Suicide Rates in Clinical Trials of SSRIs, Other Antidepressants, and Placebo: Analysis of FDA Reports
Abstract
OBJECTIVE: Previous reports suggesting that selective serotonin reuptake inhibitor (SSRI) use is associated with increased suicidal risk have not assessed completed suicides. The authors analyzed reports from randomized controlled trials to compare suicide rates among depressed patients assigned to an SSRI, other antidepressants, or placebo. METHOD: Food and Drug Administration (FDA) summary reports of the controlled clinical trials for nine modern FDA-approved antidepressants provided data for comparing rates of suicide. RESULTS: Of 48,277 depressed patients participating in the trials, 77 committed suicide. Based on patient exposure years, similar suicide rates were seen among those randomly assigned to an SSRI (0.59%, 95% confidence interval [CI]=0.31%–0.87%), a standard comparison antidepressant (0.76%, 95% CI=0.49%–1.03%), or placebo (0.45%, 95% CI=0.01%–0.89%). CONCLUSIONS: These findings fail to support either an overall difference in suicide risk between antidepressant- and placebo-treated depressed subjects in controlled trials or a difference between SSRIs and either other types of antidepressants or placebo.
A decade ago, Teicher et al. (1) suggested, on the basis of case reports, that treatment with some selective serotonin reuptake inhibitor (SSRI) antidepressants might selectively increase suicidality. This possibility continues to be raised and remains to be either definitely proven or put to rest (2–6). Data pertaining to potential suicidal risk during SSRI treatment include reports of suicidal ideation, impulses, or attempts but only anecdotal reports of suicide (1–6). To further address this question, we reviewed suicide data from summary basis of approval reports obtained from the U.S. Food and Drug Administration (FDA) through the Freedom of Information Act (7), following methods reported previously (8, 9). We tested for differences in reported rates of suicide among depressed patients randomly assigned to treatment with an investigational (but subsequently FDA-approved) SSRI antidepressant compared with similar subjects assigned to another standard antidepressant or to placebo.
Method
We obtained FDA clinical trial data for nine antidepressants (fluoxetine, sertraline, paroxetine, venlafaxine, nefazodone, mirtazapine, sustained-release bupropion, extended-release venlafaxine, and citalopram) ultimately approved for use in the United States between January 1985 and January 2000. The data were sent on microfiche or a hard paper copy for a small fee by a specific request to the FDA (FDA Freedom of Information Staff, 5600 Fishers Lane, HFI-35 Rockville, MD 20857).
Rates of suicide were classified for patients assigned to an SSRI, placebo, or another class of antidepressant. The SSRIs were fluoxetine, sertraline, paroxetine, citalopram, and one “active control” (fluvoxamine). The other antidepressants were the investigational agents nefazodone, mirtazapine, bupropion, and venlafaxine (both immediate and extended release) as well as six active controls (imipramine, amitriptyline, maprotiline, trazodone, mianserin, and dothiepin).
For purposes of data analysis, we scrutinized all the suicides from all FDA summary basis of approval reports and ascertained the antidepressant the patients were taking at the time they committed suicide. For example, a patient who was in one of the paroxetine clinical trials had been assigned to 6 weeks of clomipramine during the trial followed by 4 weeks of fluvoxamine therapy, at which time he hung himself. We classified this as a suicide while patient was receiving an SSRI. Thus, this approach is unlike our approach in earlier publications (8, 9). In our earlier reports we had combined suicide rates and suicide attempt rates based on groups of trials conducted for each investigational antidepressant.
We attempted to assess both rates of suicide and suicide attempts. From all of the FDA summary basis of approval reports, we were able to assess completed suicides. However, data for suicide attempts were either not included (fluoxetine, immediate-release venlafaxine, or bupropion) or were hard to classify by specific agent. Thus, we are including only completed suicides for this report.
We tabulated the number of suicides in relation to the total number of patients randomly assigned to a treatment condition in the antidepressant clinical trials. Additionally, we were able to estimate the incidence of suicide by patient exposure years (i.e., cumulative time that subjects were exposed to an investigational antidepressant, an active comparator, or placebo while participating in a research program). Suicide rates based on patient exposure years were not available from the trials for fluoxetine or bupropion.
Additionally, when available from the FDA summary basis of approval reports, we classified the method of suicide. This was done so that we could assess if method of suicide differed between the three trial assignments. We found six methods of suicide: hanging, overdose, drowning, gunshot, jumping, and carbon monoxide poisoning.
We used chi-square analyses to assess the statistical significance of differences in suicide frequency among the subjects receiving placebo, SSRIs, and other antidepressants. Further, we calculated 95% confidence intervals (CI) to estimate the probability of overlap of suicide risk among the three treatment assignments. Because of the six methods of suicide and small number of patients involved, we did not perform any statistical tests.
Results
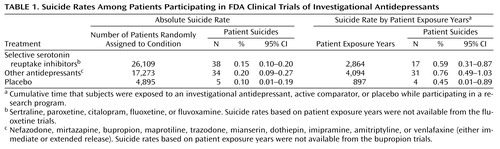
As seen in Table 1, there was no statistical difference in crude suicide rates among patients assigned to SSRIs, other antidepressants, or placebo (χ2=2.83, df=2, p>0.05). In addition, when groups were compared on the basis of patient exposure years, there was no statistical difference in suicide rate among patients assigned to SSRIs, other antidepressants, or placebo (χ2=1.39, df=2, p>0.05).
Methods of suicide were available for 39 of the 77 completed suicides. Of the 38 suicides of patients assigned to an SSRI, three were by hanging, two were by overdose, and one was by drowning. Of the 34 suicides of patients assigned to other antidepressants, nine were by hanging, six were by overdose, six were by drowning, five were by gunshot, three were by jumping, and three were by carbon monoxide poisoning. Of the five suicides of patients assigned to placebo, the one completed suicide for which the method was known was by hanging. No clear differences were observed for patients treated with SSRIs compared with patients given other antidepressants.
Discussion
In response to earlier reports that SSRIs may increase suicidal ideation, suicidal impulses, or suicide attempts (1–6), this study aimed to assess whether the suicide rate was higher among depressed patients assigned to SSRI antidepressants compared with those assigned to other antidepressants or to placebo. Our results suggest that suicide rates are similar among all of the three trial assignments. Further, method of suicide did not appear to differ among the three trial assignments.
These findings do not conclusively prove whether onset of suicidal ideation, suicidal impulses, or suicide attempts with SSRI treatment—either as a result of drug-induced akathisia or some other means—does lead to completed suicides. We could not fully assess overall suicide risk as measured by suicidal ideation, suicidal impulsivity, suicidal gestures or attempts, or the ratio of suicide attempts to completed suicides in the study group, since much of these data were missing. Thus, the only possible conclusion supported by the present data is that prescription of SSRI antidepressants is not associated with greater risk of completed suicide. Also, no specific form of assessment or vigilance is warranted as to means of suicide with SSRI antidepressants, since method of suicide does not appear to be any more impulsive for these drugs than for other antidepressants.
Antidepressant clinical trial participants are not identical to routine clinical samples of depressed patients. They are mild to moderately depressed, not actively suicidal when they enter the trial, and are mostly outpatients without comorbid psychiatric or medical illnesses or substance abuse disorders. This database only included patients who lacked psychotic features and who never had a hypomanic or manic episode. Further, the length of participation in clinical trials is generally shorter than what patients receive in general clinical practice. Routine clinical practice is also usually associated with higher doses of medication as well as the use of other medications. Thus, there are limitations to extrapolating our findings to all depressed patients. Nonetheless, it is noteworthy that the annual suicide rate for depressed clinical trial participants (0.66%) was similar to annual suicide rates reported for patients diagnosed with major affective disorders over years of risk and varied treatment conditions (0.30%–0.80%) and was nearly 40 times higher than the recent estimate for the general international population (0.0166%) (10, 11).
The numerically lower (but not significantly lower) suicide rate in depressed patients assigned to placebo may be in part due to the relatively short duration of exposure to placebo inantidepressant clinical trials, particularly for patients who only received placebo during the run-in phase (a period in which all of the trial participants were given placebo for about 1 week before random assignment). Paradoxically, we found the suicide rate to be numerically higher in non-SSRI-treated patients but not significantly different from suicide rates in the placebo- or SSRI-treated groups.
These findings need replication from other databases. Also, the question as to whether SSRIs induce suicidal ideation, suicidal impulses, and suicide attempts needs clarification. This report is based on suicide rates with antidepressants conventionally described as SSRIs and those conventionally assigned to other classes. These designations do not accurately describe the intrinsic pharmacological properties of antidepressants. Thus, our findings do not address the relationship between serotonin activity and suicide. Last, these data indicate that greater suicide risk is not associated with any type/class of antidepressants.
In conclusion, data from our analysis suggest that use of antidepressants from any of the existing subclasses of drugs has little effect on suicide rates in clinical trials. Prescription of SSRIs does not seem to be associated with higher suicide rates.
 |
Received Aug. 6, 2002; revision received Oct. 29, 2002; accepted Dec. 2, 2002. From the Northwest Clinical Research Center, Bellevue, Wash.; the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, N.C.; the Department of Psychology, Eastern Washington University, Cheney, Wash.; the Department of Psychiatry, Brown University, Providence, R.I.; and Tufts University, Boston. Address reprint requests to Arif Khan, M.D., 1900 116th Ave. NE #112, Bellevue, WA 98004; [email protected] (e-mail). The authors thank Ross J. Baldessarini, M.D., for editorial assistance.
1. Teicher MH, Glod C, Cole JO: Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry 1990; 147:207-210Link, Google Scholar
2. Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH, Heiligenstein JH, Thompson VL, Murphy DJ, Masica DN: Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. Br Med J 1991; 303:685-692Crossref, Medline, Google Scholar
3. Healy D, Langmaak C, Savage M: Suicide in the course of the treatment of depression. J Psychopharmacol 1999; 13:94-99Crossref, Medline, Google Scholar
4. Healy D: A failure to warn. Int J Risk and Safety in Med 1999; 12:151-156Google Scholar
5. Healy D: Emergence of antidepressant induced suicidality. Primary Care Psychiatry 2000; 6:23-28Crossref, Google Scholar
6. Tranter R, Healy H, Cattell D, Healy D: Functional effects of agents differentially selective to noradrenergic or serotonergic systems. Psychol Med 2002; 32:517-524Crossref, Medline, Google Scholar
7. Freedom of Information Act:5 US Congress. 552 (1994 and Supplement II 1996). Available at http://www.usdoj.gov/04foia/Google Scholar
8. Khan A, Warner HA, Brown WA: Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration Database. Arch Gen Psychiatry 2000; 57:311-317Crossref, Medline, Google Scholar
9. Khan A, Khan SR, Leventhal RM, Brown WA: Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: a replication analysis of the Food and Drug Administration Database. Int J Neuropsychopharmacol 2001; 4:113-118Crossref, Medline, Google Scholar
10. Harris EC, Barraclough B: Suicide as an outcome for mental disorders: a meta analysis. Br J Psychiatry 1997; 170:205-208Crossref, Medline, Google Scholar
11. Baldessarini RJ, Tondo L, Hennen J: Reduced suicide risk during long-term treatment with lithium. Ann NY Acad Sci 2001; 932: 24-43Google Scholar



