods. Arch Gen Psychiatry 1994; 51:519–524Crossref, Medline, Google Scholar
6. Lemke MR, Puhl P, Koethe N, Winkler T: Psychomotor retardation and anhedonia in depression. Acta Psychiatr Scand 1999; 99:252–256Crossref, Medline, Google Scholar
7. Zimmerman M, Black DW, Coryell W: Diagnostic criteria for melancholia: the comparative validity of DSM-III and DSM-III-R. Arch Gen Psychiatry 1989; 46:361–368Crossref, Medline, Google Scholar
8. Tremblay LK, Naranjo Claudio A, Cardenas L, Herrmann N, Busto UE: Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry 2002; 59:409–417Crossref, Medline, Google Scholar
9. Kendler KS, Ochs AL, Gorman AM, Hewitt JK, Ross DE, Mirsky AF: The structure of schizotypy: a pilot multitrait twin study. Psychiatry Res 1991; 36:19–36Crossref, Medline, Google Scholar
10. Parker G: Classifying depression: should paradigms lost be regained? Am J Psychiatry 2000; 157:1195–1203Link, Google Scholar
11. Chrousos GP, Gold PW: The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA 1992; 267:1244–1252Crossref, Medline, Google Scholar
12. Joyce PR, Mulder RT, Luty SE, McKenzie JM, Sullivan PF, Abbott RM, Stevens IF: Melancholia: definitions, risk factors, personality, neuroendocrine markers and differential antidepressant response. Aust NZ J Psychiatry 2002; 36:376–383Crossref, Medline, Google Scholar
13. Raskin A, Crook TA: The endogenous-neurotic distinction as a predictor of response to antidepressant drugs. Psychol Med 1976; 6:59–70Crossref, Medline, Google Scholar
14. Simpson GM, Lee JH, Cuculic Z, Kellner R: Two dosages of imipramine in hospitalized endogenous and neurotic depressives. Arch Gen Psychiatry 1976; 33:1093–1102Crossref, Medline, Google Scholar
15. Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, Iyengar S, Twomey J: The clinical picture of major depression in children and adolescents. Arch Gen Psychiatry 1987; 44:854–861Crossref, Medline, Google Scholar
16. Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T: Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry 2002; 41:928–937Crossref, Medline, Google Scholar
17. Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Hessler M, Spitznagel E: Modification of DSM-IV criteria for depressed preschool children. Am J Psychiatry 2003; 160:1169–1172Link, Google Scholar
18. Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E: Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry 2003; 60:1248–1255Crossref, Medline, Google Scholar
19. Mrakotsky C: Visual perception, spatial cognition and affect recognition in preschool depressive syndromes (dissertation). Vienna, University of Vienna/Washington University School of Medicine, Austrian National Library, 2001Google Scholar
20. Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Wallis J, Spitznagel E: The clinical picture of depression in preschool children. J Am Acad Child Adolesc Psychiatry 2003; 42:340–348Crossref, Medline, Google Scholar
21. Darwin CR: The Expression of Emotions in Man and Animals. London, John Murray, 1872Google Scholar
22. Shaffer D, Fisher P, Lucas C (NIMH DISC Editorial Board): Diagnostic Interview Schedule for Children, version IV. New York, Division of Psychiatry, Columbia University, 1998Google Scholar
23. Lucas C, Fisher P, Luby J: Young-Child DISC-IV Research Draft: Diagnostic Interview Schedule for Children. New York, Columbia University, Division of Child Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders, 1998Google Scholar
24. Maxwell E: The Family Interview for Genetic Studies: Manual. Washington, DC, National Institute of Mental Health, Intramural Research Program, Clinical Neurogenetics Branch, 1992Google Scholar
25. Goldsmith HH, Reilly J, Lemery KS: Laboratory Temperament Assessment Battery: Preschool Version. Eugene, Department of Psychology, University of Oregon, 1995Google Scholar
26. Harman HH: Modern Factor Analysis. Chicago, University of Chicago Press, 1976Google Scholar
27. Everitt BS, Landau S, Leese M: Cluster Analysis. London, Edward Arnold, 2001Google Scholar
28. Carroll BJ, Curtis GC, Mendels J: Neuroendocrine regulation in depression. Arch Gen Psychiatry 1976; 33:1051–1058Crossref, Medline, Google Scholar
29. Hirschfeld RM, Klerman GL, Andreasen NC, Clayton PJ, Keller MB: Situational major depressive disorder. Arch Gen Psychiatry 1985; 42:1109–1114Crossref, Medline, Google Scholar
30. Glass RM: Situational and neurotic-reactive depression. Arch Gen Psychiatry 1985; 42:1126–1127Crossref, Medline, Google Scholar
31. Parker G, Hadzi-Pavlovic D, Roussos J, Wilhelm K, Mitchell P, Austin M-P, Hickie I, Gladstone J, Eyers K: Non-melancholic depression: the contribution of personality, anxiety and life events to subclassification. Psychol Med 1998; 28:1209–1219Crossref, Medline, Google Scholar
32. Masi G, Favilla L, Mucci M, Poli P, Romano R: Depressive symptoms in children and adolescents with dysthymic disorder. Psychopathology 2001; 34:29–35Crossref, Medline, Google Scholar



