Concomitant Psychotropic Medication for Youths
Abstract
OBJECTIVE: This study reviewed the clinical research and practice literature relating to the prevalence and patterns of concomitant psychotropic medication given to youths with emotional and behavioral disorders. METHOD: A MEDLINE search from 1996 through spring 2002, including a review of references from relevant published articles and reports, was undertaken to identify available information on concomitant psychotropic medication for youths. RESULTS: The data supporting concomitant psychotropic medication for youths are almost exclusively based on case reports and small-scale, nonblind assessments. In the mid-1990s, over 20% of outpatient youths treated in community mental health centers and over 40% of youths treated in inpatient psychiatric facilities were given concomitant psychotropic medication. The rate has since increased. Psychiatrists more than primary care physicians prescribe concomitant psychotropic medication, and they show great variability in their prescribing habits. Youths with aggressive behavior, male gender, severe emotional illness, and disabling social maladjustment are most likely to receive concomitant psychotropic medication. CONCLUSIONS: Substantive systematic evidence is needed to clarify this increasingly common, inadequately researched child psychopharmacologic practice.
The use of concomitant psychotropic medication, defined here as the simultaneous use of two or more psychotropic medications (either for the same or different emotional/behavioral target symptoms), has been a common treatment practice in adults for a number of decades in many countries (1, 2). The evidence clearly suggests that the use of concomitant psychotropic medication has been steadily increasing (3, 4). This increase parallels the overall increase in the use of psychotropic medication, a phenomenon particularly prominent in the 1990s (5, 6). In relation to individuals, concomitant psychotropic medication is prescribed more often to patients with the following characteristics and patterns: 1) a greater number and severity of comorbid disorders, 2) a greater number and severity of symptoms, 3) unsuccessful or partially successful medical treatment interventions, 4) visits to multiple physicians, 5) visits to physicians who are more prone to prescribe medications, 6) visits to psychiatrists, and 7) advancing age (7, 8).
Although the use of concomitant psychotropic medication for youths has trailed the practice for adults, its use has expanded prominently since the late 1980s (9–11). Primarily because of the very limited scientific data on the use of concomitant psychotropic medication in youths, the practice has received little or no mention in child psychiatry and child psychopharmacology textbooks (12–16). Nonetheless, the very sizable increase in the use of concomitant psychotropic medication among youths merits a presentation and synthesis of the evidence on the use, efficacy, and safety of this practice.
Method
A MEDLINE search from 1996 through October 2001 was conducted and augmented by a review of references from relevant journals and textbooks. Many recent presentations at psychiatry and child psychiatry meetings that would not have been identified in MEDLINE were also reviewed. No studies of the use of concomitant psychotropic medication for youth were excluded on the basis of group size, study design, or outcome measures. The age range, as defined for this review, was 0–19 years old.
This review was structured according to three main objectives. The first was to describe the extent of use of concomitant psychotropic medication by examining patterns of use in terms of the prevalence, proportional rates, trends, and factors associated with variation in use. The second goal was to review the evidence base for the effectiveness of concomitant psychotropic medication. The final objective was to identify the known potential risks associated with the use of concomitant psychotropic medication for youths.
Concomitant psychotropic medication was assessed in terms of four aspects of use. The prevalence of use of concomitant psychotropic medication refers to the percentage of individuals who received concomitant psychotropic medication in a defined population of youths. By comparison, the proportional rate of use of concomitant psychotropic medication refers to the percentage of the total number of youths who received concomitant psychotropic medication in a treated group (e.g., psychiatric outpatients and/or inpatients). A concomitant psychotropic medication trend refers to a temporal change in the prevalence or proportional patterns of use. Variations in use of concomitant psychotropic medication are examined in relation to 1) physician specialty, 2) special populations, 3) physician-reported symptoms and behavioral features, and 4) type and frequency of concomitant psychotropic medication regimens.
The evidence base for the effectiveness of concomitant psychotropic medication is derived from clinical studies. A meaningful categorization of the evidence base for psychiatric treatment based on the relative strength of research has recently been proposed by Jensen et al. (17). Concomitant psychotropic medication for youth is therefore presented as controlled double-blind research, open case series reports—with and without outcome rating measures—and descriptive case reports.
The evidence base for the risks associated with the use of concomitant psychotropic medication is far less extensive. The data comprise published case reports of adverse drug events (18) from concomitant psychotropic medication use in youth. In this report, an adverse drug event refers to the occurrence of an undesired effect after the use of concomitant psychotropic medication.
Results
Proportional Rates and Prevalence
We were able to locate two national population assessments that measured use of concomitant psychotropic medication in U.S. children. One revealed that in 1997–1998, 24.7% of the representative physician office visits for youths in which a stimulant prescription was written were also associated with the use of concomitant psychotropic medication. This represented a fivefold increase over the 1993–1994 rate of 4.8% (19). The other was based on national parent report surveys in 1987 and 1996 pertaining to medication use by children and adolescents. Olfson et al. (11), by comparing these 1-year estimates, reported that the use of multiple psychotropic medications increased eightfold. For youths (younger than 19) who were given at least one psychotropic medication, the rate of receipt of additional psychotropic medication rose 2.5-fold (4.7% to 11.7%) over that decade. Outside the United States, a recent prevalence study from the Netherlands (20) indicated that psychotropic therapy for youths is almost exclusively monotherapeutic. Published data on the rate of use of concomitant psychotropic medication in youths come mainly from selected groups, and the findings vary considerably by diagnosis and treatment setting. Medical records of youths from psychiatric outpatient and inpatient settings have been the source of data for most of these studies.
Rates by Diagnostic Profile
The majority of concomitant psychotropic medication studies have focused on youths with attention deficit hyperactivity disorder (ADHD). Rappley and colleagues (21) found the proportional rate of use of concomitant psychotropic medication to be 20% among 2- and 3-year-old children who were enrolled for 15 consecutive months in a Medicaid insurance program in 1995–1996 and were diagnosed with ADHD (N=223). Ghuman et al. (22), using data from 1995–1999, reported a concomitant psychotropic medication rate of 26% for 3- to 5-year-old children referred to a developmental disorder clinic who were diagnosed with ADHD and who received stimulant medication. In an older cohort of youths in a health maintenance organization (HMO) setting, use of concomitant psychotropic medication in 1997–1998 was identified in 21% of 5–12-year-old children who were diagnosed with ADHD and who were receiving stimulant medication (23). A 1996 U.S. practice research network survey compiled by Zarin and colleagues (24) found that 49% of 166 youths (aged less than 15 years) who were treated by private psychiatrists for ADHD were receiving concomitant psychotropic medication, with 12% of that total receiving three or more psychotropic medications.
Medical chart audits from specialty outpatient clinics in Massachusetts revealed relatively high rates of use of concomitant psychotropic medication. In 1990, additional psychotropic medication was prescribed for 47% of youths diagnosed with ADHD (25) who were being treated with nortriptyline. A medical chart audit in 1991 (26) revealed the use rate of concomitant psychotropic medication to be 39% for youths diagnosed with obsessive-compulsive disorder (OCD). In some specialty clinics, even higher rates of concomitant psychotropic medication were observed. For example, Cohen et al. (27) reported a use rate of concomitant psychotropic medication of 68% from 1992–1996 among youths with ADHD, which was similar to the 71% use rate of concomitant psychotropic medication that Biederman and colleagues (28) found from 1991–1995 among youths with bipolar disorder.
Reports of use of concomitant psychotropic medication are also high among youths diagnosed with schizophrenia. In an early 1990s treatment program for youths with schizophrenia, Kumra et al. (29) noted that the mean number of psychotropic medications prescribed for each applicant youth was 2.1.
On the basis of parent reporting from a group referred to a center for social learning disabilities in July through December 1997, Martin et al. (30) reported a 29% rate of concomitant psychotropic medication among 109 youths (mean age=13.9 years) who had a pervasive developmental disorder. Similarly, in the early 1990s, Aman et al. (31) mailed a survey to parents of autistic youths (a 53% response) and reported that 28% of the identified youths (median age=13 years) given psychotropic medication were receiving concomitant psychotropic medication.
Proportional Use by Treatment Setting
Concomitant psychotropic medication use findings based primarily on medical chart audits also vary considerably by treatment site and setting. In 1990, a systematic review of medical charts in community mental health centers (CMHCs) located in Maryland, New York, and Ohio revealed that 9% (9), 11%, and 22% (32), respectively, of treated youths were receiving concomitant psychotropic medication. More recently, medical chart reviews conducted in different CMHCs in Maryland found that concomitant psychotropic medication was prescribed for 21% of treated youths in 1994 (9) and 22% of treated youths in 1997 (33).
The rate of use of concomitant psychotropic medication has been consistently higher in inpatient than in standard outpatient psychiatric settings. In 1976–1977, 30% of 100 Canadian psychiatric inpatient youths received concomitant psychotropic medication (34). In a 1991 medical chart audit from three hospitals, Kaplan and Busner (35) similarly reported a use rate of concomitant psychotropic medication of 36%. A slightly higher rate of concomitant psychotropic medication of 42% was noted with 1994 data from seven youth inpatient units in Maryland (9).
Trends
The use of concomitant psychotropic medication among youths increased substantially during the 1990s. For example, the rate of use of concomitant psychotropic medication rose 133% (9% to 21%) from 1990 to 1994 in four Maryland CMHCs (9). In a national sample of physician office visits for youth (under age 18), the rate of combined antidepressant and stimulant use increased from 4% in 1994 to 29% in 1997, reflecting a sevenfold increase (36). Rushton and Whitmire (10) noted a similar increase from 1992 through 1998 for combined stimulant and selective serotonin reuptake inhibitor (SSRI) treatment among youths aged 1–19 years. Although most rates of concomitant psychotropic medication for psychiatric clinic youths in the early and mid-1990s were below 50%, several posters presented in 2001 at national psychiatric meetings showed an increase in this respect to over 50% (37–39).
An important trend to note is that the higher rate of use of concomitant psychotropic medication among youths has occurred simultaneously with the overall increase in psychotropic use. On the basis of findings from an HMO and two state Medicaid databases covering 1987 through 1996, the prevalence of psychotropic use among youths less than 20 years old increased 4–10-fold for antidepressants, 36–153-fold for alpha agonists, and three- to sevenfold for stimulants (40). Likewise among youths (younger than age 20) in one state Medicaid program from 1996 through 2000, there was a threefold increase in neuroleptic treatment (41). The increase was most prominent after atypical neuroleptics were introduced in the early 1990s (42).
Variations
Physician specialty
Psychiatrists prescribe far more concomitant psychotropic medications for youths than do primary care physicians (43, 44). This relates primarily to the fact that psychiatrists treat comparatively more seriously disturbed children (43, 44). In all likelihood, the severity of the disorder also explains the higher rates of use of concomitant psychotropic medication in psychiatric specialty clinics, in which more severely impaired youths are seen than in CMHCs.
Besides the expected variation by physician specialty, prominent variations in concomitant psychotropic medication and psychotropic medication prescribing have been reported for individual physicians. Ahsanuddin et al. (34), for example, noted that the use rate of concomitant psychotropic medication varied from 0% to 66% among five individual child psychiatrists.
Special youth populations
Youths with a greater degree of emotional, educational, or social impairment are more likely to receive concomitant psychotropic medication. Special populations whose use of concomitant psychotropic medication has been noted include youths in special education classrooms, in foster care, and in residential treatment centers. Mattison (45) reported that 17% of 84 elementary school students in a special education classroom for children with emotional disorders were receiving concomitant psychotropic medication in 1993–1994. Bussing et al. (44) similarly found the use rate of concomitant psychotropic medication to be 20% among elementary school students in a special education program (N=102) in 1995. Zima et al. (46) reported that 45% of foster care youths aged 6–12 years who were taking psychotropic medication in 1996–1998 (N=38) were receiving two or more psychotropic medications. Likewise, Anderson et al. (47) reported that 52% of a consecutive group of Illinois children in state legal guardianship (wards of the state) who were given psychotropic medication received more than one type concomitantly in 2001. Connor and colleagues (48) assessed use of concomitant psychotropic medication during 1991–1992 in a Massachusetts residential treatment center and found that the rate was 57%–60% from the youth’s medication history and 40% at the time of admission.
Major Symptoms and Behaviors
The use of concomitant psychotropic medication has been found to be highly associated with aggressive behavior in youths, particularly the coadministration of neuroleptic medications (32, 49). Ahsanuddin et al. (34) noted that aggressive behavior disorders were the primary diagnoses associated with the use of concomitant psychotropic medication. Likewise, in a study of youths in residential treatment, Connor et al. (48) found that the major symptom pattern associated with use of concomitant psychotropic medication was aggressive behavior.
Insomnia is another target symptom associated with the use of concomitant psychotropic medication in youths. Wilens et al. (50) and Prince et al. (51) both reported that clonidine is frequently added to stimulant treatment to aid sleep. In recent years, risperidone has been added to other psychotropic medications to both aid sleep and lessen aggressiveness (52).
Common Combinations
A particularly common concomitant psychotropic medication combination for youths has been methylphenidate and clonidine. In the early 1990s, Swanson et al. (53) estimated from a national pharmaceutical market source that 41% of surveyed youths in 1994–1995 who were receiving clonidine were also receiving methylphenidate. Prince et al. (51), in a psychiatric clinic survey from 1992 to 1995, reported the rate of this combination to be 68%. In a 2–3-year-old Medicaid population, Rappley et al. (54) similarly found this to be the most common psychotropic combination.
Another common concomitant psychotropic medication prescribing pattern involves antidepressants with stimulants. Pathiyal et al. (55), in an 18-month longitudinal analysis of pharmacy claims data, reported that 22% of youths receiving methylphenidate in 1993–1995 also received antidepressants concomitantly. On the basis of 1998 North Carolina Medicaid claims data, Rushton and Whitmire (10) reported that 30% of youths receiving an SSRI in 1998 also had a stimulant prescription during the same year. Similarly, Zito et al. (56) found that one-third of Medicaid youths in one state who were receiving antidepressant medication in 1994 had also been given a stimulant that year. It should be noted that these prevalence findings for a 1-year period may not reflect the use of concomitant psychotropic medication. However, Rushton and Whitmire (10) reported that 83% of stimulant/SSRI prescriptions were filled in the same month, suggesting concomitant use.
Evidence of Effectiveness
Controlled clinical trials
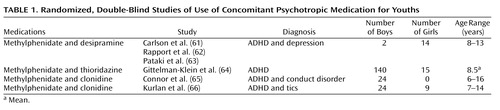
There are a number of controlled studies of adults that support the use of concomitant psychotropic medication for psychotic depression (57), treatment-resistant depression (58), bipolar I disorder (59), and OCD (60). By comparison, the published controlled, double-blind studies of concomitant psychotropic medication in youth (listed in Table 1) are few in number. The first study (61) evaluated a combination of methylphenidate and desipramine, which improved the clinical picture of ADHD to a modest degree over that of each drug individually. However, in this study of 7–12-year-old hospitalized youths, the combination resulted in impaired vigilance (62) and more side effects (63). Another study of youths (mean age=8.5 years) with ADHD (64) noted that the combination of methylphenidate and thioridazine was rated as more satisfactory by parents—although not by teachers—than methylphenidate alone and thioridazine alone. However, the methylphenidate-thioridazine combination resulted in no greater overall clinician-rated improvement than methylphenidate alone and produced more side effects. A 3-month pilot study involving 24 youths aged 6–16 years with ADHD and an aggressive behavior disorder (65) measured the behavioral effects of clonidine alone, methylphenidate alone, and the combination of the two. On all measured indices, the combination of clonidine and methylphenidate (N=8) was found to be equal to or inferior to that of methylphenidate alone.
A recent report describing a double blind, placebo-controlled study of 136 youths (aged 7–17 years) with both ADHD and tic disorders (66) found that clonidine in combination with methylphenidate decreased teacher-reported ADHD impulsivity and hyperactivity symptom ratings somewhat more than either drug alone. A study by Turgay et al. (67) noted that in youths (aged 5–12 years, N=45) with a disruptive behavior disorder and a subaverage IQ, the combination of a stimulant and risperidone resulted in no additional symptomatic benefit over risperidone alone.
In a systematic, individualized, carefully evaluated, blind assessment of seven hospitalized youths diagnosed with both a mood and a disruptive behavior disorder, the addition of lithium to methylphenidate resulted in no significant benefits over methylphenidate alone, and the outcomes of the combination were, for the most part, inconclusive (68).
Two double-blind, controlled studies (see Table 1) assessed methylphenidate augmented with either thioridazine or desipramine. The two augmenting medications have been used less of late because of potentially problematic cardiac conduction side effects (69, 70). Consequently, combinations of these add-on drugs would need to be initiated with an unusual degree of caution. Also, using concomitant psychotropic medication to augment stimulant medication for most youths with ADHD is usually unnecessary since evidence from the Elia et al. study (71) indicated that stimulants at high doses can be effective in 91% of the patients selected for an incomplete stimulant response.
Augmentation
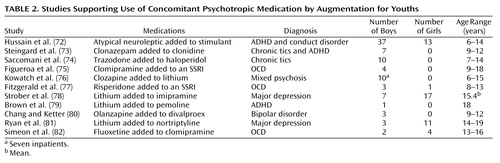
Studies that support the use of concomitant psychotropic medication for youths usually justify it in terms of using a second medication to augment an inadequate clinical response from the primary medication and treating comorbid psychiatric disorders. With the partial exception of the large study by Hussain et al. (72), the concomitant psychotropic medication augmentation studies listed in Table 2 are quite similar. They are generally small series, open reports covering up to 11 patients, with follow-up periods ranging from 1 to 48 months. Except for the study by Strober et al. (78), all noted generally positive results. The patient outcomes are composed of either descriptions of individual outcomes (two reports) or average clinician- or patient-rated changes in target symptoms (nine reports). The Hussain et al. report (72) was also open and uncontrolled, but it differed in that it covered 50 patients and contained independent teacher ratings.
Comorbid treatment
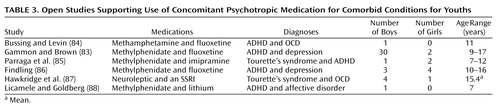
With the exception of the 32 patients in the study by Gammon and Brown (83), the five other studies listed in Table 3 covering the use of concomitant psychotropic medication for comorbid indications were small series and open studies covering up to seven patients. The evaluations were accomplished through the use of standard rating scales in five of the six studies; these were completed by clinicians (N=4) and/or by teachers (N=3). All of the reports covering the use of concomitant psychotropic medication for comorbidity were uncontrolled, and all concluded that the concomitant psychotropic medication was beneficial. It is noteworthy that in Table 1, Table 2, and Table 3 over one-half of the case reports of the use of concomitant psychotropic medication involved prepubertal youths, a majority of male youths, and youths with the diagnosis of ADHD. Also of note is that all of the reports listed in Table 2, Table 3, and Table 4 reflect research that the study by Jensen et al. (17) would categorize as level C in scientific merit—on a scale from a high of A to a low of C.
Evidence of Associated Risks
A fundamental underlying concern with concomitant psychotropic medication for youths is the virtual lack of rigorous systematic research to support its use and to understand its potential risks (99, 100). This and certain publicized cases of use of extreme concomitant psychotropic medication led the Council of the American Academy of Child and Adolescent Psychiatry (101) to recommend judicious and cautious use of concomitant psychotropic medication for youths.
The cases of concomitant psychotropic medication cited by Wagner (100), Sallee et al. (89), and Behr (102) of prepubertal youths receiving four to seven psychotropic medications concomitantly that were associated with adverse drug events understandably “raised eyebrows” in the field. In part, this was due to the consistent finding in the adult literature indicating that the risk of adverse drug events increases with the number of concomitant medications administered (103, 104). Turner et al. (105) and Martinez-Mir et al. (104), evaluating pediatric inpatients, likewise reported a significant increase in adverse drug events in relation to the number of concomitant medications used.
Other safety concerns with use of concomitant psychotropic medication in youths include 1) the greater possibility of untoward drug interactions (106, 107) and 2) the creation of drug-induced behavioral toxicity after the addition of another psychotropic medication—a consequence not often recognized as such, which can then lead to even more complex drug therapy to treat that side effect (100).
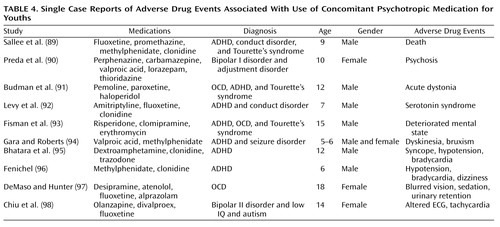
Table 4 presents a brief list of reports of adverse drug events with the use of concomitant psychotropic medication in youths. It is noteworthy that in nearly all cases, three or more psychotropic medications were administered concomitantly. The majority of the youths in these reports were boys younger than 13 years old.
Risks and Differences by Age
The risk of adverse drug events with the use of concomitant psychotropic medication in relation to age can be appreciated from data on valproate (used primarily for seizure control). From 1978 to 1984, the rate of fatal hepatotoxicity due to valproate monotherapy in youths under the age of 11 was five in 42,618. The rate of hepatic fatality when valproate was administered with one or more additional anticonvulsants increased prominently to 22 in 47,864, a fourfold increase. Moreover, during that survey period, there were no hepatic deaths among youths aged 11–21 years that were associated with valproate monotherapy, compared to five with valproate polytherapy (108). One should also consider that, overall, youths aged 9 years or younger are substantially more at risk for adverse drug events than are older youths (109).
Although differences in prescribing patterns in concomitant psychotropic medication in relation to age are incompletely studied, there are a number of findings in this area that are of interest. The use of concomitant psychotropic medication is more common with advancing age, in female adults, and in male youths (110). Also, compared to adults, youths are prescribed different concomitant psychotropic medication combinations, have fewer multiple providers, and have different problematic behaviors targeted by concomitant psychotropic medication (13, 110–112).
Discussion
Drawing inferences from the wide range of findings presented in this review with their modest generalizability is challenging. Several issues seem germane in considering the published concomitant psychotropic medication reports: namely, the rationale for concomitant psychotropic medication, U.S. academic perspectives, the emphasis on the treatment for aggression, drug and target symptom specificity, and the implications for better methods of assessment.
Rationale
Adding a second psychotropic medication to augment a partial response to the first could be useful if the first had been given at a customary therapeutic dose for a reasonable period of time and if that treatment and its subsequent augmentation received a systematically monitored assessment. The evaluation of the first medication and of the combination of medications should optimally be performed with reliable measures, baseline and subsequently rated targeted assessments, and preferably with at least one blind independent rater. “N of 1” research protocols for evaluating single patients in an experimental study might be developed to evaluate use of concomitant psychotropic medication more rigorously, beginning in teaching hospital settings under rigid research conditions and moving out to the community setting when methods are better developed.
Treating two comorbid psychiatric disorders with two or more different medications has been recommended if each of the disorders has a unique pattern and an optimal treatment. This rationale applies in certain instances (e.g., with tics and ADHD), but in most other circumstances, the diagnostic patterns are far less specific. In practice, there is often a sizable symptom overlap in comorbid diagnoses in child psychiatry (e.g., bipolar disorder and ADHD, disruptive behavior disorders and ADHD) (28, 113). In addition, although a 100% treatment response would obviously be optimal, it is uncommon (particularly in chronic cases), and “doctors who cannot accept this level of imperfection put their patients at risk in a futile search for the holy grail through tactics like adding more drugs” (114, p. 17). Green (16, p. xi) similarly advised clinicians to “resist the tempting but erroneous notion that the right combination of drugs will solve any problem.” Furthermore, a number of investigators have reported successfully treating comorbid disorders with only one psychotropic medication by exploiting its range of effects, as has been shown by Klein et al. (115), Spencer et al. (116), Steingard et al. (117), and Scahill et al. (118).
Some writers in the field identify rational as opposed to irrational use of concomitant psychotropic medication (119, 120). Essentially, a rational concomitant psychotropic medication approach is based on the weight of scientific evidence in its favor. However, as one notes from the available reports (Table 1, Table 2, and Table 3), the evidence base for the use of concomitant psychotropic medication is profoundly limited. Consequently, the use of the term “rational” for the use of concomitant psychotropic medication applied to youths appears to be premature.
Academic Perspectives
Almost without exception, leaders in the field support use of concomitant psychotropic medication for specific indications in child psychopharmacology. Biederman (121, p. 11) stated, “Examples of the rational use of combined treatment include the use of an antidepressant plus a stimulant for ADHD and comorbid depression, the use of clonidine to ameliorate stimulant-induced insomnia, and the use of a mood stabilizer plus an anti-ADHD agent to treat ADHD comorbid with bipolar disorder.” Popper (122, p. 497) noted that “multiple agents are needed for treating comorbid presentations often seen in children, including ADHD with Tourette’s syndrome or obsessive-compulsive disorder (OCD), major depression with psychotic features, and certain depressive/anxiety disorders.” Although generally critical of many instances of use of concomitant psychotropic medication, Wagner (100, p. 78) found it “very appropriate for co-morbid conditions in which there is a first-line treatment for each disorder.” She cited using an SSRI for major depression with a stimulant for ADHD as an appropriate example.
There have also been statements of support for specific combinations of concomitant psychotropic medications. For example, Carlson et al. (123, p. 409) noted that “certain combinations appear to be useful.” These include 1) antidepressants with lithium for bipolar disorder, 2) lithium augmentation for refractory depression, 3) lithium and neuroleptics for acute psychoses, 4) lithium and anticonvulsants for refractory mania, 5) sustained-release methylphenidate with short-acting methylphenidate to increase potency at drug onset, and 6) methylphenidate and a late afternoon or evening neuroleptic for severely hyperactive children. Pliszka et al. (113, p. 112) recommended use of concomitant psychotropic medication for youths with disruptive behavior disorders who are partially responsive to stimulant treatment, King (124, p. 41S) recommended clonazepam or a low dose of a neuroleptic to augment the effects of SSRIs for youths with treatment-resistant OCD, and Emslie et al. (125, p. 828) supported one of four psychotropic drug groups to augment inadequate medication responses to an SSRI for depressed youths.
Essentially, Biederman (121) and Wagner (100) promoted treating comorbid disorders as the major justification for the use of concomitant psychotropic medication in youths, whereas Carlson et al. (123) recommended concomitant psychotropic medication to augment the treatment of partial medication responders. Others, such as Popper (122), Theesen (126), and Wilens et al. (127), indicated that both augmentation and comorbidity justify the use of concomitant psychotropic medication in youths. Still other reasons to use concomitant psychotropic medication include allowing a lower dose of one agent to be used, reversing side effects, and alleviating symptoms while waiting for another medication to become effective (16, 114, 120, 128).
Treating Aggressive/Disruptive Behavior
The admission of youths to inpatient psychiatric facilities in the United States is precipitated largely by aggressive/disruptive behavior (34, 129–131). These youths receive concomitant psychotropic medication at a significantly higher rate (34) and proportionally more neuroleptic medication (32, 34, 49, 132) than nonaggressive youths. In fact, Connor et al. (48, p. 27) wrote that “neuroleptics were used primarily for aggression regardless of diagnosis,” a finding previously noted (32, 34). In addition to neuroleptics, psychotropic drugs used individually and concomitantly to treat aggressive behavior in youths included stimulants, lithium, antidepressants, anticonvulsants, and alpha agonists (133).
Specificity of Drug Action and Symptoms
Child psychopharmacologists specifically aim to ameliorate discrete pathologically impairing target symptoms associated with diagnostic entities (134). Generally, sleep impairment, enuresis, tics, depressive symptoms, hyperactivity, obsessive-compulsive features, intermittent impulsive behavior, and delusions are commonly targeted for reduction with psychotropic medication. The usual treatment approach is to focus on the reduction of one symptom or one symptom complex by using a low dose of one psychotropic medication initially (13). However, when presented with an array of symptoms, one can easily extend this approach and add a different medication for each target symptom. A review of the adverse drug event reports comprising Table 4 reveals that a very common aim of concomitant psychotropic medication in these patients had been to treat multiple target symptoms.
Implications for Better Assessment
Kutcher (135, p. 257) noted that “in child and adolescent psychopharmacology, augmentation strategies are poorly researched, and in many cases, clinicians use adult literature as guidelines.” Sanchez et al. (136, p. 639) similarly noted that most concomitant psychotropic medication for youths is “still based on studies in adults.” Extrapolation from adult studies to children and adolescents has numerous limitations because emotional disorders and psychotropic medication responses are often quite different in children and adolescents than in adults (13, 137). Even mixing groups of late adolescents with younger youths can blur age-specific outcomes (138). Likewise, concomitant psychotropic medication prescribing patterns for adults and youths prominently differ.
After reviewing the various methods for establishing evidence of efficacy, we propose that a systematic evaluation of concomitant psychotropic medication should take place on three levels. First, a national survey using probability sampling techniques can be conducted to estimate the prevalence and prescribing patterns of concomitant psychotropic medication across a spectrum of child and adolescent behavioral and emotional disorders as well as in different treatment settings, including primary care, psychiatry, and subspecialty practices. Second, the effectiveness, safety, and level of satisfaction with certain widely used drug combinations can be systematically evaluated by using multiple informants as well as standardized rating scales and questionnaires. Obtaining long-term outcome data on concomitant psychotropic medication in behavioral, academic, and social domains will, of course, depend greatly on the ability to retain subjects for extended periods. Third, patients receiving complex concomitant psychotropic medication could be addressed by using “N of 1” methods (139). The methods for evaluating single patients in an experimental design originated in clinical psychology and randomly assigns the treatment interventions under double-blind conditions to successive time periods. The results of these trials generalize to the single patient under study; the goal is to produce an objective assessment of the benefit of the various interventions (drug 1 alone, drug 1 plus placebo, drug 1 plus drug 2). Applications of this approach have already been reported in the adult psychiatry and pediatric literature (140, 141). If the approach is applied to concomitant psychotropic medication, careful monitoring of functioning and side effects could determine if the outcome is altered after a series of sequential evaluations of the concomitant medication or a placebo substitution. In regard to improving methods for assessing safety for rarely occurring events (less than 1 per 10,000 exposures), much work is needed. This will initially require an examination of weaknesses in the existing infrastructure for reporting adverse drug events and in the methods for producing evidence on risk assessment (142, 143).
Comments
Drug combinations in general medicine in adulthood are a common, almost standard, practice for the treatment of certain conditions, such as seropositive HIV, intractable seizures, congestive heart failure, and hypertension (144–146). The level of pathology of these disorders can be monitored regularly, thus allowing medication treatments to be adjusted so as to reach or approximate the desired endpoint. In child psychiatry, although a sizable number of psychopathologic features are quantifiable, systematic monitoring in this regard is uncommon in clinical practice. Furthermore, the evidence base for the use of concomitant psychotropic medication, by comparison with adult practice, is quite weak (17).
The trend for use of concomitant psychotropic medication in youths will probably increase at its present lively pace, far outpacing the capacity for better evaluation and assessment. Efforts in academic institutions to slow the use of multiple psychotropic medications have been tried and are usually partially successful. However, these results appear to be poorly maintained over time (147–149), a consequence due in all likelihood to an array of complex factors that continue to stimulate the use of concomitant psychotropic medication. These include a relatively weak response to the initial psychotropic agent, the influence of open studies reporting that concomitant psychotropic medication for youths is useful, the ready application of findings from adult concomitant psychotropic medication studies to youths, the failure to publish negative findings, a reluctance to gradually withdraw an add-on psychotropic medication that initially appeared to be beneficial, and the inability of many physicians to reduce a complex regimen that the previous clinician initiated. Added to these are the pressures for medication treatments from managed care organizations, direct-to-consumer and physician-directed pharmaceutical advertising, and distraught parents and beleaguered child care staff (100, 150). Although this review cannot reverse the trend for the use of concomitant psychotropic medication in youths, it is hoped that it can aid in encouraging needed research in this area.
 |
 |
 |
 |
Received Sept. 24, 2001; revision received April 30, 2002; accepted May 8, 2002. From the Division of Child Psychiatry, Johns Hopkins Medical Institutions, Baltimore, and the Schools of Pharmacy and Medicine, University of Maryland School of Medicine, Baltimore. Address reprint requests to Dr. Safer, 7702 Dunmanway, Baltimore, MD 21222; [email protected] (e-mail). The authors thank the Pediatric Psychopharmacology Work Group at the University of Maryland for their contribution to their review, particularly Drs. Laurel Kiser and Davis Pruitt.
1. Rittmannsberger H, Meise U, Schauflinger K, Horvath E, Donat H, Hinterhuber H: Polypharmacy in psychiatric treatment: patterns of psychotropic drug use in Austrian psychiatric clinics. Eur Psychiatry 1999; 14:33-40Crossref, Medline, Google Scholar
2. Tyrer P: Drug treatment of psychiatric patients in general practice. Br Med J 1978; 2:1008-1010Crossref, Medline, Google Scholar
3. Baldessarini RJ, Kando JC, Centorrino F: Hospital use of antipsychotic agents in 1989 and 1993: stable dosing with decreased length of stay. Am J Psychiatry 1995; 152:1038-1044Link, Google Scholar
4. Frye MA, Ketter TA, Leverich GS, Huggins T, Lantz C, Denicoff KD, Post RM: The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. J Clin Psychiatry 2000; 61:9-15Crossref, Medline, Google Scholar
5. Foote SM, Etheredge L: Increasing use of new prescription drugs: a case study. Health Affairs 2000; 19:165-170Crossref, Google Scholar
6. Zuvekas SH: Trends in mental health services use and spending, 1987-1996. Health Affairs 2001; 20:214-224Crossref, Google Scholar
7. Colley CA, Lucas LM: Polypharmacy: the cure becomes the disease. J Gen Intern Med 1993; 8:278-283Crossref, Medline, Google Scholar
8. Nichol MB, Stimmel GL, Lange SC: Factors predicting the use of multiple psychotropic medications. J Clin Psychiatry 1995; 56:60-66Medline, Google Scholar
9. Safer DJ: Changing patterns of psychotropic medication prescribed by child psychiatrists in the 1990’s. J Child Adolesc Psychopharmacol 1997; 7:267-273Crossref, Medline, Google Scholar
10. Rushton JL, Whitmire JT: Pediatric stimulant and SSRI prescription trends: 1992-1998. Arch Pediatr Adolesc Med 2001; 155:560-565Crossref, Medline, Google Scholar
11. Olfson M, Marcus SC, Weissman MW, Jensen PS: National trends in the use of psychotropic medication by children. J Am Acad Child Adolesc Psychiatry 2002; 41:514-521Crossref, Medline, Google Scholar
12. Graham P, Turk J, Verhulst FC: Child Psychiatry, 3rd ed. Oxford, UK, Oxford University Press, 1999Google Scholar
13. Kutcher SP: Child and Adolescent Psychopharmacology. Philadelphia, WB Saunders, 1997Google Scholar
14. Weiner JM: Textbook of Child and Adolescent Psychiatry, 2nd ed. Washington, DC, American Psychiatric Press and American Academy of Child and Adolescent Psychiatry, 1997Google Scholar
15. Barker P: Basic Child Psychiatry, 6th ed. Oxford, UK, Blackwell Science, 1995Google Scholar
16. Green WH: Child and Adolescent Clinical Psychopharmacology. Baltimore, Williams & Wilkins, 1995Google Scholar
17. Jensen PS, Bhatara VS, Vitiello B, Hoagwood K, Feil M, Burke LB: Psychoactive medication prescribing practices for US children: gaps between research and clinical practice. J Am Acad Child Adolesc Psychiatry 1999; 38:557-565Crossref, Medline, Google Scholar
18. Levine J: Ascertainment of side effects in psychopharmacologic clinical trials, in Methodology of the Evaluation of Psychotropic Drugs. Edited by Benton O, Maier W, Rickels K. Heidelburg, Germany, Springer-Verlag, 1990, pp 130-135Google Scholar
19. Bhatara VS, Feil M, Hoagwood K, Vitiello B, Zima B: Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002; 53:244Link, Google Scholar
20. Schirm E, Tobi H, Zito JM, deJong-van den Berg L: Psychotropic medication in children: a study from the Netherlands. Pediatrics 2001; 108:e25Google Scholar
21. Rappley MD, Mullan PB, Alvarcz FJ, Eneli IU, Wang J, Gardiner JC: Diagnosis of attention-deficit/hyperactivity disorder and use of psychotropic medication in very young children. Arch Pediatr Adolesc Med 1999; 153:1039-1045Crossref, Medline, Google Scholar
22. Ghuman JK, Ginsberg GS, Subramaniam G, Ghuman HS, Kau AS, Riddle MA: Psychostimulants in preschool children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2001; 40:516-524Crossref, Medline, Google Scholar
23. Boles M, Lynch FL, DeBar LL: Variations in pharmacotherapy for attention deficit hyperactivity disorder in managed care. J Child Adolesc Psychopharmacol 2001; 11:43-52Crossref, Medline, Google Scholar
24. Zarin DA, Suarez AP, Pincus HA, Kupersanin BA, Zito JM: Clinical and treatment characteristics of children with attention deficit/hyperactivity disorder in psychiatric practice. J Am Acad Child Adolesc Psychiatry 1998; 37:1262-1270Crossref, Medline, Google Scholar
25. Wilens TE, Biederman J, Geist DE, Steingard R, Spencer T: Nortriptyline in the treatment of ADHD: a chart review of 58 cases. J Am Acad Child Adolesc Psychiatry 1993; 32:343-349Crossref, Medline, Google Scholar
26. Geller DA, Biederman J, Reed ED, Spencer T, Wilens TE: Similarities in response to fluoxetine in the treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1995; 34:36-44Crossref, Medline, Google Scholar
27. Cohen LG, Biederman J, Gibson S, Whitt S, Frazier J, Wozniak J, Wilens TE, Spencer T, Mick E: Pharmacotherapy of attention deficit hyperactivity disorder (ADHD) in psychiatrically referred girls, in Abstracts of the 39th New Clinical Drug Evaluation Unit Meeting. Bethesda, Md, National Institute of Mental Health, 1999Google Scholar
28. Biederman J, Mick E, Bostic JQ, Prince J, Daly J, Wilens TE, Spencer T, Garcia-Jetton J, Russell R, Wozniak J, Faraone SV: The naturalistic course of pharmacologic treatment of children with maniclike symptoms: a systematic chart review. J Clin Psychiatry 1998; 59:628-637Crossref, Medline, Google Scholar
29. Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Rapoport JL: Childhood-onset schizophrenia. Arch Gen Psychiatry 1996; 53:1090-1097Crossref, Medline, Google Scholar
30. Martin A, Scahill L, Klin A, Volkmar FR: Higher-functioning pervasive developmental disorders: rates and patterns of psychotropic drug use. J Am Acad Child Adolesc Psychiatry 1999; 38:923-931Crossref, Medline, Google Scholar
31. Aman MG, Van Bourgondien ME, Wolford PL, Sarphane G: Psychotropic and anticonvulsant drugs in subjects with autism: prevalence and patterns of use. J Am Acad Child Adolesc Psychiatry 1995; 34:1672-1681Crossref, Medline, Google Scholar
32. Kaplan SL, Simms RM, Busner J: Prescribing practices of outpatient child psychiatrists. J Am Acad Child Adolesc Psychiatry 1994; 33:35-44Crossref, Medline, Google Scholar
33. Storch DD: Outpatient pharmacotherapy in a community mental health center. J Am Acad Child Adolesc Psychiatry 1998; 37:249-250Crossref, Medline, Google Scholar
34. Ahsanuddin KM, Ivey JA, Schlotzhauer D, Hall K, Prosen H: Psychotropic medication prescription patterns in 100 hospitalized children and adolescents. J Am Acad Child Psychiatry 1983; 22:361-364Crossref, Medline, Google Scholar
35. Kaplan SL, Busner J: Prescribing practices of inpatient child psychiatrists under three auspices of care. J Child Adolesc Psychopharmacol 1997; 7:275-286Crossref, Medline, Google Scholar
36. Bhatara V, Feil M, Hoagwood K, Vitiello B, Zima BT: Concomitant pharmacotherapy in youths receiving antidepressants or stimulants: national trends, in Abstracts of Posters Presented at the 47th Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 2000Google Scholar
37. Hussain MZ, Chaudry ZA, Hussain S: Evaluation and intervention of prodromal symptoms of bipolar disorder, in Abstracts of the 41st New Clinical Drug Evaluation Unit Meeting. Bethesda, Md, National Institute of Mental Health, 2001Google Scholar
38. Baumgartner JL, Emslie GJ, Crismon ML: Citalopram use in 17 children and adolescents: a case series. IbidGoogle Scholar
39. Cohen LG, Doyle RL, Prince JB, Wozniak J, Wilens TE, Spencer TJ, Biederman J: Pharmacoepidemiology of citalopram in youth with mood disorders. IbidGoogle Scholar
40. Zito JM, Safer DJ, dosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MAM: Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003; 57:17-25Crossref, Google Scholar
41. Patel NC, Sanchez RJ, Johnsrud MT, Crismon ML: Trends in antipsychotic use in a Texas Medicaid population of children and adolescents: 1996 to 2000. J Child Adolesc Psychopharmacol 2002; 12:221-229Crossref, Medline, Google Scholar
42. Malone RP, Sheikh R, Zito JM: Novel antipsychotic medications in the treatment of children and adolescents. Psychiatr Serv 1999; 50:171-174Link, Google Scholar
43. Epstein MA, Shaywitz SE, Shaywitz BA, Woolston JL: The boundaries of attention deficit disorder. J Learn Disabil 1991; 24:78-86Crossref, Medline, Google Scholar
44. Bussing R, Zima BT, Belin T: Variations in ADHD treatment among special education students. J Am Acad Child Adolesc Psychiatry 1998; 37:968-976Crossref, Medline, Google Scholar
45. Mattison RE: Use of psychotropic medications in special education students with serious emotional disturbance. J Child Adolesc Psychopharmacol 1999; 9:149-155Crossref, Medline, Google Scholar
46. Zima BT, Bussing R, Crecelius GM, Kaufman A, Belin TR: Psychotropic medication treatment patterns among school-aged children in foster care. J Child Adolesc Psychopharmacol 1999; 9:135-147Crossref, Medline, Google Scholar
47. Anderson TR, Naylor M, Kruesi M, Stoewe J: Co-pharmacy and polypharmacy in children and adolescents in substitute care. Abstracts of the 49th Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 2002Google Scholar
48. Connor DF, Ozbayrak KR, Harrison RJ, Melloni RH: Prevalence and patterns of psychotropic and anticonvulsant medication use in children and adolescents referred to residential treatment. J Child Adolesc Psychopharmacol 1998; 8:27-38Crossref, Medline, Google Scholar
49. Hogg J: The administration of psychotropic and anticonvulsant drugs to children with profound intellectual disability and multiple impairments. J Intellect Disabil Res 1992; 36:473-488Crossref, Medline, Google Scholar
50. Wilens TE, Biederman J, Spencer T: Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1994; 33:424-427Crossref, Medline, Google Scholar
51. Prince JB, Wilens TE, Biederman J, Spencer TJ, Wozniak JR: Clonidine for sleep disturbances associated with attention deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry 1996; 35:599-605Crossref, Medline, Google Scholar
52. Hill P: The hyperactive child, in Difficult Clinical Problems in Psychiatry. Edited by Lader M, Naber D. London, Martin Dunitz, 1999, pp 213-228Google Scholar
53. Swanson JM, Connor DF, Cantwell D: Combining methylphenidate and clonidine: ill-advised. J Am Acad Child Adolesc Psychiatry 1999; 38:617-619Crossref, Google Scholar
54. Rappley MD, Eneli IU, Mullan PB, Alvarez FJ, Wang J, Luo Z, Gardiner JC: Patterns of psychotropic medication use in very young children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 2002; 23:23-30Crossref, Medline, Google Scholar
55. Pathiyal A, Miwa LJ, Sverdiov LS, Gardner E, Jones JK: Patterns of methylphenidate use, in Abstracts of the 1998 Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics. Alexandria, Va, ASCPT, 1998Google Scholar
56. Zito JM, Safer DJ, dosReis S, Gardner JF, Soeken K, Boles M, Lynch F: The rising prevalence of antidepressants among US youths. Pediatrics 2002; 109:721-727Crossref, Medline, Google Scholar
57. Minter RE, Mandel MR: The treatment of psychotic major depressive disorder with drugs and electroconvulsive therapy. J Nerv Ment Dis 1979; 167:726-733Crossref, Medline, Google Scholar
58. Dufresne RL: Issues in polypharmacotherapy: focus on major depression. Psychopharmacol Bull 1996; 32:547-553Medline, Google Scholar
59. Freeman MP, Stoll AL: Mood stabilizer combinations: a review of safety and efficacy. Am J Psychiatry 1998; 155:12-21Link, Google Scholar
60. McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH: A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57:794-801Crossref, Medline, Google Scholar
61. Carlson GA, Rapport MD, Kelly KL, Pataki CS: Methylphenidate and desipramine in hospitalized children with comorbid behavior and mood disorders: separate and combined effects on behavior and mood. J Child Adolesc Psychopharmacol 1995; 5:191-204Crossref, Google Scholar
62. Rapport MD, Carlson GA, Kelly KL, Pataki C: Methylphenidate and desipramine in hospitalized children, I: separate and combined effects on cognitive function. J Am Acad Child Adolesc Psychiatry 1993; 32:333-342Crossref, Medline, Google Scholar
63. Pataki CS, Carlson GA, Kelly KL, Rapport MD, Biancaniello TM: Side effects of methylphenidate and desipramine alone and in combination in children. J Am Acad Child Adolesc Psychiatry 1993; 32:1065-1072Crossref, Medline, Google Scholar
64. Gittelman-Klein R, Klein DF, Katz S, Saraf K, Pollack E: Comparative effects of methylphenidate and thioridazine in hyperkinetic children, I: clinical results. Arch Gen Psychiatry 1976; 33:1217-1231Crossref, Medline, Google Scholar
65. Connor DF, Barkley RA, Davis HT: A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr 2000; 39:15-25Crossref, Medline, Google Scholar
66. Kurlan R (Tourette’s Syndrome Study Group): Treatment of ADHD in children with tics. Neurology 2002; 58:527-536Crossref, Medline, Google Scholar
67. Turgay A, Binder C, Snyder R, Fisman S: Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics 2002; 110:e34Google Scholar
68. Carlson GA, Rapport MD, Kelly KL, Pataki CS: The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992; 31:262-270Crossref, Medline, Google Scholar
69. Bess A, Cunningham S: Important Drug Warning Regarding Mellaril: Letter to Physicians. East Hanover, NJ, Novartis Pharmaceutical Corp, 2000Google Scholar
70. Riddle MA, Geller B, Ryan N: Another sudden death in a child treated with desipramine. J Am Acad Child Adolesc Psychiatry 1994; 32:792-797Crossref, Google Scholar
71. Elia J, Borcherding BG, Rapoport JL, Keysor CS: Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders? Psychiatry Res 1991; 36:141-155Crossref, Medline, Google Scholar
72. Hussain MZ, Zubaido A, Chaudhry MB: Novel antipsychotic in ADHD with conduct disorder, in 2000 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 2000Google Scholar
73. Steingard RJ, Goldberg M, Lee D, DeMaso DR: Adjunctive clonazepam treatment of tic symptoms in children with comorbid tic disorders and ADHD. J Am Acad Child Adolesc Psychiatry 1994; 33:394-399Crossref, Medline, Google Scholar
74. Saccomani L, Rizzo P, Nobili L: Combined treatment with haloperidol and trazodone in patients with tic disorders. J Child Adolesc Psychopharmacol 2000; 10:307-310Crossref, Medline, Google Scholar
75. Figueroa Y, Rosenberg DR, Birmaher B, Keshavan MS: Combination treatment with clomipramine and selective serotonin reuptake inhibitors for obsessive-compulsive disorder in children and adolescents. J Child Adolesc Psychopharmacol 1998; 8:61-67Crossref, Medline, Google Scholar
76. Kowatch RA, Suppes T, Gilfillan SK, Fuentes RM, Grannemann BD, Emslie GJ: Clozapine treatment of children and adolescents with bipolar disorder and schizophrenia. J Child Adolesc Psychopharmacol 1995; 5:241-253Crossref, Google Scholar
77. Fitzgerald KD, Stewart CM, Tawile V, Rosenberg DR: Risperidone augmentation of serotonin reuptake inhibitor treatment of pediatric obsessive compulsive disorder. J Child Adolesc Psychopharmacol 1999; 9:115-123Crossref, Medline, Google Scholar
78. Strober M, Freeman R, Rigali J, Schmidt S, Diamond R: The pharmacotherapy of depressive illness in adolescence, II: effects of lithium augmentation in nonresponders to imipramine. J Am Acad Child Adolesc Psychiatry 1992; 31:16-20Crossref, Medline, Google Scholar
79. Brown RP, Ingber PS, Tross S: Pemoline and lithium in a patient with attention deficit disorder. J Clin Psychiatry 1983; 44:146-148Medline, Google Scholar
80. Chang KD, Ketter TA: Mood stabilizer augmentation with olanzapine in acutely manic children. J Child Adolesc Psychopharmacol 2000; 10:45-49Crossref, Medline, Google Scholar
81. Ryan ND, Meyer V, Dachille S, Mazzie D, Puig-Antich J: Lithium antidepressant augmentation in TCA-refractory depression in adolescents. J Am Acad Child Adolesc Psychiatry 1988; 27:371-376Crossref, Medline, Google Scholar
82. Simeon JG, Dinicolo VF, Ferguson HB, Copping W: Adolescent depression: a placebo-controlled fluoxetine treatment study and follow-up. Prog Neuropsychopharmcol Biol Psychiatry 1990; 14:791-795Crossref, Medline, Google Scholar
83. Bussing R, Levin GM: Methamphetamine and fluoxetine treatment of a child with attention deficit hyperactivity disorder and obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 1993; 3:53-58Crossref, Medline, Google Scholar
84. Gammon GD, Brown TE: Fluoxetine and methylphenidate in combination for treatment of attention deficit disorder and comorbid depressive disorder. J Child Adolesc Psychopharmacol 1991; 3:1-10Crossref, Google Scholar
85. Parraga HC, Kelly DP, Parraga MI, Cochran MK, Maxim LT: Combined psychostimulant and tricyclic antidepressant treatment of Tourette’s syndrome and comorbid disorders in children. J Child Adolesc Psychopharmacol 1994; 4:113-122Crossref, Google Scholar
86. Findling RL: Open-label treatment of comorbid depression and attentional disorders with co-administration of serotonin reuptake inhibitors and psychostimulants in children, adolescents, and adults: a case series. J Child Adolesc Psychopharmacol 1996; 6:165-175Crossref, Medline, Google Scholar
87. Hawkridge S, Stein DJ, Bouwer C: Combined pharmacotherapy for TS and OCD (letter). J Am Acad Child Adolesc Psychiatry 1996; 35:703-704Crossref, Medline, Google Scholar
88. Licamele WL, Goldberg RL: The concurrent use of lithium and methylphenidate in a child. J Am Acad Child Adolesc Psychiatry 1989; 28:785-787Crossref, Medline, Google Scholar
89. Sallee FR, DeVane CL, Ferrell RE: Fluoxetine-related death in a child with cytochrome P-450 2D6 genetic deficiency. J Child Adolesc Psychopharmacol 2000; 10:27-34Crossref, Medline, Google Scholar
90. Preda A, Madlener A, Hetherington P: Premature polypsychopharmacology. J Am Acad Child Adolesc Psychiatry 1998; 37:348-349Crossref, Medline, Google Scholar
91. Budman CL, Sherling M, Bruun RD: Combined pharmacotherapy risk. J Am Acad Child Adolesc Psychiatry 1995; 34:263-264Crossref, Medline, Google Scholar
92. Levy F, Einfeld S, Looi J: Combined pharmacotherapy or polypharmacy? J Paediatr Child Health 1996; 32:265-266Crossref, Medline, Google Scholar
93. Fisman S, Reniers D, Diaz P: Erythromycin interaction with risperidone or clomipramine in an adolescent. J Child Adolesc Psychopharmacol 1996; 6:133-138Crossref, Medline, Google Scholar
94. Gara L, Roberts W: Adverse response to methylphenidate in combination with valproic acid. J Child Adolesc Psychopharmacol 2000; 10:39-43Crossref, Medline, Google Scholar
95. Bhatara VS, Kallepalli BR, Misra LK, Awadallah S: A possible clonidine-trazodone-dextroamphetamine interaction in a 12-year-old boy. J Child Adolesc Psychopharmacol 1996; 6:203-209Crossref, Medline, Google Scholar
96. Fenichel RR: Combining methylphenidate and clonidine: the role of post-marketing surveillance. J Child Adolesc Psychopharmacol 1995; 5:155-156Crossref, Google Scholar
97. DeMaso DR, Hunter TA: Combining fluoxetine with desipramine (letter). J Am Acad Child Adolesc Psychiatry 1990; 29:151Crossref, Medline, Google Scholar
98. Chiu S, Welch C, Corwin RD, Ziegler J, Sachs H: Symptomatic electrocardiography changes from combining olanzapine, divalproex sodium and antidepressants, in Abstracts of the 46th Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 1999Google Scholar
99. Vitiello B, Jensen PS: Medication development and testing in children and adolescents: current problems, future directions. Arch Gen Psychiatry 1997; 54:871-876Crossref, Medline, Google Scholar
100. Wagner KD: Polypharmacy for children. Psychiatr Times, Nov 2000, pp 78-79Google Scholar
101. Council of the American Academy of Child and Adolescent Psychiatry: Policy statement: prescribing psychoactive medications for children and adolescents. AACAP News, July 2000, p 207Google Scholar
102. Behr R: Overzealous prescribing of medications. J Am Acad Child Adolesc Psychiatry 1998; 37:900-901Medline, Google Scholar
103. Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U, Stocker DN, Braunschweig S, Kullak-Ublick GA, Galeazzi RL, Follath F, Gasser T, Meier PJ: Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 2000; 49:158-167Crossref, Medline, Google Scholar
104. Martinez-Mir I, Garcia-Lopez M, Palop V, Ferrer JM, Rubio E, Morales-Olivas FJ: A prospective study of adverse drug reactions in hospitalized children. Br J Clin Pharmacol 1999; 47:681-688Crossref, Medline, Google Scholar
105. Turner S, Nunn AJ, Fielding K, Choonara I: Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr 1999; 88:965-968Crossref, Medline, Google Scholar
106. Geller B, Cooper TB, Farooki ZQ, Chestnut EC: Dose and plasma levels of nortriptyline and chlorpromazine in delusionally depressed adolescents and of nortriptyline in nondelusionally depressed adolescents. Am J Psychiatry 1985; 142:336-338Link, Google Scholar
107. Ambrosini PJ, Sheikh RM: Increased plasma valproate concentrations when coadministered with guanfacine. J Child Adolesc Psychopharmacol 1998; 8:143-147Crossref, Medline, Google Scholar
108. Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB: Valproic acid hepatic fatalities: a retrospective review. Neurology 1987; 37:379-385Crossref, Medline, Google Scholar
109. Ghose K: The need for a review journal of drug use and the elderly. Drugs Aging 1991; 1:2-5Crossref, Medline, Google Scholar
110. McMillan DA, Harrison PM, Rogers LJ, Tong N, McLean AJ: Polypharmacy in an Australian teaching hospital: preliminary analysis of prevalence, types of drugs and associations. Med J Aust 1986; 145:339-342Crossref, Medline, Google Scholar
111. Hack S, Chow B: Pediatric psychotropic medication compliance. J Child Adolesc Psychopharmacol 2001; 11:59-67Crossref, Medline, Google Scholar
112. Bjerrum L, Sogaard J, Hallas J, Kragstrup J: Polypharmacy: correlations with sex, age and drug regimen. Eur J Clin Pharmacol 1998; 54:197-202Crossref, Medline, Google Scholar
113. Pliszka SR, Carlson CL, Swanson JM: ADHD With Comorbid Disorders. New York, Guilford, 1999Google Scholar
114. Werry JS: Introduction, in Practitioner’s Guide to Psychoactive Drugs for Children and Adolescents, 2nd ed. Edited by Werry JS, Aman MG. New York, Plenum, 1999, pp 3-22Google Scholar
115. Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S: Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry 1997; 54:1073-1080Crossref, Medline, Google Scholar
116. Spencer T, Biederman J, Kerman K, Steingard R, Wilens TE: Desipramine treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1993; 32:354-360Crossref, Medline, Google Scholar
117. Steingard R, Biederman J, Spencer T, Wilens TE, Gonzalez A: Comparison of clonidine response in the treatment of attention-deficit hyperactivity disorder with and without comorbid tic disorders. J Am Acad Child Adolesc Psychiatry 1993; 32:350-353Crossref, Medline, Google Scholar
118. Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF: A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 2001; 158:1067-1074Link, Google Scholar
119. Kingsbury SJ, Yi D, Simpson GM: Rational and irrational polypharmacy. Psychiatr Serv 2001; 52:1033-1036Link, Google Scholar
120. Preskorn SH, Lacey RL: Polypharmacy: when is it rational? J Prac Psychiatr Behav Health 1995; 1:92-98Google Scholar
121. Biederman J: Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 1998; 59:4-16Crossref, Medline, Google Scholar
122. Popper C: Balancing knowledge and judgment. Child Adolesc Psychiatr Clin North Am 1995; 4:483-523Crossref, Google Scholar
123. Carlson GA, Ranade L, Qadir A: Management of psychopharmacologic agents in children and adolescents. Psychiatr Q 1992; 63:391-411Crossref, Medline, Google Scholar
124. King RA: Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998; 37:27S-45SCrossref, Medline, Google Scholar
125. Emslie GJ, Mayes TL, Hughes CW: Updates in the pharmacologic treatment of childhood depression. Psychiatr Clin North Am 2000; 23:813-835Crossref, Google Scholar
126. Theesen KA: The Handbook of Psychiatric Drug Therapy for Children and Adolescents. New York, Pharmaceutical Products Press, 1995Google Scholar
127. Wilens TE, Spencer T, Biederman J, Wozniak J, Connor DF: Combined pharmacotherapy: an emerging trend in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry 1995; 34:110-112Crossref, Medline, Google Scholar
128. Rapoport JL: An evidence-based approach to pediatric psychiatry in 2001 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 2001Google Scholar
129. Dalton R, Forman MA: Psychiatric Hospitalization for School-Age Children. Washington, DC, American Psychiatric Press, 1992Google Scholar
130. Safer DJ: An outpatient/inpatient comparison of child psychiatric diagnosis. Am J Orthopsychiatry 1995; 65:298-303Crossref, Medline, Google Scholar
131. Aronet ET, Noam CG, Weinstein SR: Structured diagnostic interviews and clinicians’ discharge diagnoses in hospitalized adolescents. J Am Acad Child Adoles Psychiatry 1993; 32:674-681Crossref, Medline, Google Scholar
132. Connor DF, Ozbyrak KR, Kusiak KA, Caponi AB, Melloni RH: Combined pharmacotherapy in children and adolescents in a residential treatment center. J Am Acad Child Adolesc Psychiatry 1997; 36:248-254Crossref, Medline, Google Scholar
133. Weller E, Rowan A, Elia J, Weller R: Aggressive behavior in patients with attention-deficit/hyperactivity disorder, conduct disorder, and pervasive developmental disorders. J Clin Psychiatry 1999; 60:5-11Crossref, Google Scholar
134. Green WH: Principles of psychopharmacology and specific drug treatments, in Child and Adolescent Psychiatry, 2nd ed. Edited by Lewis M. Baltimore, Williams & Wilkins, 1996, pp 772-801Google Scholar
135. Kutcher S: Practical clinical issues regarding child and adolescent psychopharmacology. Child Adolesc Psychiatr Clin North Am 2000; 9:245-260Crossref, Medline, Google Scholar
136. Sanchez L, Hagino O, Weller E, Weller R: Bipolarity in children. Psychiatr Clin North Am 1999; 22:629-648Crossref, Medline, Google Scholar
137. Vitiello B, Jensen PS: Developmental perspectives in pediatric psychopharmacology. Psychopharmacol Bull 1995; 31:75-81Medline, Google Scholar
138. Milin RP, Simeon J, Spenst BA: Double-blind study of paroxetine in adolescents with unipolar major depression, in Abstracts of the 46th Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 1999Google Scholar
139. Guyatt GH, Jaeschke R, Roberts R: N-of-1 randomized clinical trials in pharmacoepidemiology, in Pharmacoepidemiology, 3rd ed. Edited by Strom BL. Chichester, UK, John Wiley & Sons, 2000, pp 615-632Google Scholar
140. Kent MA, Camfield CS, Camfield PR: Double-blind methylphenidate trials. Arch Pediatr Adolesc Med 1999; 153:1291-1296Crossref, Google Scholar
141. Field CJ, Aman MG, White AJ, Vaithianathan C: A single-subject study of imipramine in a mentally retarded woman with depressive symptoms. J Ment Defic Res 1986; 30:191-198Medline, Google Scholar
142. Woosley R: Opportunities in phase IV to improve drug development. Food Drug Law J 1997; 52:185-188Medline, Google Scholar
143. Nelson R: We need a postmarketing drug development process. Pharmacoepidemiol Drug Saf 2000; 9:253-255Crossref, Medline, Google Scholar
144. Jallon P: The problem of intractability: the continuing need for new medical therapies in epilepsy. Epilepsia 1997; 38:S37-S42Google Scholar
145. Zanchetti A, Hansson L: Introduction: the role of combination therapy in modern antihypertensive therapy. J Cardiovasc Pharmacol 2000; 35(suppl 3):S1Google Scholar
146. Gulick RM, Mellors JW, Havur D, Eron JJ, Gonzalez C, McMahon D: Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997; 337:734-739Crossref, Medline, Google Scholar
147. de Girolamo G, Williams P, Cappiello V: Psychotropic drug utilization and audit in two Italian psychiatric services. Psychol Med 1987; 17:989-997Crossref, Medline, Google Scholar
148. Meyer TJ, Van Kooten D, Marsh S, Prochazka AV: Reduction of polypharmacy by feedback to clinicians. J Gen Intern Med 1991; 6:133-136Crossref, Medline, Google Scholar
149. Pitkala KH, Strandberg TE, Tilvis RS: Is it possible to reduce polypharmacy in the elderly? a randomized, controlled trial. Drugs Aging 2001; 18:143-149Crossref, Medline, Google Scholar
150. Woolston JL: Combined pharmacotherapy: pitfalls of treatment. J Am Acad Child Adolesc Psychiatry 1999; 38:1455-1457Crossref, Medline, Google Scholar



