Low Olfactory Bulb Volume in First-Degree Relatives of Patients With Schizophrenia
Abstract
OBJECTIVE: There is a substantial genetic contribution to schizophrenia but no way to readily identify individuals at risk. Biological abnormalities reflecting greater genetic vulnerability may be discovered by examining healthy family members of patients with schizophrenia. There is evidence that olfactory impairments are common in patients. The authors previously reported that patients have abnormal olfactory bulbs, assessed by magnetic resonance imaging (MRI). This study examined olfactory bulbs in patients’ relatives to determine whether low bulb volume represents an endophenotypic marker of genetic vulnerability. METHOD: Olfactory psychophysical measures and MRI scans of olfactory bulbs were acquired from 19 healthy first-degree relatives, 20 healthy comparison subjects with similar age and gender distributions, and the 11 patient probands of these relatives. Olfactory bulb volumes were measured by using a reliable region-of-interest procedure. RESULTS: The patients had impaired ability to detect odors and had lower olfactory bulb volumes than the comparison subjects. Although the family members had normal olfactory ability, they exhibited low right bulb volume. The patients had smaller left, but not right, olfactory bulbs than their own healthy relatives. CONCLUSIONS: The findings in family members suggest that structural abnormalities of the olfactory system in schizophrenia may partly reflect preexisting genetic vulnerability to illness. Preliminary analyses suggest that right olfactory bulb volume may serve as an endophenotypic marker of genetic vulnerability, while left bulb volume may reflect overt disease among individuals who share genetic vulnerability. Bulb abnormalities in patients are consistent with reports of cellular abnormalities affecting peripheral olfactory receptor neurons.
Epidemiological studies have established that there is a substantial genetic contribution to the transmission of schizophrenia (1). However, a genetic vulnerability need not result in clinical symptoms. The precise nature and mode of inheritance of this genetic risk are not clear. Thus, it is not possible to identify individuals who carry the genetic diathesis for schizophrenia. One approach to this problem has been to look for biological or behavioral measures that denote greater than average genetic predisposition to the disorder. For example, eye movement dysfunctions (2), cognitive impairments (3), sensory gating deficits (4, 5), and low brain volumes (6) have all been proposed as markers of genetic susceptibility to schizophrenia. The identification of such “latent trait” or “endophenotypic” markers can contribute to understanding of the disorder by illuminating the mechanisms of gene action, distinct from the clinical phenotype. This can facilitate linkage analyses designed to identify the loci of genes associated with these endophenotypes (7).
There is increasing evidence that impairments in olfactory function are common in patients with schizophrenia (8, 9). This is not surprising, as olfactory processing is mediated by structures also implicated in schizophrenia, including the ventromedial temporal lobe and basal forebrain. The olfactory system thus shares a common neural substrate with cognitive and emotional processes linked to schizophrenia. Behavioral studies of the olfactory system have demonstrated impairments in odor detection, odor identification, and odor recognition memory. These deficits are present early in the course of the disorder and are unrelated to illness severity, neuroleptic use, or smoking (8). The olfactory system is highly genetically predetermined (10). Although findings in studies of unaffected family members of patients with schizophrenia (11–13) have been inconsistent, some deficits have been found in this cohort (11, 12). The olfactory deficits noted in schizophrenia may, therefore, partly reflect a heritable vulnerability factor. We have reported the presence of a structural abnormality in the olfactory system (14). Patients with schizophrenia had lower olfactory bulb volumes than age- and gender-matched comparison subjects. In this study, we examined the olfactory bulb volumes of healthy first-degree relatives of patients with schizophrenia. If these subjects also exhibited low bulb volume, it would provide strong evidence for a genetically mediated abnormality affecting the structural integrity of the olfactory system.
Method
Subjects
Nineteen first-degree relatives (12 male, seven female) of patients with schizophrenia were recruited by the University of Pennsylvania Schizophrenia Research Center. Twenty healthy individuals (12 male, eight female), without history of schizophrenia or affective illness in a first-degree relative, were recruited from the community. The family member cohort included six parents and 13 siblings related to 11 patients (seven male, four female). Three of these 11 patients were included in our previous study of olfactory bulb deficits (14). All subjects received a psychiatric interview with the Structured Clinical Interview for DSM-IV, Patient or Nonpatient Edition (15, 16), and a physical examination, including routine laboratory tests. The patient probands were rated on the Brief Psychiatric Rating Scale (BPRS) (17), the Scale for Assessment of Negative Symptoms (18), and the Scale for Assessment of Positive Symptoms (19). Ratings were completed by investigators trained to a criterion reliability of 0.90 (intraclass correlation). The family members and normal comparison subjects were assessed for axis II psychopathology with the Structured Clinical Interview for DSM-III-R Personality Disorders (20). All of the probands met the DSM-IV criteria for schizophrenia and had no other concurrent diagnoses. The family members and healthy comparison subjects were free of any current axis I diagnosis. The comparison subjects were also free of any axis II cluster A (i.e., schizotypal, schizoid, or paranoid) personality disorder. Although family members were not excluded on the basis of axis II diagnosis, none of these 19 subjects met the criteria for a cluster A disorder. Two family members had past histories of childhood attention deficit disorder, two had histories of past alcohol abuse, and one had a history of prior cannabis abuse.
Subjects were excluded if they had a history of neurological disorder (including head trauma with loss of consciousness), a history of substance abuse or dependence within the past year (as assessed by history, record review, and serum toxicology tests), any medical condition that might alter cerebral functioning, or a recent respiratory infection or any other condition that could affect olfactory functioning (e.g., common cold or allergies). The family members ranged in age from 17 to 54 years (mean=36.9, SD=14.9). The comparison subjects were comparable to the family members in gender and age distribution. Their ages ranged from 18 to 56 years (mean=36.0, SD=13.4). The patients’ ages ranged from 20 to 53 years (mean=30.7, SD=11.0), and their mean age did not differ significantly from that of either the family members (t=–1.19, df=28, p=0.24) or the healthy comparison subjects (t=–1.10, df=29, p=0.28). The patients were all stable outpatients at the time of testing, and their mean duration of illness was 7.3 years (SD=6.4, range=3–25). Eight were receiving atypical antipsychotic medications (risperidone, olanzapine, or clozapine), one was receiving a typical antipsychotic (fluphenazine hydrochloride), and two were unmedicated at the time of testing. Their mean BPRS score was 28.9 (SD=9.1), indicating a relatively low level of acute symptoms.
Experimental Procedures
After a complete description of the study, written informed consent was obtained from the subjects, who then underwent scanning with magnetic resonance imaging (MRI). The images of the olfactory bulbs were acquired on a 1.5-T GE Signa scanner (GE Medical Systems, Waukesha, Wis.) with a standard head coil. After a sagittal localizing scan, 3-mm interleaved coronal images were acquired with a 512×512 matrix and an 18-cm field of view, TR=500 msec, TE=24 msec. The individual voxel size was 0.3516×0.3516 mm within-plane, with 3-mm slice thickness. Although they are small structures, the olfactory bulbs can be clearly identified by using this protocol (Figure 1). Whole-brain dual-echo axial scans (5-mm slice thickness with no gap, TR=3000 msec, TE=30 and 80 msec) were also obtained for 10 patients, 17 family members, and 20 healthy comparison subjects.
In addition to MR scanning, nearly all subjects (10 patients, 15 comparison subjects, 17 family members) also underwent standardized psychophysical assessment of their olfactory abilities. Separate tests of the right and left nostrils were conducted, and the other nostril was occluded by tape. The order of nostril testing was counterbalanced across subjects. A single staircase, forced-choice odor-detection task was used to estimate basal detection sensitivity to phenethyl alcohol (21), a compound with relatively low trigeminal stimulation properties (22). The subjects were required to smell successive pairs of odorants, one containing different concentrations of phenethyl alcohol and the other containing odorless mineral oil, and to identify which vial of odorant “smells stronger.” Each correct identification was followed by a concentration that was 0.5 log step lower (weaker odor); an incorrect identification was followed by a concentration 0.5 log step higher (stronger odor). A change in direction from increasing concentration to decreasing concentration, or vice versa, on successive presentations was considered a “staircase reversal.” The test uses the geometric mean of the last four staircase reversal points, out of a total of seven, as an estimate of the odor detection threshold sensitivity. The ability to recognize and identify odors was assessed with the University of Pennsylvania Smell Identification Test (23). This is a standardized and reliable (test-retest r=0.95) 40-item forced-choice test of olfactory identification (24). The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page, and four response alternatives for each item are located above the odorant strip. The subjects were asked to smell each scratched microencapsulated strip and then pick the one response alternative that best fit the odor. Two booklets of the test were administered to the left nostril and two to the right; the order of the booklets and nostril presentation was systematically counterbalanced across subjects.
Image processing was performed by a trained operator (B.I.T.) who manually traced the left and right olfactory bulbs on adjacent slices at the level of the anterior cribriform plate. The reliability and reproducibility of the measurements of olfactory bulb volume were established as previously reported (14). Intraclass correlation coefficients (ICCs) for repeated measurements by a single operator were 0.919 or higher; the ICCs for measurements across operators were 0.924 or higher. Total cranial volume was determined for 47 of the 50 subjects, from the 5-mm axial images, by using well-established procedures (25, 26).
Data Analysis
Statistical analysis of the bulb volume data was conducted by using multivariate analysis of variance (MANOVA), with the left and right olfactory bulb volumes as the dependent measures, group membership as the independent measure, and age as a covariate. A significant omnibus MANOVA (p<0.05) was followed by pairwise group contrasts (patients versus comparison subjects, family members versus comparison subjects, patients versus family members), separately for left and right bulb volumes. An additional analysis assessed differences between the patients and family members in a matched-pairs design, by which each patient was compared to his or her own family members. This intrafamilial analysis allowed us to identify the features that are characteristic of schizophrenia, while partially controlling for other genetic and environmental influences that contribute to nonspecific interfamilial differences. Since olfactory identification scores are not normally distributed (Shapiro-Wilk W=0.88, p<0.001), relationships between olfactory bulb volumes and psychophysical olfactory measures were assessed by nonparametric Spearman correlation coefficients. The healthy comparison subjects smoked less than the ill probands and the family members (F=4.40, df=2, 47, p=0.02). Since the effect of smoking on olfactory bulb size is unknown, we included smoking status, as indicated by the self-reported number of packs smoked per day, as an additional covariate.
Results
The mean volume measures for the left and right olfactory bulbs, by group, are presented in Table 1. MANOVA revealed a significant overall effect of group membership on bulb volume (F=4.15, df=2, 45, p=0.03). There was also a significant group-by-side (left versus right) interaction (F=3.88, df=2, 45, p=0.03). Pairwise contrasts revealed a significantly lower right olfactory bulb volume in both the patients (F=10.04, df=1, 45, p=0.003) and family members (F=6.08, df=1, 45, p=0.02) than in the healthy comparison subjects, but there was no significant difference between the patients and relatives (F=0.99, df=1, 45, p=0.33). The difference in left olfactory bulb volume between the patients and the comparison subjects was nearly significant (F=4.05, df=1, 45, p=0.05, two-tailed), but the family members did not differ substantially from the comparison subjects (F=0.07, df=1, 45, p=0.80, two-tailed). The patients did differ significantly on left bulb volume from the family members (F=5.25, df=1, 45, p=0.03) (Figure 2).
Compared to their own healthy family members in a matched-pairs design, the patients again had a significantly lower volume on the left side (F=15.43, df=1, 16, p=0.001) but not on the right (F=1.40, df=1, 16, p=0.26). Repeat analyses with total cranial volume as an additional covariate produced the same results. There was also no main effect of gender on olfactory bulb volume and no interaction between gender and group membership.
In the psychophysical testing (Table 2), the patients with schizophrenia exhibited less ability to detect the presence of an odor than their own, otherwise healthy relatives. This deficit was found for both the left nostril (F=6.80, df=1, 14, p=0.03) and the right nostril (F=7.71, df=1, 14, p=0.02). Compared to the unrelated healthy comparison subjects, the patients had a significant deficit for the left nostril (F=5.34, df=1, 36, p=0.03) but not the right nostril (F=0.99, df=1, 36, p=0.33). Surprisingly, the patients did not have impaired odor identification, despite having lower odor detection threshold sensitivities. There were no differences between the family members and the healthy comparison subjects on either odor detection or odor identification. There were also no significant associations between olfactory bulb volume and olfactory behavioral performance, either for the entire study group or for any of the three groups examined separately.
Discussion
The MRI data demonstrate that there is a structural abnormality of the olfactory bulb in patients with schizophrenia. The results also provide evidence for a similar, although less severe, impairment in their otherwise healthy first-degree relatives. Since first-degree relatives share, on average, 50% of their genetic material with their ill family member, the presence of the deficit in these relatives without schizophrenia is consistent with a genetically transmitted vulnerability factor. It is noteworthy, in this regard, that all of the relatives were carefully screened for the presence of current axis I diagnoses, substance abuse, or neurological deficits and that none had a cluster A axis II personality disorder. Although the patients had bilaterally lower volumes than unrelated healthy comparison subjects, they had lower volumes than their own first-degree relatives only on the left side. This suggests that the presence of the illness itself, in people who otherwise share genetic vulnerability, is associated with a left bulb abnormality (and a parallel deficit in odor detection threshold sensitivity in the left nostril). However, genetic vulnerability to the disorder, as evidenced by deficits in the otherwise healthy family members, is associated with a right bulb abnormality. Low right olfactory bulb volume may therefore be an endophenotypic marker of an inherited neuronal abnormality that conveys risk for the development of schizophrenia.
How might such an abnormality arise? Embryonic development of both the olfactory bulbs and the epithelium is critically dependent on the interaction between peripheral olfactory receptor neurons and their dendritic targets. Penetration of the mitral/tufted cell dendritic zone by olfactory receptor cell axons is the initial event triggering mitral cell differentiation and the formation of glomeruli in the olfactory bulb (27). Focal denervation of these peripheral inputs results in reduced bulb size (28). It is notable, therefore, that evidence now supports the presence of abnormalities in these olfactory receptor neurons in patients with schizophrenia. Feron and colleagues (29), in an in vitro study of material obtained from biopsies of the olfactory epithelium, found that patients’ tissue was less able to attach to the culture slide than was tissue of well comparison subjects and that a greater proportion of the patients’ cells were undergoing mitosis. Using autopsy material, we found a high density, in patients, of immature neurons expressing growth-associated protein GAP-43 (30). This abnormality has been experimentally replicated in rodents by disrupting synaptic connectivity between olfactory receptor neurons and the bulb, either by destroying the olfactory epithelium and then allowing it to reconstitute itself (31) or by removing the bulb so that axons emanating from the epithelium cannot establish connections with their targets (32). If, as these studies suggest, patients with schizophrenia have some as yet undefined difficulty in establishing healthy synaptic connections between the olfactory epithelium and the bulb, this could result in both an increased density of immature receptor neurons and smaller bulb size. Our findings are, therefore, consistent with these reported cellular abnormalities.
Two notable features of the family data are the restriction of the structural deficit to only the right bulb and the absence of associated deficits in olfactory identification or threshold. With regard to the behavioral measures, it is important to note that previous studies of olfactory psychophysical impairments in family members (11, 12) were conducted by using simultaneous presentation of stimuli to both nostrils, rather than the unilateral presentation used here. Bilateral presentation appears to elicit additional facilitative mechanisms, which may influence the level of observed impairment (33). The absence of expected behavioral deficits may also reflect, in part, the lack of sufficient statistical power to detect small group differences.
Finally, the absence of behavioral deficits among the relatives, despite the presence of structural deficits, may be explained as a threshold phenomenon. That is, behavioral impairments in olfaction may not be observed until bulb volumes diminish below some critical threshold. Such structure-function relationships are well established in many organ systems, including the brain. In Parkinson’s disease, for example, near-total degeneration of nigrostriatal bundle neurons is required before neurological symptoms emerge (34). The family members in our study may retain enough functionally intact olfactory bulb substrate to adequately detect and identify odorants. It is also possible that the changes in the olfactory bulb that give rise to these lower MRI volumes are ones that do not compromise function, at least as assessed by these psychophysical instruments. However, given other reports of psychophysical olfactory impairments in first-degree relatives of schizophrenic patients (11, 12), we consider this latter explanation to be less likely. Whatever the explanation, though, these findings suggest that the volumetric measures may be more sensitive indicators of a heritable deficit.
Medication effects may have been a factor in the absence of odor identification deficits in the patients. Nine of the eleven patients were medicated, eight with atypical antipsychotic agents. Although a meta-analytic review (8) of the published literature did not support a significant effect of medication on olfactory psychophysical measures, a more recent study (35) demonstrated normalization of a threshold deficit for the left nostril after 8 weeks of treatment with predominantly atypical agents. Differences in the effects of typical and atypical agents on olfactory performance have received little consideration up to now. Since the patients in our study group were also being treated with primarily atypical agents, this may have contributed to the lack of robust psychophysical impairments. There is also the possibility of a selection bias. Patients who remain in close contact with their families may be in a relatively early stage of their illness and/or be less severely affected than the general population of patients, many of whom are disconnected from their families of origin.
Low volume, in family members, was restricted to the right bulb. It is well known that the right hemisphere is better adapted to processing olfactory inputs than the left (36). This functional asymmetry presumably extends to the level of the bulb. Studies have demonstrated larger right olfactory bulbs, as a consequence of normal development, in species as diverse as the rat (37) and the winter flounder (38). There is also evidence that the two bulbs contain different levels of modulating neurotransmitters (39) and the enzymes involved in their synthesis (40). If, as these studies suggest, the two bulbs are structurally and functionally distinct, then the underlying abnormality that gives rise to low MRI-measured volumes could be one that is manifest primarily in the right, rather than the left, bulb. If this is the case, then an understanding of the normal structural and functional asymmetry of the olfactory bulb could provide an important clue to the etiology of the abnormality in schizophrenia.
Our finding of a lateralized deficit for family members stands in contrast to the bilaterally symmetric low bulb volume that we observed for patients. Lateralized deficits, with the left side exhibiting more impairment than the right, are now well established in schizophrenia (41–43). There is evidence that this may also be true for olfactory deficits (34, 44). However, we have also reported (45) that olfactory dysfunction is not stable but, rather, declines over the course of schizophrenia in tight association with the duration of illness. These two sets of findings can be integrated into one, albeit speculative, model that posits a genetically mediated abnormality affecting primarily the right bulb, coupled with illness-related deteriorative changes that also affect the left.
Collectively, the accumulating data on olfactory deficits in schizophrenia provide convincing evidence of fundamental impairments in the integrity of this neural system. The current study reinforces our earlier conclusion, based on an analysis of olfactory bulb volume in patients, that this abnormality manifests itself as a structural, as well as behavioral, deficit. It also argues strongly, in conjunction with earlier behavioral studies, that this deficit is at least in part genetically mediated. Further investigations of the olfactory system may, therefore, shed light on both the molecular and genetic underpinnings of schizophrenia.
 |
 |
Received June 12, 2002; revision received Nov. 7, 2002; accepted Nov. 25, 2002. From the Schizophrenia Research Center, Department of Psychiatry, and the Smell and Taste Center, Department of Otorhinolaryngology, University of Pennsylvania. Address reprint requests to Dr. Turetsky, Neuropsychiatry Program, Department of Psychiatry, 10th Floor, Gates Bldg., University of Pennsylvania, Philadelphia, PA 19104; [email protected] (e-mail). Supported by NIMH grant MH-59852.
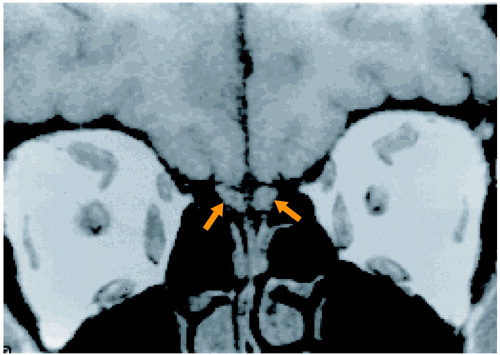
Figure 1. MRI Scan of Olfactory Bulbs
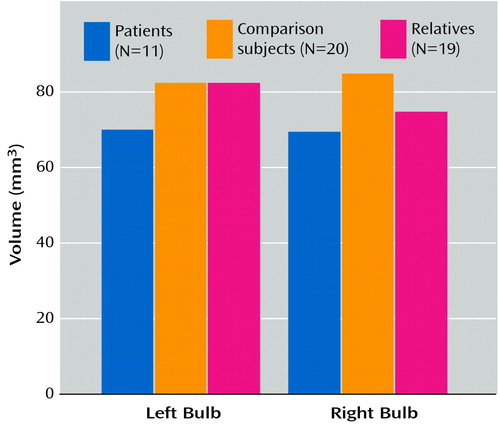
Figure 2. Mean Volumes of the Left and Right Olfactory Bulbs for Patients With Schizophrenia, First-Degree Relatives, and Healthy Comparison Subjects
1. Kendler KS, Diehl SR: The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull 1993; 19:261-285Crossref, Medline, Google Scholar
2. Levy DL, Holzman PS, Matthysse S, Mendell NR: Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull 1993; 19:461-536Crossref, Medline, Google Scholar
3. Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price RA: Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 1994; 51:651-661Crossref, Medline, Google Scholar
4. Waldo MC, Adler LE, Freedman R: Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr Res 1988; 1:19-24Crossref, Medline, Google Scholar
5. Clementz BA, Geyer MA, Braff DL: Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry 1998; 155:1691-1694Link, Google Scholar
6. Cannon TD, Mednick SA, Parnas J: Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry 1989; 46:883-889Crossref, Medline, Google Scholar
7. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587-592Crossref, Medline, Google Scholar
8. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL: Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325-340Crossref, Medline, Google Scholar
9. Martzke JS, Kopala LC, Good KP: Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry 1997; 42:721-732Crossref, Medline, Google Scholar
10. Barinaga M: Olfaction: smell’s course is predetermined. Science 2001; 294:1269-1271Crossref, Medline, Google Scholar
11. Kopala LC, Good KP, Torrey EF, Honer WG: Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry 1998; 155:134-136Link, Google Scholar
12. Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG: Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry 2001; 158:1286-1290Link, Google Scholar
13. Moberg PJ, Doty RL, Turetsky BI, Wylonis L, Cannon TD, Acosta TA, Gur RE: Olfactory functioning in siblings discordant for schizophrenia (abstract). Biol Psychiatry 1996; 39:571-572Crossref, Google Scholar
14. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE: Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 2000; 157:828-830Link, Google Scholar
15. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
16. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
17. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
18. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
19. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
20. Maffei C, Fossati A, Agostoni I, Barraco A, Bagnato M, Deborah D, Namia C, Novella L, Petrachi M: Interrater reliability and internal consistency of the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), version 2.0. J Personal Disord 1997; 11:279-284Crossref, Medline, Google Scholar
21. Deems DA, Doty RL: Age-related changes in the phenyl ethyl alcohol odor detection threshold. Trans Pa Acad Ophthalmol Otolaryngol 1987; 39:646-650Medline, Google Scholar
22. Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD: Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 1978; 20:175-185Crossref, Medline, Google Scholar
23. Doty RL, Shaman P, Dann M: Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32:489-502Crossref, Medline, Google Scholar
24. Doty RL, McKeown DA, Lee WW, Shaman P: A study of the test-retest reliability of ten olfactory tests. Chem Senses 1995; 20:645-656Crossref, Medline, Google Scholar
25. Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE: Frontal and temporal lobe brain volumes in schizophrenia: relationship to symptoms and clinical subtype. Arch Gen Psychiatry 1995; 52:1061-1070Crossref, Medline, Google Scholar
26. Gur RE, Mozley PD, Shtasel DL, Cannon TD, Gallacher F, Turetsky B, Grossman R, Gur RC: Clinical subtypes of schizophrenia: differences in brain and CSF volume. Am J Psychiatry 1994; 151:343-350Link, Google Scholar
27. Treloar HB, Purcell AL, Greer CA: Glomerular formation in the developing rat olfactory bulb. J Comp Neurol 1999; 413:289-304Crossref, Medline, Google Scholar
28. Leo JM, Devine AH, Brunjes PC: Focal denervation alters cellular phenotypes and survival in the developing rat olfactory bulb. J Comp Neurol 2000; 417:325-336Crossref, Medline, Google Scholar
29. Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A: Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res 1999; 40:211-218Crossref, Medline, Google Scholar
30. Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, Hahn CG: Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry 2001; 58:829-835Crossref, Medline, Google Scholar
31. Verhaagen J, Oestreicher AB, Gispen WH, Margolis FL: The expression of the growth associated protein B50/GAP43 in the olfactory system of neonatal and adult rats. J Neurosci 1989; 9:683-691Crossref, Medline, Google Scholar
32. Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL: Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res 1990; 26:31-44Crossref, Medline, Google Scholar
33. Cain WS: Bilateral interaction in olfaction. Nature 1977; 268:50-52Crossref, Medline, Google Scholar
34. Zigmond MJ, Stricker EM: Parkinson’s disease: studies with an animal model. Life Sci 1984; 35:5-18Crossref, Medline, Google Scholar
35. Purdon SE, Flor-Henry P: Asymmetrical olfactory acuity and neuroleptic treatment in schizophrenia. Schizophr Res 2000; 44:221-232Crossref, Medline, Google Scholar
36. Doty RL, Bromley SM, Moberg PJ, Hummel T: Laterality in human nasal chemoreception, in Cerebral Asymmetries in Sensory and Perceptual Processing. Edited by Christman S. Amsterdam, North Holland, 1997, pp 497-542Google Scholar
37. Heine O, Galaburda AM: Olfactory asymmetry in the rat brain. Exp Neurol 1986; 91:392-398Crossref, Medline, Google Scholar
38. Prasada Rao PD, Finger TE: Asymmetry of the olfactory system in the brain of the winter flounder, Pseudopleuronectes americanus. J Comp Neurol 1984; 225:492-510Crossref, Medline, Google Scholar
39. Dluzen DE, Kreutzberg JD: Norepinephrine is lateralized within the olfactory bulbs of male mice. J Neurochem 1996; 66:1222-1226Crossref, Medline, Google Scholar
40. Rodriguez-Gomez FJ, Rendon-Unceta MC, Sarasquete C, Munoz-Cueto JA: Localization of tyrosine hydroxylase-immunoreactivity in the brain of the Senegalese sole, Solea senagalensis. J Chem Neuroanat 2000; 19:17-32Crossref, Medline, Google Scholar
41. McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA: Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 1993; 50:190-197Crossref, Medline, Google Scholar
42. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
43. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618-624Crossref, Medline, Google Scholar
44. Good KP, Martzke JS, Honer WG, Kopala LC: Left nostril olfactory identification impairment in a subgroup of male patients with schizophrenia. Schizophr Res 1998; 33:35-43Crossref, Medline, Google Scholar
45. Moberg PJ, Doty RL, Turetsky BI, Arnold SE, Mahr RN, Gur RC, Bilker W, Gur RE: Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry 1997; 154:1016-1018Link, Google Scholar



