Comparative Efficacy and Safety of Atypical and Conventional Antipsychotic Drugs in First-Episode Psychosis: A Randomized, Double-Blind Trial of Olanzapine Versus Haloperidol
Abstract
OBJECTIVE: Few long-term studies have compared the efficacy and safety of typical and atypical antipsychotic medications directly in patients with a first episode of psychosis who met the criteria for schizophrenia or a related psychotic disorder. This study compared the acute and long-term effectiveness of haloperidol with that of olanzapine in patients with first-episode psychosis in a large, controlled clinical trial. METHOD: Patients with first-episode psychosis (N=263) were randomly assigned under double-blind conditions to receive haloperidol or olanzapine and were followed for up to 104 weeks. Domains measured included psychopathology, psychosocial variables, neurocognitive functioning, and brain morphology and metabolism. This report presents data from clinical measures of treatment response and safety data from the 12-week acute treatment phase. RESULTS: Haloperidol and olanzapine were associated with substantial and comparable baseline-to-endpoint reductions in symptom severity, which did not differ significantly in last-observation-carried-forward analyses. However, in a mixed-model analysis, olanzapine-treated subjects had significantly greater decreases in symptom severity as measured by the Positive and Negative Syndrome Scale total score and negative and general scales and by the Montgomery-Åsberg Depression Rating Scale but not as measured by the Positive and Negative Syndrome Scale positive scale and by the Clinical Global Impression severity rating. Olanzapine-treated patients experienced a lower rate of treatment-emergent parkinsonism and akathisia but had significantly more weight gain, compared with the haloperidol-treated patients. Overall, significantly more olanzapine-treated subjects than haloperidol-treated subjects completed the 12-week acute phase of the study (67% versus 54%). CONCLUSIONS: As expected on the basis of previous studies, both olanzapine and haloperidol were effective in the acute reduction of psychopathological symptoms in this group of patients with first-episode psychosis. However, olanzapine had several relative advantages in therapeutic response. Although the nature of adverse events differed between the two agents, retention in the study was greater with olanzapine. Retention in treatment is important in this patient population, given their risk of relapse. Longer-term results are needed to determine whether treatment with atypical antipsychotics results in superior outcomes for a first episode of schizophrenia.
Shortly after their introduction, first-generation antipsychotic medications were shown to suppress the acute psychotic symptoms of schizophrenia and related psychotic disorders and prevent their recurrence (1). However, many patients with chronic disorders were found to be unresponsive to antipsychotic drugs, and it was generally believed that, despite the ability of these drugs to suppress acute psychotic symptoms and prevent relapse, they did not positively change the long-term course of the disorder or subsequently improve outcome (2). Moreover, antipsychotic drugs were associated with high rates of neurological side effects (i.e., acute extrapyramidal signs and tardive dyskinesia) that compromised the therapeutic effects of treatment and impelled many patients to discontinue their use, thus increasing the risk for relapse (3).
In contrast, studies of patients in the first episode of illness demonstrated higher relative rates of therapeutic response and symptom remission with use of first-generation antipsychotics, compared to patients with multiple prior episodes (4–7), but first-episode patients experienced high rates of extrapyramidal side effects (8, 9). Taken together, these observations suggested that first-episode patients may be more sensitive to the pharmacologic effects of antipsychotic drugs and may thus require lower drug doses (7, 10). Despite a favorable acute response profile, first-episode patients have been shown to experience a high rate of psychotic relapse, particularly if they have discontinued antipsychotic medication (11–15). In addition, although generally high rates of symptom remission have been reported for first-episode psychotic patients treated with first-generation antipsychotics, these medications have been associated with a low rate of functional recovery (13, 14).
The introduction of second-generation or so-called “atypical” antipsychotic drugs has been heralded as a therapeutic advance in the treatment of schizophrenia and related psychotic disorders. Evidence suggests that atypical antipsychotics affect a broader range of schizophrenic psychopathology and are generally better tolerated than conventional antipsychotics (16). However, these claims have not been consistently demonstrated by empirical data; thus, researchers and clinicians vary in their opinions about the comparative effectiveness of first- and second-generation drugs (17–19). Despite the lack of consensus, atypical antipsychotic drugs have become the most commonly used class of antipsychotic drugs in clinical practice.
Comparison between second-generation and classic antipsychotics has been almost entirely conducted in studies of chronically ill patients whose symptoms may have been (partially) resistant to treatment. The question of their comparative efficacy and safety has been examined in very few studies of first-episode patients and only in short-term studies (6 weeks) (20, 21). This question has considerable importance because the first treatment intervention for patients with a recent onset of psychotic disorder is a critical therapeutic opportunity that may potentially influence the course and outcome of what is often a lifelong illness (22, 23).
For these reasons we designed the study reported here to compare the therapeutic efficacy and outcome of treatment with olanzapine or haloperidol in patients who were experiencing their first episode of psychosis while in an early stage of illness. The study was designed to evaluate treatment effects over short- and long-term periods by using three outcome dimensions: 1) clinical measures of treatment response based on psychopathology, side effects, and treatment adherence; 2) cognitive function as measured by neuropsychological test performance; and 3) biomarkers of treatment effects measured by structural magnetic resonance imaging and proton magnetic resonance spectroscopy. This article reports the methods and results of the clinical measures of treatment response based on psychopathology, side effects, and treatment adherence in the study’s initial 12-week acute treatment phase.
Method
Study Setting and Sponsorship
The project was conducted at 14 academic medical centers in North America and Western Europe. Lilly Research Laboratories provided funding. The study was directed by a steering committee comprised of the study principal investigator (J.L.), co-principal investigators (Cecil Charles, Ph.D., Richard Keefe, Ph.D., G.T., M.T.), the principal study biostatistician (R.M.H.), and the principal investigators from the 14 sites.
Subjects
The study subjects were selected from patients who presented to clinical services (inpatient, emergency, outpatient) for evaluation and treatment of psychotic symptoms. Patients were included in the study if they met all of the following criteria: 1) were ages 16–40 years; 2) had onset of psychotic symptoms before age 35 years; 3) met the DSM-IV diagnostic criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder as assessed by using the Structured Clinical Interview for DSM-IV, Research Version; 4) experienced psychotic symptoms (delusions, hallucinations, thought disorder, and grossly bizarre behavior) for at least 1 month but not more than 60 months, 5) scored ≥4 on at least two Positive and Negative Syndrome Scale (24) psychosis items (P1, P2, P3, P5, or P6) or scored ≥5 on one psychosis item; 6) had a Clinical Global Impression (CGI) severity score ≥4 (moderately ill); 7) required treatment with antipsychotic drugs on a clinical basis; 8) had a level of understanding sufficient to communicate with the research staff and to cooperate with all tests and examinations required by the protocol; 9) understood the nature of the study and signed an informed consent document (required of each patient or the patient’s authorized legal representative); and 10) for female patients of childbearing potential, had been using a medically accepted means of contraception.
Patients were excluded from the study for any of the following reasons: they 1) had previously received antipsychotic drug treatment for more than 16 cumulative weeks, had been treated with clozapine at anytime in their lifetime, or had been treated with an injectable depot neuroleptic within less than three dosing intervals before study entry; 2) were either pregnant or nursing; 3) had serious, unstable medical illnesses or findings from a medical examination that suggested a contraindication to antipsychotic drug treatment; 4) had a history of allergic or severe adverse reactions to study medications; 5) met the DSM-IV criteria for substance dependence (except caffeine and nicotine) within 1 month before the first visit; 6) were judged clinically to be at suicidal risk too serious to be included in this study; 7) required treatment with anticonvulsants, benzodiazepines (except as allowed for agitation and control of extrapyramidal signs), antidepressants, psychostimulants, or other antipsychotic drugs concurrently with study medications beyond those permitted as concomitant treatments; 8) had contraindications for neuroimaging per current regulations of the local regulatory agency (e.g., if the patient had a metal prosthesis); or 9) had a past history of any DSM-IV psychotic disorder with recovery (Recovery, although based on the clinical impression of the patient’s history, was defined as the cessation of positive and negative symptoms and return of functioning for 6 months or longer. For example, a patient who had an episode of a brief psychotic disorder, recovered [no longer had positive and negative symptoms], then had another episode 6.5 months later was considered to have a past history of a DSM-IV psychotic disorder with recovery.), 10) had a premorbid IQ of ≤70, or 11) had received ECT within 1 month (30 days) before study entry.
Study Design and Procedures
Patients who met the inclusion criteria were assessed for competence to provide informed consent. If they were found to be competent, the study was explained and they were asked to participate and provide informed consent. Patients currently receiving antipsychotics underwent a washout period of between 2 and 14 days, depending on their clinical status, after which they were randomly assigned to treatment with olanzapine or haloperidol under double-blind conditions. During the first 6 weeks of the 12-week acute treatment phase, the initial dose titration ranges were 5–10 mg/day for olanzapine and 2–6 mg/day for haloperidol. During the second 6 weeks, the dose titration ranges were 5–20 mg/day for olanzapine and 2–20 mg/day for haloperidol. The dose titration schedule and philosophy was to go slowly and target the lowest effective dose while still enabling patients to receive active treatment. Medication doses could be adjusted as clinically indicated within the prescribed range after week 6 of treatment. Certain concomitant medications were permitted during the washout and acute treatment phase for the management of agitation, general behavior disturbances, and/or insomnia but only for a cumulative duration no greater than 21 days. These medications included 500–2000 mg/day of chloral hydrate, 1–8 mg/day of lorazepam, and 5–40 mg/day of diazepam.
Antiparkinsonian medications were not administered prophylactically. However, if clinically significant extrapyramidal signs occurred, anticholinergic medication (benztropine or biperiden at a dose of up to 6 mg/day), 10–80 mg/day of propranolol, or procyclidine (oral or intramuscular administration of 5–10 mg, two to three times/day, for a maximum dose of 30 mg/day) was allowed. Antidepressants and mood stabilizers were not permitted in the acute treatment phase of the study. Patients requiring these medications were withdrawn from the study.
Assessments
Efficacy
Efficacy was measured in four domains: 1) psychopathology, 2) psychosocial measures of social and vocational function and quality of life, 3) neurocognitive function, and 4) brain morphology and metabolism. (Findings for the latter three domains are reported separately.) During the washout and acute treatment phases, patients were assessed for psychopathology weekly through week 6 of treatment and biweekly during weeks 7 through 12.
Psychopathology was assessed by using the Positive and Negative Syndrome Scale (30 items, 1–7 severity scale), the Montgomery-Åsberg Depression Rating Scale (10 items, 0–6 severity scale) (25), and the CGI severity rating (one item, 1–7 scale). Patients who met the following criteria, defined a priori, were classified as treatment responders: 1) had no rating of >3 (mild) on items P1, P2, P3, P5, and P6 of the Positive and Negative Syndrome Scale, and 2) had a ≥30% reduction from baseline in the Positive and Negative Syndrome Scale total score, and 3) had a CGI severity score ≤4 (moderately ill).
Safety
At study entry all patients provided their medical history and were assessed with a physical examination and laboratory tests. Vital signs (blood pressure, pulse, weight, and temperature) were measured at each study visit. Side effects were recorded by using the Coding Symbols for a Thesaurus for Adverse Reaction Terms (COSTART) classification terms (26) at each assessment visit. Extrapyramidal signs and abnormal involuntary movements were assessed by examinations of patients and scored on the Simpson-Angus Rating Scale (27) (including an additional dystonia item), the Abnormal Involuntary Movement Scale (28), and the Barnes Rating Scale for Drug-Induced Akathisia (29) at every assessment visit.
Statistical Methods
All analyses were done on an intent-to-treat basis, meaning that all patients were included in the treatment groups to which they were randomly assigned no matter how long they adhered to the protocol. All total scores from rating scales and subscales were derived from the individual scale items. Patients were included in the efficacy analyses only if they had a baseline measure and at least one postbaseline measure.
For the analyses of continuous efficacy data, we used both a traditional last-observation-carried-forward analysis of variance (ANOVA) model and a mixed random coefficients model. Within-group comparisons were also examined by using t tests to analyze baseline-to-endpoint differences. Categorical data were evaluated by using Fisher’s exact and chi-square tests. Continuous safety data were analyzed by using last-observation-carried-forward ANOVA. All hypothesis tests were performed by using a two-sided significance level of 0.05.
For the mixed-models analysis, we fit linear mixed random regression coefficients models to the longitudinal data for the primary efficacy variable (Positive and Negative Syndrome Scale total score) and secondary efficacy variables (Positive and Negative Syndrome Scale positive, negative, general psychopathology scale, CGI severity, and Montgomery-Åsberg Depression Rating Scale scores). Such model fitting involves iterative estimation and fitting of covariance structures and fixed effects before final estimation and hypothesis testing. The modeling process is complex, with choices to be made about the form of the covariance structure among the random effects and error terms and about the fixed effects to be included in the model. We first tested for the presence of a center effect. This difference was nonsignificant, and the center effect was removed from any further consideration in the modeling. We then used modeling of the Positive and Negative Syndrome Scale total score (the primary efficacy variable) to arrive at a final covariance structure and a final model, which included fixed effects for therapy (olanzapine versus haloperidol), visit (as a numeric variable, with a separate slope for each treatment), and a common quadratic slope for visit. Random coefficients were allocated for the intercept and linear slope for each subject. The preliminary model fixed effects included two intercepts (one for each treatment), two linear slopes (one for each treatment), and two quadratic slopes (one for each treatment). (The quadratic slopes were included because efficacy generally is not a linear but a curved function of time because the change in response decreases over time.) The preliminary model random effects included a random subject intercept and a random subject linear slope. The preliminary covariance structure was unstructured. The quadratic slopes did not differ significantly from each other, and the intercepts did differ significantly from each other, resulting in a final model with a single intercept, a linear slope for each treatment, and a single quadratic slope coefficient. This model structure was then applied to the Positive and Negative Syndrome Scale total scores and to the other Positive and Negative Syndrome Scale subscale scores, the CGI severity score, and the Montgomery-Åsberg Depression Rating Scale score. The same model was used for all efficacy variables in order to control the complexity of fitting different models to different efficacy variables. Since the Positive and Negative Syndrome Scale total score was the primary outcome variable, we examined this variable without correction for multiplicity. Since the other efficacy variables were secondary, we also examined them without correction for multiplicity, but their interpretation should be considered supportive and exploratory.
Results
Subject Characteristics
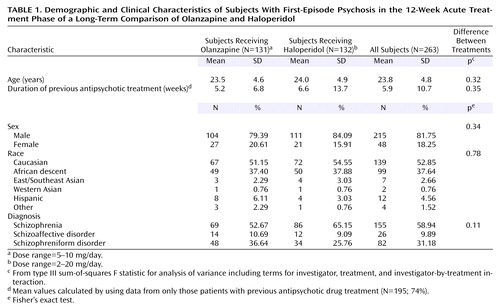
Two hundred sixty-three subjects entered the study and were randomly assigned to treatment. Their demographic and clinical characteristics by treatment group are described in Table 1. Eighty-two percent of the subjects were male, 53% were white, 38% were black, and 9% were from other racial groups. The mean age was about 24 years (SD=5). Fifty-nine percent had a diagnosis of schizophrenia, 31% had schizophreniform disorder, and 10% had schizoaffective disorder. The mean duration of illness (from self-reported date of onset of symptoms to date of study entry) was 62.5 weeks (SD=60.6), with a median duration of 38.1 weeks (range=1.3–250.1). At baseline, 26% of the subjects had never received antipsychotic treatment, and 74% reported some prior treatment with antipsychotics. For those subjects reporting prior antipsychotic treatment, the mean self-reported duration of prior treatment was 5.9 weeks (SD=10.7), with a median duration of 2.9 weeks (range=0.1–10.7).
Efficacy
The acute-phase mean modal doses were 9.1 mg/day of olanzapine and 4.4 mg/day of haloperidol. Sixty-eight percent of the olanzapine-treated subjects and 54% of the haloperidol-treated subjects completed the 12-week acute phase (p=0.03, Fisher’s exact test). Reasons for discontinuation for the olanzapine-treated subjects and the haloperidol-treated subjects, respectively, included adverse events for 3.1% and 6.8%, lack of efficacy for 6.1% and 12.1%, patient’s decision for 9.2% and 8.3%, physician’s decision for 5.3% and 6.8%, and other reasons for 9.2% and 12.1%. The duration of time in the study for each treatment group is illustrated in the survival curve displayed in Figure 1.
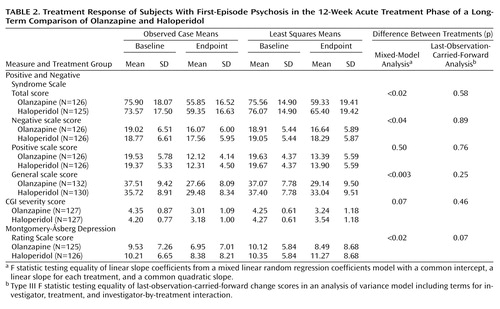
The analyses of the psychopathology scale scores are reported in Table 2. The last-observation-carried-forward analyses found significant reductions in symptoms for both treatments and no significant differences between the two groups for any of the efficacy measures. In the random regression coefficients model, both treatments produced significant symptom reductions as reflected by the baseline-to-endpoint total change scores on the psychopathology scales. There were statistically significant differences between treatments in the rates of change in the Positive and Negative Syndrome Scale total score and negative and general scale scores and in the Montgomery-Åsberg Depression Rating Scale score, with olanzapine associated with greater reductions (Table 2).
Each of the linear slopes differed significantly from zero and differed from each other. Both linear coefficients were negative (indicating declines in Positive and Negative Syndrome Scale total score over time), but the linear coefficient for olanzapine (–4.7) was significantly more extreme than the coefficient for haloperidol (–4.2). Thus, the model demonstrated that subjects receiving both treatments began with similar baseline scores and that the Positive and Negative Syndrome Scale total score declined significantly more for subjects taking olanzapine than for those taking haloperidol. Figure 2 shows plots of the means for the observed cases (the data used in the mixed models) and the least squares means predicted from the models for each of the two treatments. For the observed case means, the week-0 measurement represents baseline, while in the plot of the least squares means, the week-0 measurement represents the common intercept at baseline. The week-12 least squares mean estimates were 65.4 for haloperidol and 59.3 for olanzapine, a difference of 6.1 points in the Positive and Negative Syndrome Scale total score. (Since this was a constant intercept model, the hypothesis tests of the difference between least squares means at any time point are isomorphic to the hypothesis test on the slope.)
For secondary efficacy variables, the linear slopes for the two groups differed significantly for all measures except the Positive and Negative Syndrome Scale positive scale score and the CGI severity score, with olanzapine treatment associated with greater symptom reductions (Table 2).
In a categorical analysis of response rate that used the definition of response described in the Method section, the proportion of study subjects responding by week 12 was 55% for those assigned to olanzapine treatment, compared with 46% for those receiving haloperidol (χ2=1.76, df=1, p=0.19). The median time to response was 7.9 weeks (SE=0.4) for olanzapine-treated patients and 8.4 weeks (SE=0.4) for haloperidol-treated patients. This difference was not significant.
Safety
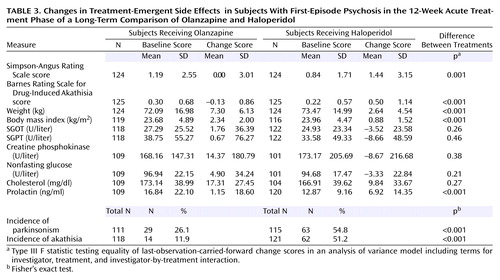
Significant between-treatment differences in the incidence of side effects were seen. Treatment-emergent extrapyramidal side effects were significantly more frequent and severe in the haloperidol-treated group, as reflected by the change in scores on the Simpson-Angus Rating Scale (F=11.96, df=1, 235, p<0.001) and Barnes Rating Scale for Drug-Induced Akathisia (F=25.99, df=1, 237, p<0.001) (Table 3). This difference was also evident from the two groups’ rates of treatment with concomitant medications for extrapyramidal signs. Of the haloperidol-treated patients, 52% received anticholinergics, 74% received benzodiazepines (administered for control of agitation as well as extrapyramidal signs), and 18% received propranolol, compared to 17%, 63%, and 2%, respectively, of the olanzapine-treated patients. These differences were statistically significant for anticholinergics (χ2=36.57, df=1, p<0.01) and propranolol (χ2=16.90, df=1, p<0.01) and marginally significant for benzodiazepines (χ2=4.13, df=1, p<0.05). The incidence of treatment-emergent parkinsonism (defined by a change in the Simpson-Angus Rating Scale score from ≤3 at baseline to >3 postbaseline) was significantly higher in the haloperidol group (54.8%) than in the olanzapine group (26.1%) (p<0.001, Fisher’s exact test). Similarly, the incidence of treatment-emergent akathisia (defined by a change in the Barnes Rating Scale for Drug-Induced Akathisia score from <2 at baseline to ≥2 postbaseline) was greater in the haloperidol group (51.2%) than in the olanzapine group (11.9%) (p<0.001, Fisher’s exact test). Haloperidol was also associated with greater increases in prolactin than olanzapine (mean=6.9 ng/ml, SD=14.4, versus mean=1.2 ng/ml, SD=18.6) (F=11.2, df=1, 203, p<0.001).
In contrast, the olanzapine-treated patients experienced more weight gain than the haloperidol-treated patients (last-observation-carried-forward mean=7.3 kg, SD=6.1, versus mean=2.6 kg, SD=4.5) (t=4.58, df=255, p<0.001). In the olanzapine group, 61.5% of the patients had a >7% increase in their body weight from baseline, compared to 22.7% of haloperidol patients (p<0.001, Fisher’s exact test). The patients’ body mass index increased after treatment by 2.39 in the olanzapine-treated group and 0.88 in the haloperidol-treated group (t=8.38, df=209, p<0.001). Olanzapine was also associated with mild but nonsignificant elevations of SGOT, SGPT, creatine phosphokinase, nonfasting glucose, and cholesterol levels.
Discussion
These results are consistent with those of prior studies of patients with first-episode schizophrenia and schizoaffective disorder that have found favorable rates of therapeutic response to antipsychotic drugs (4–6, 13, 20, 21). In a study that examined response to a standardized treatment protocol with conventional antipsychotic drugs and used response criteria similar to those in the current study, a median time to response of 9 weeks and a 52-week response rate of 87% were found in a cohort of 118 patients with first-episode schizophrenia, schizophreniform disorder, and schizoaffective disorder (5). To our knowledge, the first study of response to atypical drugs among first-episode patients was a 6-week, randomized, controlled trial comparing the effects of risperidone with those of haloperidol in 183 patients with schizophrenia and schizophreniform disorder (20).The response rates were 63% for risperidone and 56% for haloperidol. Sanger et al. (21) examined a subgroup of first-episode patients with schizophrenia, schizophreniform disorder, and schizoaffective disorder (N=83) from a large (total N=1,996) prospective, multicenter, international, double-blind, 6-week acute treatment study comparing olanzapine and haloperidol. The rate of clinical response was significantly higher for the olanzapine-treated patients (67.2%) than for the haloperidol-treated patients (29.2%). Recently, the results of a randomized, controlled trial of clozapine and chlorpromazine in a group of 160 Chinese patients with first-episode schizophrenia and schizophreniform disorder were reported (30). The 12- and 52-week response rates were 62% and 81%, respectively, for clozapine and 50% and 79%, respectively, for chlorpromazine; the median response times were 8 weeks for clozapine and 12 weeks for chlorpromazine. Although the response criteria of these studies varied, overall these results indicate that patients with first-episode psychosis are highly responsive to antipsychotic drug treatment, with a trend suggesting that atypical drugs exhibit some degree of superiority in response rate and time to response.
In the current study, both treatments produced substantial and significant within-treatment-group reductions in psychopathology from baseline by week 12. Although a statistically significant difference between the two antipsychotics was not found in the last-observation-carried-forward analysis, a random regression coefficient model demonstrated that olanzapine was superior to haloperidol in reducing symptoms, as measured by a difference between groups of approximately 6 points in the reduction of the Positive and Negative Syndrome Scale total score, the primary response variable. This modest difference approximates the 5-point mean difference observed in both groups at 12 weeks in the last-observation-carried-forward analysis. In addition, in the random regression coefficient model, olanzapine was associated with superior results on three of the secondary variables—the Positive and Negative Syndrome Scale negative and general scale scores and the Montgomery-Åsberg Depression Rating Scale score—but not on the CGI severity score and the Positive and Negative Syndrome Scale positive scale score.
There are several possible explanations for the differences in results between the last-observation-carried-forward analysis and random regression analysis. Last-observation-carried-forward analyses use only baseline and endpoint values, while random regression analyses use all data points. The mixed-model method has been used increasingly for the analysis of longitudinal data from clinical trials because it is capable of addressing more sophisticated questions about the rate and the pattern of change over time and because it makes different and perhaps more realistic assumptions about the meaning of missing data resulting from dropout. Last-observation-carried-forward analyses assume that the patients who drop out would continue to maintain their last score and that dropouts occur randomly and are not influenced by the treatment assignment or the level of symptoms. Neither assumption is necessarily justified. Another possible explanation may be that the mixed model does not assume a zero slope for subjects who drop out, and thus the effects for subjects who terminate early are decreased. The random regression model fits the rate of change and then assesses the differences between the two treatment groups in this rate of change. Although there may be no difference between the two drugs, as suggested by the last-observation-carried-forward results, this interpretation is not consistent with the differences in the raw observed case means. However, since mixed models assume that the data are missing at random (but not completely at random), these conclusions should be interpreted with caution.
In addition, significantly more patients in the olanzapine treatment group than in the haloperidol treatment group were retained in the study. This superiority in retention in treatment is consistent with the pattern of results of previous studies of atypical and conventional antipsychotic drugs in first-episode patients (20, 21, 30). This pattern of results suggests that atypical drugs are more likely to be associated with better long-term adherence and a potentially lower risk of symptomatic recurrence, as has been demonstrated with patients with chronic disorders (31).
Consistent with a greater pharmacodynamic sensitivity among first-episode patients, even the relatively low doses of haloperidol used in the current study were associated with rates of treatment-emergent parkinsonism and akathisia of 55% and 51%, respectively. In contrast, the rates of extrapyramidal signs associated with olanzapine were significantly lower. This difference may have contributed to the respective study retention rates. Among the 104 patients who discontinued before week 12, extrapyramidal signs were the reason for discontinuation for seven patients receiving haloperidol and no patients receiving olanzapine (p<0.04, Fisher’s exact test). The rates of extrapyramidal signs also reflect the well-known safety concerns about the tolerability of conventional antipsychotic drugs at therapeutic doses and the associated risk for subsequent development of tardive dyskinesia. Olanzapine-treated patients, on the other hand, gained a mean of 7.3 kg in 12 weeks, reflecting this phenomenon as seen in patients with a chronic disease history. The weight gain associated with olanzapine can also affect tolerability. However, in the present study, of the 104 subjects who discontinued at or before week 12, only two subjects receiving olanzapine and no subjects receiving haloperidol did so due to weight gain (p<0.17, Fisher’s exact test). Further research is necessary to characterize the relative medical risk that could occur as a consequence of weight gain associated with use of atypical antipsychotics. No significant differences in treatment effects on glucose or cholesterol were seen. These findings may suggest that metabolic disturbances are caused by independent mechanisms from those associated with weight change or that the measures and timing of assessments in this study were not sensitive enough to detect these effects.
These results warrant some additional comments. First, in acutely ill and severely symptomatic patients, relatively low doses of olanzapine and haloperidol were both effective in reducing symptom severity by the end of the 12-week acute phase. This finding does not support the premise that atypical drugs are less potent than conventional antipsychotics in controlling the symptoms of acutely ill and severely psychotic patients. Moreover, the use of adjunctive medication as a potentially confounding factor in comparing the relative efficacies of the two drugs actually demonstrated a lower frequency of coadministration, particularly of benzodiazepines, among the olanzapine-treated patients. Second, the fact that the doses of both drugs, and particularly of haloperidol, were kept low and yet they were still effective demonstrates the sensitivity of first-episode patients to pharmacologic effects. This pattern was reflected by the rates of both therapeutic effects and side effects. The use of low doses also served to minimize any potential bias against the conventional antipsychotic that could result from the neurological and/or behavioral toxicity that could be caused by higher doses. Third, the modest between-group differences in symptom improvement could be due to the fact that first-episode patients, in general, have such a good response to antipsychotic drugs. This pattern of good response may create a “ceiling effect” in measuring the comparative efficacies of treatments by using traditional measures of psychopathology. Thus, the challenge in treating first-episode patients may not be in getting them to respond to treatment but in sustaining treatment, as these patients may tend to discontinue treatment soon after recovery due to lack of insight or due to side effects. Finally, we observed other benefits associated with olanzapine treatment that were (relatively) independent of the treatment effects on psychopathology. These benefits, including improvement in cognitive function, greater relapse prevention, and reduced loss of gray matter volume, have been reported separately (32, 33), as well as with other drugs in different patient populations (34, 35), suggesting potentially superior therapeutic actions of atypical antipsychotic drug treatment.
In summary, this report of the 12-week acute treatment portion of a 104-week study comparing olanzapine to haloperidol adds to the literature on the treatment response of patients with first-episode psychosis and the comparative efficacy and safety of the atypical and conventional antipsychotic drugs. The present study results demonstrate some limited therapeutic advantages for the atypical drug olanzapine relative to haloperidol in terms of symptom reduction and treatment retention. In addition, olanzapine exhibited a lower incidence of extrapyramidal signs. However, olanzapine was associated with a greater weight gain. Additional studies will be needed to determine whether atypical medications reliably result in superior therapeutic response, tolerability, and overall outcome for patients being treated for a first episode of schizophrenia and related psychotic disorders.
 |
 |
 |
Received July 8, 2002; revision received Jan. 22, 2003; accepted Feb. 6, 2003. From the Department of Psychiatry, University of North Carolina School of Medicine; Lilly Research Laboratories, Indianapolis; the Department of Psychiatry, Duke University School of Medicine, Durham, N.C.; the Department of Psychiatry, Harvard Medical School, Boston; the Department of Psychiatry, University of Utrecht Medical School, Utrecht, the Netherlands; the Institute of Psychiatry, Maudsley Hospital, London; the Department of Psychiatry, University of Toronto School of Medicine, Toronto, Ont.; and the Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia. Address correspondence to Dr. Lieberman, Department of Psychiatry, C.B. 7160, University of North Carolina School of Medicine, Chapel Hill, NC 27599-7160; [email protected] (e-mail). Supported by Lilly Research Laboratories, Indianapolis, Ind., NIMH grants MH-00537 and MH-33127 to Dr. Lieberman, the UNC Mental Health and Neuroscience Clinical Research Center, the North Carolina Foundation of Hope, and NIMH grants MH-52376 and MH-62157 to Dr. Green. This paper was based partly on results from the Comparative Efficacy and Safety of Atypical and Conventional Antipsychotic Drugs in First-Episode Psychosis study conducted by the HGDH Study Group sponsored by Eli Lilly and Company. The HGDH Study Group consists of Drs. Jeffrey A. Lieberman and Diana Perkins, University of North Carolina School of Medicine, Chapel Hill, N.C.; Dr. Charles B. Nemeroff, Emory University School of Medicine, Atlanta; Drs. Franca Centorrino and Bruce Cohen, McLean Hospital, Harvard Medical School, Belmont, Mass.; Drs. Gary Tollefson, Todd Sanger, and Mauricio Tohen, Lilly Research Laboratories, Indianapolis; Drs. Joseph P. McEvoy, Cecil Charles, and Richard Keefe, John Umstead Hospital, Duke University Health System, Butner, N.C.; Dr. John Kuldau, University of Florida, Gainesville, Fla.; Dr. Alan I. Green, Massachusetts Mental Health Center, Harvard Medical School, Boston; Dr. Anthony J. Rothschild and Jayendra K. Patel, University of Massachusetts Medical Center, Worchester, Mass.; Dr. Raquel E. Gur, University of Pennsylvania Medical Center, Philadelphia; Drs. Robert B. Zipursky and Zafiris J. Daskalakis, University of Toronto School of Medicine, Toronto; Dr. Stephen M. Strakowski, University of Cincinnati, Cincinnati; Dr. Ira Glick, Stanford University School of Medicine, Stanford, Calif.; Dr. John De Quardo, University of Michigan Medical Center, Ann Arbor, Mich.; Prof. Dr. R.S. Kahn, University Hospital Utrecht, Utrecht, the Netherlands; and Dr. Tonmoy Sharma and Prof. Robin Murray, Institute of Psychiatry, London.
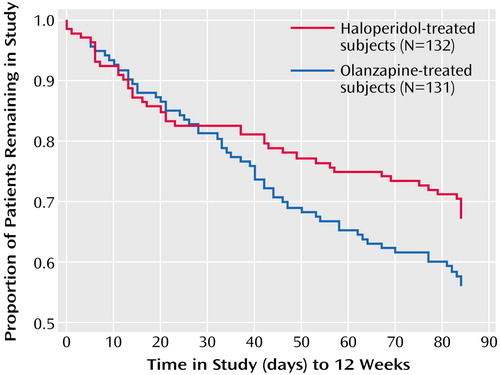
Figure 1. Time to Study Discontinuation for Any Reason of Subjects With First-Episode Psychosis in the 12-Week Acute Treatment Phase of a Long-Term Comparison of Olanzapine and Haloperidola
aNo significant difference between treatment groups in time to discontinuation (p=0.06, log rank test).
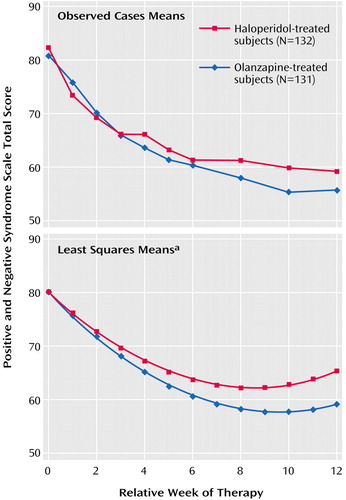
Figure 2. Observed Case Mean and Least Squares Mean Total Scores on the Positive and Negative Syndrome Scale of Subjects With First-Episode Psychosis in the 12-Week Acute Treatment Phase of a Long-Term Comparison of Olanzapine and Haloperidol
aThe fact that the least squares mean curves begin to slope upward toward the right of the plot should be interpreted as an artifact of the shared quadratic slope. The difference (and significance of the difference) between the least squares means for the two groups over the 12 weeks is invariant to that slope.
1. National Institute of Mental Health Psychopharmacology Service Center Collaborative Study Group: Phenothiazine treatment in acute schizophrenia. Arch Gen Psychiatry 1964; 10:246–261Crossref, Medline, Google Scholar
2. Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G: One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry 1994; 151:1409–1416Link, Google Scholar
3. Kane JM: Drug Therapy, Schizophrenia. N Engl J Med 2001:34–41Google Scholar
4. May PR, Tuma AH, Dixon WJ, Yale C, Thiele DA, Kraude WH: Schizophrenia: a follow-up study of the results of five forms of treatment. Arch Gen Psychiatry 1981; 38:776–784Crossref, Medline, Google Scholar
5. Sheitman BB, Lee H, Strous R, Lieberman JA: The evaluation and treatment of first-episode psychoses. Schizophr Bull 1997; 23:653–661Crossref, Medline, Google Scholar
6. Robinson DG, Woerner MG, Alvir JMJ, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA: Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999; 156:544–549Link, Google Scholar
7. Lieberman JA, Sheitman B, Chakos M, Robinson D, Schooler N, Keith S: The development of treatment resistance in patients with schizophrenia: a clinical and pathophysiological perspective. J Clin Psychopharmacol 1998; 18:20S-24SCrossref, Medline, Google Scholar
8. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar
9. Chakos MH, Alvir JM, Woerner MG, Koreen A, Geisler S, Mayerhoff D, Sobel S, Kane JM, Borenstein M, Lieberman JA: Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry 1996; 53:313–319Crossref, Medline, Google Scholar
10. McEvoy JP, Hogarty GE, Steingard S: Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991; 48:739–745Crossref, Medline, Google Scholar
11. Kane JM, Rifkin A, Woerner M, Reardon G, Sarantakos S, Schiebel D, Ramos-Lorenzi J: Low-dose neuroleptic treatment of outpatient schizophrenics, I: preliminary results for relapse rates. Arch Gen Psychiatry 1983; 40:893–896Crossref, Medline, Google Scholar
12. Crow TJ, MacMillan JF, Johnson AL, Johnstone EC: A randomised controlled trial of prophylactic neuroleptic treatment. Br J Psychiatry 1986; 148:120–127Crossref, Medline, Google Scholar
13. Tohen M, Stoll AL, Strakowski SM, Faedda GL, Mayer PV, Goodwin DC, Kolbrener ML, Madigan AM: The McLean first-episode psychosis project: six-month recovery and recurrence outcome. Schizophr Bull 1992; 18:172–185Crossref, Google Scholar
14. Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, Bratzokis G, Aravagiri M: Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001; 158:1835–1842Link, Google Scholar
15. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247Crossref, Medline, Google Scholar
16. Kinon BJ, Lieberman JA: Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl) 1996; 124:2–34Crossref, Medline, Google Scholar
17. Leucht S, Pitschel-Walz G, Abraham D, Kissling W: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res 1999; 35:51–68Crossref, Medline, Google Scholar
18. Geddes J, Freemantle N, Harrison P, Bebbington P: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Br Med J 2000; 321:1371–1376Crossref, Medline, Google Scholar
19. Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B: Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001; 158:518–526Link, Google Scholar
20. Emsley RA (Risperidone Working Group): Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull 1999; 25:721–729Crossref, Medline, Google Scholar
21. Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley C Jr, Tollefson GD: Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry 1999; 156:79–87Link, Google Scholar
22. Lieberman JA: Atypical antipsychotic drugs as a first-line treatment of schizophrenia: rationale and hypothesis. J Clin Psychiatry 1996; 57(suppl 11):68–71Medline, Google Scholar
23. Green AI, Schildkraut JJ: Should clozapine be a first-line treatment for schizophrenia? the rationale for a double-blind clinical trial in first-episode patients. Harv Rev Psychiatry 1995; 3:1–9Crossref, Medline, Google Scholar
24. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
25. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
26. COSTART: Coding Symbols for Thesaurus of Adverse Reaction Terms, 5th ed. Rockville, Md, Food and Drug Administration, Center for Drug Evaluation and Research, 1995Google Scholar
27. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
28. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
29. Barnes TRE: A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
30. Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji Z, Koch G, Hamer RM: Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 2003; 28:995–1003Crossref, Medline, Google Scholar
31. Wahlbeck K, Tuunainen A, Ahokas A, Leucht S: Dropout rates in randomised antipsychotic drug trials. Psychopharmacology (Berl) 2001; 155:230–233Crossref, Medline, Google Scholar
32. Keefe RS, Seidman LJ, Christensen BK, Yurgelun-Todd DA, Lewine RR, Sitskoorn MM, Lieberman JA: Treatment of neurocognitive deficits with olanzapine or low-dose haloperidol in first episode psychosis, in Abstracts of the VIII International Congress on Schizophrenia Research. Schizophrenia Research 2001; 49(1–2) [Suppl]:234Google Scholar
33. Lieberman J, Charles HC, Sharma T, Zipursky R, Kahn R, Gur R, Tohen M, Green AI, McEvoy J, Perkins D, Hamer RM, Nemeroff C, Rothschild A, Kuldau J, Strakowski S, Tollefson G: Antipsychotic treatment effects on progression of brain pathomorphology in first episode schizophrenia, in Abstracts of the IXth International Congress on Schizophrenia Research. Schizophrenia Research 2003; 60(1):293Google Scholar
34. Csernansky JG, Mahmoud R, Brenner R (Risperidone-USA-79 Study Group): A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002; 346:16–22Crossref, Medline, Google Scholar
35. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S: Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60:82–91Crossref, Medline, Google Scholar



