Dexamethasone Suppression Test Findings in Subjects With Personality Disorders: Associations With Posttraumatic Stress Disorder and Major Depression
Abstract
OBJECTIVE: Previous studies using the 1.0-mg dexamethasone suppression test (DST) in subjects with personality disorders have produced mixed results. However, these studies focused on major depression and did not consider the possible effects of the comorbidity of posttraumatic stress disorder (PTSD). PTSD has been shown to be associated with increased cortisol suppression. To investigate the effect of PTSD, the authors conducted a 0.5-mg DST, which is more sensitive than the 1.0-mg DST for detection of increased cortisol suppression, in a group of subjects with personality disorders. METHOD: Subjects with personality disorders (N=52) ingested 0.5 mg of dexamethasone. Pre- and postfasting blood samples were drawn for measurement of cortisol levels. A three-way analysis of covariance was used to test for the main effects of major depression, PTSD, and gender on percent cortisol suppression, with plasma dexamethasone concentration as a covariate. Secondary analyses assessed for main and interaction effects of age at which trauma(s) occurred and a diagnosis of borderline personality disorder. RESULTS: Neither major depression nor gender had a significant effect on percent cortisol suppression. Subjects with PTSD had significantly higher percent cortisol suppression than subjects with major depression. Age at which trauma(s) occurred and a borderline personality disorder diagnosis had no significant main or interaction effects on cortisol suppression. CONCLUSIONS: A high level of cortisol suppression was associated with PTSD in subjects with personality disorder. This finding is similar to published findings for PTSD subjects without personality disorders. Major depression, gender, age when trauma(s) occurred, and a diagnosis of borderline personality disorder did not have significant main or interaction effects on cortisol suppression.
More than 20 years ago, the high prevalence of depressive symptoms in patients with personality disorders led researchers to explore the relationship between affective disorders and borderline personality disorder by using phenomenological, epidemiological, and biological approaches (1). As the number of readily available biological tools was small, investigators used the 1.0-mg dexamethasone suppression test (DST), which had been found to demonstrate cortisol nonsuppression (postdexamethasone cortisol level >5 μg/dl) in approximately 40%–60% of depressed subjects. This test was used in studies of subjects with personality disorders (primarily borderline personality disorder) in an attempt to examine the biological interface between affective disorder and personality disorder. The results were equivocal and did not support the conclusion that borderline personality disorder was biologically similar to major depression (2).
Over the past few decades, it has become recognized that approximately 70% of individuals with personality disorders report a history of severe childhood abuse (3–8). Approximately 30% of subjects with personality disorders also meet the diagnostic criteria for posttraumatic stress disorder (PTSD) (9, 10). In view of these findings, some investigators have suggested that individuals with borderline personality disorder or related personality disorders who have childhood trauma histories should more parsimoniously be viewed as having a variant of PTSD, which they termed “complex PTSD” (4–6). Thus, it is tempting to apply the DST to examine the biological interface between personality disorders and PTSD. Since PTSD is associated with DST findings that are essentially the opposite of those in major depression, the question of the interface becomes more compelling, particularly as both current major depression and current PTSD can be considered as independent variables.
The DST provides a measure of hypothalamic-pituitary-adrenal (HPA) axis negative feedback sensitivity. HPA axis findings in major depression include: increased baseline cortisol (11), increased 24-hour urinary cortisol excretion (12, 13), decreased cortisol suppression after ingestion of 1.0 mg of dexamethasone (14–16), and decreased glucocorticoid receptor density on peripheral lymphocytes (17, 18). Among subjects with major depression, the findings are most robust in those with psychotic or melancholic depression.
In contrast, subjects with PTSD show lower levels of plasma cortisol than normal comparison subjects (19), decreased 24-hour urinary cortisol excretion (17, 20–23), and increased density of lymphocyte glucocorticoid receptors (18, 24, 25). As designed, the 1.0-mg DST is optimized for the detection of decreased HPA axis feedback sensitivity. By decreasing the dexamethasone dose from 1.0 mg to 0.5 mg, the DST is made more sensitive for the detection of increased HPA axis feedback sensitivity. Using a 0.5-mg DST, several studies have found that subjects with PTSD display enhanced suppression of cortisol, compared to both nontraumatized and trauma-exposed subjects without PTSD (18, 24–30).
Borderline personality disorder has been the most frequently studied personality disorder in research on HPA axis variables. Some studies have reported low rates of cortisol nonsuppression (2, 31–33), whereas others have reported high rates of cortisol nonsuppression (34–37) after the 1.0-mg DST. Although the subjects were evaluated for major depression, trauma history/comorbid PTSD was not evaluated and, therefore, was not included as an independent variable. A review of these studies found that nondepressed borderline personality disorder subjects were almost all suppressors of cortisol in response to the 1.0-mg DST, and subjects with comorbid borderline personality disorder and major depression had rates of nonsuppression lower than those of subjects with major depression alone (38). The 1-mg dose of dexamethasone given in these studies was too high and not appropriate for detection of enhanced cortisol suppression.
We hypothesized that among subjects with personality disorders, those with comorbid PTSD would show enhanced cortisol suppression after a 0.5-mg DST. We also hypothesized that major depression would be associated with decreased cortisol suppression. On the basis of pilot data (39), we did not anticipate that borderline personality disorder alone would be associated with any effects on cortisol suppression.
Method
Subjects
After complete description of the study to subjects, written informed consent was obtained. The protocol was approved by the institutional review board of the Mount Sinai School of Medicine. Fifty-two subjects (12 women and 40 men) between the ages of 19 and 60 years (inclusive) participated in this study. Subjects were recruited by newspaper advertisements and from referrals from mental health professionals who were aware of the research program. All subjects underwent a comprehensive medical evaluation to ensure that they were free of major medical/endocrine disorders. None of the subjects was more than 20% over or under their ideal body weight. Most of the subjects had never been treated with psychotropic medications. The others had not been taking any medications, including oral contraceptives, for at least 1 month (3 months for fluoxetine). None of the subjects had current or past alcohol or drug dependence or had an alcohol intake that exceeded 1 pint a day of distilled liquor for 10 years or a half-pint per day for 20 years. None of the subjects met the criteria for substance abuse for at least 6 months before participation. Subjects with prior heroin or persistent cocaine use or with intravenous drug use were excluded from the study. Urine samples for toxicology screening were obtained from all subjects, and the screening was repeated if an index of suspicion for recent substance use was present.
Psychological Testing
After initial screening determined their eligibility, subjects were given a packet of self-report questionnaires. Trauma history was assessed in all subjects by using two instruments with good reliability and validity. The Trauma History Questionnaire (40) was used because it assesses a wide range of traumatic experiences, including childhood abuse, assaults, and natural disasters. Subjects were also given the Childhood Trauma Questionnaire (41), which reliably assesses and yields a severity score for five types of traumatic childhood experiences (physical neglect, emotional neglect, physical abuse, sexual abuse, and emotional abuse). The information from these two instruments was used by the clinical interviewers for their assessment of PTSD. For diagnostic assessment, subjects were interviewed by a graduate-level clinical psychologist using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) and the Structured Clinical Interview for DSM-III-R Personality Disorders. PTSD diagnosis by SCID testing was confirmed and PTSD symptoms were quantified by using the Clinician-Administered PTSD Scale (42). The Clinician-Administered PTSD Scale is a structured clinical interview that is given after the trauma history assessment, since the latter facilitates subjects’ recollection of traumatic events. This instrument allows for a quantitative measure of symptom severity in each of the three symptom clusters and yields an overall symptom severity score. Twelve subjects did not complete the Clinician-Administered PTSD Scale. Subjects were also given the Beck Depression Inventory (43) for quantification of depressive symptoms.
Axis I and II diagnoses were established in a consensus meeting moderated by a senior clinician (J.S.). As we recognized the limitations of self-report data, we sought at least one interview of a family member for all participants. If we had any doubt about whether the subject met the inclusion criteria, the subject was not included in the study. Subjects who met the DSM-IV criteria or Research Diagnostic Criteria for schizophrenia or any schizophrenia-related psychotic disorders or for bipolar I disorder were excluded.
HPA Axis Assessments
On two consecutive mornings at 8:00 a.m., the subjects, who had fasted since midnight, reported to the General Clinical Research Center at the Mount Sinai Medical Center and at 8:15 a.m. had blood drawn and saved for analysis of cortisol level. Before leaving the General Clinical Research Center, each subject was given a 0.5-mg tablet of dexamethasone. The subject was telephoned at 10:55 p.m. and reminded to take the dexamethasone tablet. On day 2, the subjects returned to the General Clinical Research Center and at 8:15 a.m. had blood drawn for analysis of cortisol and dexamethasone levels. For female participants, no data were collected on the particular phase of the menstrual cycle at the time of the DST.
Cortisol level was determined by using a commercial radioimmunoassay kit (DiaSorin, Stillwater, Minn.). The inter- and intra-assay coefficients of variation were 4.0% and 6.8%, respectively. IgG Corporation (Nashville, Tenn.) radioimmunoassay kits were used for determination of dexamethasone concentrations. 3H-Dexamethasone was purchased from Amersham Pharmacia Biotech (Piscataway, N.J.). The cross-reactivity of the antibody with cortisol was 0.04% and less than 0.01% with other endogenous steroids. The inter- and intra-assay coefficients of variation were 4.9% and 5.9%, respectively. All subjects had detectible dexamethasone concentrations in day-2 blood samples.
Data Analysis
Although directional hypotheses were stated, tests of significance were two-sided.
PTSD, major depression, and gender
The main analysis was a three-way analysis of covariance (ANCOVA) for the effects of current comorbid PTSD, current comorbid major depression, and gender on percent cortisol suppression, with plasma dexamethasone concentration on day 2 as a covariate.
Childhood and adulthood trauma
In a secondary analysis, the effects of criterion A traumas occurring during childhood and those occurring during adulthood were analyzed by using a three-way ANCOVA for main effects of PTSD, childhood trauma, and adulthood trauma, with dexamethasone concentration on day 2 as a covariate. Since the previous analysis did not find major depression and gender to have a significant effect on the dependent variable, they were not included in this analysis.
Borderline personality disorder
Since it has been suggested that there may be a unique relationship between borderline personality disorder and PTSD, we conducted a secondary analysis using a two-way ANCOVA to examine the effects of borderline personality disorder and current comorbid PTSD, with plasma dexamethasone concentration as a covariate. Again, since the primary analysis did not find major depression and gender to have a significant effect on the dependent variable, they were not included in this secondary analysis.
For all these analyses, there were no significantly heterogeneous regressions of the dependent variable on the covariates.
To assess gender differences for dichotomous and continuous variables, chi-square and Student’s t tests were used, respectively. Partial correlation analyses, with dexamethasone concentration controlled, were used to assess for associations of percent cortisol suppression with continuous measures of severity of childhood trauma exposure (Childhood Trauma Questionnaire) and PTSD symptoms (Clinician-Administered PTSD Scale) among subjects who reported childhood or lifetime criterion A exposure, respectively.
Results
Clinical Characteristics of Subjects
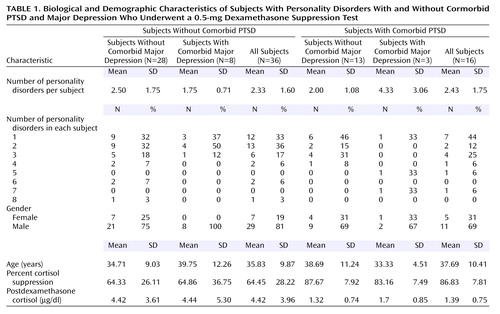
Fifty-two subjects participated in this study (12 women, 40 men). The mean number of DSM-III-R personality disorders per subject was 2.4 (SD=1.6). Twenty-one subjects met the criteria for borderline personality disorder. Table 1 summarizes data on the subjects’ demographic and biological characteristics.
Major depression
Eleven (21.2%) of the subjects met the DSM-III-R criteria for current major depression (five of these had melancholic-type depressions). An additional 18 subjects (34.6%) met the criteria for past major depression. There was no significant difference between female and male subjects in the rate of major depression. The mean Beck Depression Inventory score was significantly higher for subjects with current major depression (mean=25.44, SD=11.24, compared with mean=12.94, SD=9.38, for subjects without current major depression) (t=3.44, df=43, p=0.001).
Trauma and PTSD
Six subjects (12%) denied any history of a DSM-IV criterion A trauma, six subjects (12%) reported a criterion A trauma occurring only during childhood (four had PTSD), 24 subjects (46%) reported a criterion A trauma occurring only during adulthood (five had PTSD), and 16 subjects (31%) reported criterion A traumas occurring during both childhood and adulthood (seven had PTSD). In total, 46 (88%) of the subjects reported one or more criterion A traumas, which included childhood physical and sexual abuse, rape, torture, combat, violent assault, and motor vehicle accidents. Sixteen subjects (31%) met the DSM-IV criteria for PTSD. Eleven subjects (21%) had current major depression, and three of those subjects had comorbid PTSD. There were no gender differences in rates of criterion A trauma, childhood trauma, adulthood trauma, or PTSD. Current PTSD and major depression were not significantly associated.
Subjects with PTSD had a significantly higher total mean Childhood Trauma Questionnaire score (mean=65.12, SD=17.33, compared with mean=52.44, SD=15.16, for subjects without PTSD) (t=2.66, df=50, p=0.01) and a higher total mean Clinician-Administered PTSD Scale score calculated as the sum of the current frequency score and current severity score for the 17 diagnostic symptoms (mean=65.55, SD=22.42, compared with mean=17.93, SD=19.88, for subjects without PTSD) (t=6.53, df=38, p<0.0005). Female subjects had a higher mean Childhood Trauma Questionnaire score than male subjects (female subjects: mean=66.50, SD=22.84; male subjects: mean=53.30, SD=13.38) (t=2.51, df=50, p<0.02). There were no significant differences in Clinician-Administered PTSD Scale scores associated with gender (female subjects: mean=33.88, SD=27.37; male subjects: mean=30.31, SD=30.50) (t=0.30, df=38, p=0.77). A significant positive correlation was found between total Childhood Trauma Questionnaire score and total Clinician-Administered PTSD Scale score (r=0.40, N=40, p<0.02).
Effects on Cortisol Suppression
Gender, PTSD, and major depression
A three-way ANCOVA was performed to assess the effects of PTSD, major depression, and gender on percent cortisol suppression, with plasma dexamethasone concentration as a covariate. All of the female subjects with major depression also had PTSD, so it was not possible to assess the three-way interaction or adjust the estimates of other effects for this possible interaction. The effect of gender was not significant. There were no significant interactions. The covariate, dexamethasone concentration, was significantly associated with percent cortisol suppression (F=9.64, df=1, 44, p=0.003).
Compared to subjects without PTSD, subjects with PTSD had a significantly greater percent cortisol suppression, with adjustment for plasma dexamethasone concentration. The mean percent cortisol suppression values, adjusted for covariates, were 63.0 (95% confidence interval [CI]=54.3–71.8) for subjects without PTSD and 83.6 (95% CI=67.7–99.4) for subjects with PTSD (F=5.04, df=1, 44, p=0.03).
Subjects with and without major depression did not differ significantly in percent cortisol suppression values, with adjustment for plasma dexamethasone concentation (F=0.04, df=1, 44, p=0.85). The mean percent cortisol suppression values, adjusted for covariates, were 73.1 (95% CI=64.4–81.9) for subjects without major depression and 76.9 (95% CI=57.2–96.7) for subjects with major depression.
Childhood trauma, adulthood trauma, and PTSD
Results of a three-way ANCOVA showed that neither childhood history (F=2.07, df=1, 44, p=0.16) nor adulthood history (F=1.00, df=1, 44, p=0.32) of criterion A traumas had a significant effect on percent cortisol suppression. However, the effect of PTSD was highly significant (F=8.68, df=1, 44, p=0.005), and plasma dexamethasone concentration remained a significant covariate (F=9.20, df=1, 44, p=0.004). None of the interactions were significant.
Borderline personality disorder and PTSD
By a two-way ANCOVA, the factor of borderline personality disorder was not significant (F=2.02, df=1, 47, p=0.16), nor was there an interaction effect of borderline personality disorder and PTSD (F=0.22, df=1, 47, p=0.64). After the effect of borderline personality disorder was controlled, subjects with PTSD still had significantly greater percent cortisol suppression, with adjustment for plasma dexamethasone concentration (F=8.39, df=1, 47, p=0.006). The mean percent cortisol suppression values, adjusted for covariates, were 78.9 (95% CI=70.1–87.7) for subjects without borderline personality disorder and 69.2 (95% CI=58.8–79.6) for subjects with borderline personality disorder.
Correlations Between Cortisol Suppression and Trauma Measures
There were no significant correlations of percent cortisol suppression with the total Childhood Trauma Questionnaire score or any of the five Childhood Trauma Questionnaire subscale scores. Further, no significant correlations were found between percent cortisol suppression and Clinician-Administered PTSD Scale total score or any of the three Clinician-Administered PTSD Scale subscale scores.
Discussion
In a group of outpatients with personality disorders, subjects with comorbid PTSD had a significantly greater degree of cortisol suppression in response to a 0.5-mg DST than subjects without comorbid PTSD. These findings suggest that PTSD is a separate disorder that can be comorbid with personality disorders. Secondary analysis further showed that increased cortisol suppression was associated with the presence of PTSD, regardless of whether criterion A trauma(s) occurred during childhood, during adulthood, or during both periods. Although the analysis would be challenging, it may be of interest in future studies to attempt to quantify the extent of both childhood and adulthood trauma to ascertain whether these factors exert a significant effect, apart from PTSD, on cortisol suppression after ingestion of dexamethasone.
As hypothesized, neither borderline personality disorder nor the interaction of borderline personality disorder with PTSD had a significant effect on cortisol response to the 0.5-mg DST, indicating that individuals with borderline personality disorder do not constitute a “special case” within the personality disorders in terms of HPA axis function. However, it is important to consider whether the factor of personality disorder, in itself, has a direct effect on cortisol suppression. This relationship could not be directly assessed in the current study, owing to the lack of a comparison group without personality disorder pathology. Pertinent data on percent cortisol suppression after a 0.5-mg DST is available from a prior study (18). In the PTSD group in that study, the mean percent cortisol suppression was 89.7, compared with 73.4 for a non-PTSD group of nonpsychiatric subjects. Although personality disorders were not explicitly tested for, the normal comparison subjects were all high-functioning, employed individuals without a history of psychiatric problems. There were no significant differences in percent cortisol suppression between subjects with and without personality disorder among those without PTSD (t=1.11, df=48, p=0.27) or among those with PTSD (t=1.08, df=28, p=0.29), indicating the absence of a personality disorder effect whether or not PTSD was present.
Postdexamethasone 8:00 a.m. cortisol level can also be used to compare current findings with previously published reports. If the primary analysis (three-way ANCOVA for main effects of PTSD, major depression, and gender, with plasma dexamethasone concentration as a covariate) is repeated with the 8:00 a.m. postdexamethasone cortisol level as the dependent variable, a significant effect is again found for PTSD (F=4.24, df=1, 44, p<0.05) but not for major depression (F=0.002, df=1, 44, p=0.96) or gender (F=0.02, df=1, 44, p=0.88). Data are available to allow current findings for this variable to be compared with those of three prior studies that had both PTSD groups and nonpsychiatric comparison groups with low levels of personality disorder pathology. These studies found 8:00 a.m. postdexamethasone cortisol levels for nonpsychiatric comparison subjects and PTSD subjects to be 3.91 μg/dl and 1.09 μg/dl, respectively (18), 4.78 μg/dl and 1.78 μg/dl, respectively (25), and 3.22 μg/dl and 1.45 μg/dl, respectively (28) (nonweighted means=3.97 μg/dl and 1.44 μg/dl, respectively). These nonweighted means are similar to our current findings for mean 8:00 a.m. postdexamethasone cortisol levels of 4.42 μg/dl for subjects without PTSD and 1.39 μg/dl for subjects with comorbid PTSD (Table 1). An analysis of variance comparing our current findings with the three earlier reports did not indicate any significant differences among the four study groups for subjects without PTSD (F=0.70, df=3, 161, p=0.55) or for subjects with PTSD (F=1.04, df=3, 161, p=0.38). Again, these results support the assertion that the factor of personality disorder does not exert a significant effect on degree of cortisol suppression in response to the 0.5-mg DST.
It is noteworthy that just as the 0.5-mg DST is more sensitive for detection of increased HPA feedback sensitivity (increased cortisol suppression), reciprocally, it is less sensitive than the 1.0-mg DST for the detection of decreased HPA feedback sensitivity (decreased cortisol suppression). Because a lower dose of dexamethasone was used in this study, we were not able to categorize subjects by using the standard term “DST nonsuppressor.” It may be that in subjects with personality disorders, major depression is associated with decreased cortisol suppression, but our test was not sensitive enough to detect this relationship. In this light, it would be of interest to repeat the current study by using both a 0.5-mg and a 1.0-mg DST. This design would allow associations with both increased and decreased cortisol suppression to be better explored.
We have avoided use of dichotomous terms such as “hyper-” or “hyposuppressor” because percent cortisol suppression and 8:00 a.m. postdexamethasone cortisol levels occur on a continuum, and we have not endeavored to designate numerical cutoff values for such categories on the 0.5-mg DST. Further, we do not want to foster the notion that, as a whole, either of our groups fall into a category that has classically been defined by endocrinologists as physiologically pathological.
In this study, the presence of current major depression did not have a significant effect on cortisol suppression. From prior studies using the 1-mg DST in patients with major depression, it may have been anticipated that comorbid major depression would be associated with a decrease in percent cortisol suppression. However, in studies of subjects with personality disorder (primarily borderline personality disorder), findings were quite mixed and patterns of negative feedback sensitivity were unclear. The current study findings offer a potential explanation—that prior studies of dexamethasone suppression in personality disorders (and many studies that included primarily subjects with major depression) did not analyze the effects of comorbid PTSD, which is associated with an opposite effect on cortisol suppression. The prevalence of PTSD in borderline personality disorder, for example, is approximately 30% (9, 10), and the subjects with personality disorder in the current study had a similar rate of PTSD. The increased suppression of cortisol in response to dexamethasone associated with comorbid PTSD in subjects with personality disorder adds a confounding effect that interferes with assessment of the effect of personality disorder.
Typically, findings of decreased cortisol suppression in major depression are more robust in cases of severe major depression such as the psychotic or melancholic major depressive subtypes. Substantial proportions of subjects with personality disorders often experience “atypical” major depression, which is less frequently associated with decreased cortisol suppression. The fact that 50% of the subjects with comorbid major depression in this study had a melancholic-type depression mitigates the possibility that major depression subtype was a confounding factor.
An interesting possibility indicated by a previous study (44) is that there are significant neuroendocrine differences between patients with major depression who have a history of trauma and patients with major depression who do not have a trauma history. The data from only the depressed subjects without PTSD in the current study (N=8) are examined with ANCOVA, with plasma dexamethasone concentration as a covariate, the difference in percent cortisol suppression between subjects who did (N=5) and did not (N=3) endorse a past DSM-IV criterion A trauma approached significance; subjects without a trauma history had lower cortisol suppression (mean=50.33, SD=52.55, versus mean=73.58, SD=26.70 for subjects with a trauma history) (F=5.53, df=1, 5, p=0.07). The earlier study (44) did not evaluate subjects for PTSD but rather divided subjects into groups on the basis of severity of trauma history. Ideally, it would be desirable for future studies to have sufficient power to evaluate the effects of PTSD and major depression and the effect of trauma history within subjects with major depression.
 |
Received July 6, 2002; revision received Oct. 2, 2002; accepted Dec. 26, 2002. From the Departments of Psychiatry and Biomathematical Sciences, Mount Sinai School of Medicine; the Department of Psychiatry, Bronx Veterans Affairs Medical Center, Bronx, N.Y.; and the Department of Psychology, University of Pittsburgh Medical Center, Pittsburgh. Address reprint requests to Dr. Grossman, Department of Psychiatry, Box 1230, Mount Sinai School of Medicine, New York, New York 10029-6574; [email protected] (e-mail). Supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression and NIMH grant MH-58697-01 to Dr. Grossman, NIMH grant MH-49555 to Dr. Yehuda, and NIH grant 5-M01-RR-0071 to the Mount Sinai General Clinical Research Center.
1. Gunderson JG, Elliott GR: The interface between borderline personality disorder and affective disorder. Am J Psychiatry 1985; 142:277-288Link, Google Scholar
2. Lahmeyer HW, Val E, Gaviria FM, Prasad RB, Pandey GN, Rodgers P, Weiler MA, Altman EG: EEG sleep, lithium transport, dexamethasone suppression, and monoamine oxidase activity in borderline personality disorder. Psychiatr Res 1988; 25:19-30Crossref, Medline, Google Scholar
3. Bryer JB, Nelson BA, Miller JB, Krol PA: Childhood sexual and physical abuse as factors in adult psychiatric illness. Am J Psychiatry 1987; 144:1426-1430Link, Google Scholar
4. Herman JL, Perry JC, van der Kolk BA: Childhood trauma in borderline personality disorder. Am J Psychiatry 1989; 146:490-495Link, Google Scholar
5. Herman JL: Trauma and Recovery. New York, Basic Books, 1992Google Scholar
6. Ogata SN, Silk KR, Goodrich S, Lohr NE, Westen D, Hill EM: Childhood sexual and physical in adult patients with borderline personality disorder. Am J Psychiatry 1990; 147:1008-1013Link, Google Scholar
7. van der Kolk BA, Perry JC, Herman JL: Childhood origins of self-destructive behavior. Am J Psychiatry 1991; 148:1665-1671Link, Google Scholar
8. Zanarini MC, Gunderson JG, Marino MF, Schwartz EO, Frankenburg FR: Childhood experiences of borderline patients. Compr Psychiatry 1989; 30:18-25Crossref, Medline, Google Scholar
9. Swartz M, Blazer D, George L, Winfield I: Estimating the prevalence of borderline personality disorder in the community. J Personal Disord 1990; 4:257-272Crossref, Google Scholar
10. Stone M, Hurt S, Stone D: The PI500: long-term follow-up of borderline inpatients meeting DSM-III criteria, 1: global outcome. J Personal Disord 1987; 1:291-298Crossref, Google Scholar
11. Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ: Cortisol regulation in post-traumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 1996; 40:79-88Crossref, Medline, Google Scholar
12. Sacher EJ, Hellman L, Fukushima DK, Gallager RF: Cortisol production in depressive illness. Arch Gen Psychiatry 1970; 23:289-298Crossref, Medline, Google Scholar
13. Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugarman AA: Urinary free cortisol in depression. Psychol Med 1976; 6:43-50Crossref, Medline, Google Scholar
14. Carroll BJ: The dexamethasone suppression test for melancholia. Br J Psychiatry 1982; 140:292-304Crossref, Medline, Google Scholar
15. Sherman B, Pfohl B, Winokur G: Circadian analysis of plasma cortisol levels before and after dexamethasone administration in depressed patients. Arch Gen Psychiatry 1984; 41:271-275Crossref, Medline, Google Scholar
16. APA Task Force on Laboratory Tests in Psychiatry: The dexamethasone suppression test: an overview of its current status in psychiatry. Am J Psychiatry 1987; 144:1253-1262Link, Google Scholar
17. Yehuda R, Boisoneau D, Mason JW, Giller EL: Relationship between lymphocyte glucocorticoid receptor number and urinary-free cortisol excretion in mood, anxiety, and psychotic disorder. Biol Psychiatry 1993; 34:18-25Crossref, Medline, Google Scholar
18. Yehuda R, Boisoneau D, Lowry MT, Giller EL: Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995; 52:583-593Crossref, Medline, Google Scholar
19. Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ: Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Ann NY Acad Sci 1994; 746:378-380Crossref, Medline, Google Scholar
20. Bourne PG, Rose RM, Mason JW: Urinary 17-OHCS levels: data on seven helicopter ambulance medics in combat. Arch Gen Psychiatry 1967; 17:104-110Crossref, Medline, Google Scholar
21. Bourne PG, Rose RM, Mason JW: 17-OHCS levels in combat: special forces “A” team under threat of attack. Arch Gen Psychiatry 1968; 19:135-140Crossref, Medline, Google Scholar
22. Yehuda R, Southwick SM, Nussbaum G, Giller EL, Mason JW: Low urinary cortisol excretion in PTSD. J Nerv Ment Dis 1990; 178:366-369Crossref, Medline, Google Scholar
23. Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L: Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 1986; 174:145-159Crossref, Medline, Google Scholar
24. Yehuda R, Giller EL, Southwick SM, Lowry MT, Mason JW: Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry 1991; 30:1031-1048Crossref, Medline, Google Scholar
25. Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW: Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150:83-86Link, Google Scholar
26. Yehuda R, Giller EL, Levengood RA, Southwick SM, Siever LJ: Hypothalamic-pituitary-adrenal functioning in post-traumatic stress disorder: expanding the stress-response spectrum, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder. Edited by Friedman MH, Charney DS, Deutch AY. New York, Raven Press, 1995, pp 351-365Google Scholar
27. Heim C, Ehlert U, Hanker JP, Hellhammer DH: Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med 1998; 60:309-318Crossref, Medline, Google Scholar
28. Stein MB, Yehuda R, Koverola C, Hanna C: Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 1997; 42:680-686Crossref, Medline, Google Scholar
29. Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA: Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry 1996; 153:929-934Link, Google Scholar
30. Kellner M, Baker DG, Yehuda R: Salivary cortisol in Operation Desert Storm returnees. Biol Psychiatry 1997; 41:849-850Crossref, Google Scholar
31. Soloff PH, George A, Nathan RS: The dexamethasone suppression test in patients with borderline personality disorders. Am J Psychiatry 1982; 139:1621-1623Link, Google Scholar
32. Nathan RS, Soloff PH, George A, Peters JL, McCarthy T: DST and TRH tests in borderline personality disorder, in Biological Psychiatry: Proceedings of the IVth World Congress of Biological Psychiatry. Edited by Shagass C, Josiassen RG, Wagner BH, Weiss KJ, Stoff D, Simpson GM. New York, Elsevier, 1986, pp 563-565Google Scholar
33. Siever LJ, Trestman RL: The serotonin system and aggressive personality disorder. Int Clin Psychopharmacol 1993; 8:33-39Crossref, Medline, Google Scholar
34. Carroll BJ, Greden JF, Feinberg M, Lohr N, James NM, Steiner M, Haskett RF, Albala AA, DeVigne JP, Tarika J: Neuroendocrine evaluation of depression in borderline patients. Psychiatr Clin North Am 1981; 4:89-99Crossref, Medline, Google Scholar
35. Beeber AR, Kline MD, Pies RW, Manning JM Jr: Dexamethasone suppression test in hospitalized depressed patients with borderline personality disorder. J Nerv Ment Dis 1984; 172:301-303Crossref, Medline, Google Scholar
36. Krishnan KR, Davidson JR, Rayasam K, Shope F: The dexamethasone suppression test in borderline personality disorder. Biol Psychiatry 1984; 19:1149-1153Medline, Google Scholar
37. Baxter L, Edell W, Gerner R, Fairbanks L, Gwirtsman H: Dexamethasone suppression test and axis I diagnosis of inpatients with DSM-III borderline personality disorder. J Clin Psychiatry 1984; 45:150-153Medline, Google Scholar
38. Lahmeyer HW, Reynolds CF, Kupfer DJ, King R: Biologic markers in borderline personality disorder: a review. J Clin Psychiatry 1989; 50:217-225Medline, Google Scholar
39. Grossman R, Yehuda R, Siever L: The dexamethasone suppression test and glucocorticoid receptors in borderline personality disorder. Ann NY Acad Sci 1997; 821:459-464Crossref, Medline, Google Scholar
40. Green BL: Trauma History Questionnaire, in Measurement of Stress, Trauma, and Adaptation. Edited by Stamm BH. Lutherville, Md, Sidran Press, 1996, pp 366-369Google Scholar
41. Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J: Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994; 151:1132-1136Link, Google Scholar
42. Blake D, Weathers F, Nagy D, Kaloupek G, Klauminzer D, Charney D, Keane T: A clinician rating scale for current and lifetime PTSD. Behavior Therapist 1990; 13:187-188Google Scholar
43. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561-571Crossref, Medline, Google Scholar
44. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB: Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284:592-597Crossref, Medline, Google Scholar



