Response of Cortical Metabolic Deficits to Serotonergic Challenge in Familial Mood Disorders
Abstract
OBJECTIVE: In subjects with mood disorders, positron emission tomography (PET) with [18F]fluorodeoxyglucose has shown prefrontal cortical metabolism deficits, including in a subgenual region in subjects with familial illness. The authors applied a dl-fenfluramine challenge method to study metabolic response in this region to serotonergic challenge in familial major depression. METHOD: The study group consisted of 19 depressed subjects with major depressive disorder or bipolar disorder, all of whom had at least one first-degree relative with history of major depression, and 10 healthy volunteers with similar age and gender distributions. PET images were acquired under placebo and challenge conditions, and volumetric MRI scans were also obtained. Group comparisons of metabolic and volumetric data were performed. Ratings of acute mood change during serotonergic challenge were compared with the imaging data. RESULTS: Within Brodmann’s area 32, a glucose metabolism deficit in the depressed subjects on placebo day was observed by voxel-level analysis, but no volumetric deficit was found in the subgenual regions examined. Under challenge, both groups suppressed metabolism similarly. Within the patient group, the correlation between acute mood improvement during challenge and greater metabolic suppression approached significance. CONCLUSIONS: In familial mood disorders, a ventromedial prefrontal cortical deficit in baseline metabolism is not due to altered structural volume, and the response to serotonergic challenge appears predictive of acute mood response. The potential to predict treatment response can be tested by a combined challenge and treatment study.
Neuroimaging studies of mood disorders have more consistently shown functional than structural deficits (1). For example, 11 of 12 studies of the prefrontal cortex found a decreased rate of regional cerebral glucose metabolism (1), whereas one-half or fewer of published studies found alterations in the volume of key brain regions thought to play a role in mood disorders (2). We have previously reported blunted responses in relative prefrontal cortical cerebral glucose metabolism in several brain regions to the release of serotonin by fenfluramine in subjects with mood disorders in vivo by using PET and [18F]fluorodeoxyglucose (FDG) (3). Drevets et al. (4) found that a subgenual prefrontal cortical region in subjects with familial mood disorders exhibited striking deficits of blood flow, metabolism, and structural volume. Replication studies of the volumetric finding have produced mixed results (5–8). Mayberg et al. (5) found that in ventral prefrontal regions, elevations in blood flow and glucose metabolism occurred during induced sadness in comparison subjects, and decreases accompanied recovery from depression in patients. Reductions in frontal blood flow and glucose metabolism with treatment response in depression have been documented in other studies (9, 10).
The ventromedial prefrontal cortex, with its limbic (anterior cingulate) and paralimbic (ventral prefrontal cortex) components, has been implicated in mood disorders in many studies (see for example references 11–13). Humans with lesions that include the ventromedial prefrontal cortex demonstrate failure to experience emotion or exhibit abnormal autonomic responses to emotional experiences (14, 15).
Our goal was to perform FDG PET studies under placebo and dl-fenfluramine challenge conditions as well as MRI studies comparing subjects with familial mood disorders and healthy volunteers. Our purpose was to test the metabolic response of the ventromedial prefrontal cortex and subjective mood to serotonergic challenge. With this approach, we sought to test the hypothesis that there are structural or metabolic abnormalities in this region in subjects with familial mood disorders and to determine their relation to serotonergic function and mood response.
Method
Subjects
Study subjects consisted of 19 subjects experiencing a DSM-III-R major depressive episode and 10 healthy volunteers with similar age and gender distributions (Table 1 shows further details). Before and after each scan, patients were hospitalized in an inpatient research unit for the evaluation and treatment of depression. They received no payment for participation. Depression severity was in the moderate range, as determined by the 17-item Hamilton Depression Rating Scale (16) at the time of study (mean score=22.3, SD=3.9, range=17–30). Five subjects had bipolar disorder (mean age=37 years, SD=15), and 14 had major depressive disorder or unipolar depression (mean age=36 years, SD=10). Diagnoses were determined according to DSM-III-R criteria by using the Structured Clinical Interview for DSM-III-R (SCID) (17). For comparability with prior studies of familial mood disorders (e.g., Drevets et al. [4]), all patients had at least one first-degree relative with a diagnosed mood disorder. Assessment of family members was performed with a DSM-III-R checklist with information obtained from the subject and, whenever possible, one additional family member. All subjects were free of medication at the time of study for at least 14 days (6 weeks in the case of fluoxetine), except for benzodiazepines, which were discontinued at least 24 hours before scanning in 12 cases but were needed within 24 hours of scanning for the remaining seven. Prior treatment medications for 13 of the patients (six were medication-naive) were fluoxetine (N=3), sertraline (N=2), and valproate (N=2); one patient each was receiving paroxetine, bupropion, clomipramine, doxepin, tranylcypromine, and hydroxyzine. Other medications included the benzodiazepines clonazepam (N=3) and lorazepam (N=4). Nine of the 19 patients had comorbid axis I diagnoses (three had panic disorder, two had dysthymia, and one each had social phobia, simple phobia, anorexia nervosa, and posttraumatic stress disorder). The healthy volunteers were recruited through newsletter and newspaper advertisements and were admitted the day before the first scan to the Irving Center for Clinical Research and discharged after the second scan. The screening examination included the SCID (nonpatient version), history of past suicidal behavior, and evaluation of family psychiatric history to exclude axis I diagnosis in the subjects or first-degree relatives and history of suicidal behavior in the subjects. Patients and healthy volunteers were free of medical illness as assessed by medical history, physical examination, standard laboratory tests (including blood count, electrolytes, thyroid indices, and toxicology screen), and according to MRI and PET scans as reviewed by a neuroradiologist. Pregnancy was excluded by history and by testing on the morning of the PET studies, and premenopausal female subjects were studied within 5 days after onset of menses. All subjects gave written informed consent following full explanation of the study, including the risks of radiation exposure. The study and consent procedures were as approved by the medical center institutional review board.
Pharmacological Challenge and Plasma Levels
Brain response to serotonergic challenge was assessed by administration of an oral dose of placebo or 60 mg (about 0.8 mg/kg) of dl-fenfluramine as described previously (3, 18, 19). This challenge raises intrasynaptic levels of serotonin both by release from nerve terminals and by inhibiting transmitter reuptake. Levels of fenfluramine and norfenfluramine were assayed by a gas-liquid chromatography method (20) adapted as described previously (21). Prolactin levels were determined with an immunoradiometric assay (Hybritech, Inc., San Diego, Calif.).
Clinical Ratings
Acute mood symptom changes induced by the pharmacological challenge were evaluated by using the Profile of Mood States (POMS), a 65-item self-report scale that assesses aspects of current mood and is widely used in pharmacological, experimental, and treatment research (22). Scores range from –32 to 208, with higher scores indicating greater mood disturbance. POMS ratings were obtained within 1 hour after each of the two PET scanning sessions.
PET Scans
Subjects fasted from midnight before the PET scan until shortly after scan completion. Scans were of 1-hour duration and began approximately 40 minutes after intravenous bolus injection of FDG, 5 mCi, which followed drug administration (placebo or fenfluramine, see reference 3) by 3 hours. The 3-hour period was chosen on the basis of prior data demonstrating robust prolactin and symptomatic response to fenfluramine at this interval (21). Subjects were provided with minimal, uniform sensory stimulus for the 40 minutes of the distribution phase between FDG injection and scanning. Subjects were then positioned supine on the scanning table with an individually molded, thermoplastic mask to minimize head movement, which was reused for the second PET study to enhance reproducibility of head positioning. Scans were acquired with a Siemens ECAT EXACT 47 scanner (in-plane spatial resolution 5.8 mm, axial resolution 4.3 mm full width at half maximum at center) in two-dimensional mode in a series of 12 5-minute frames. Images were reconstructed as 12 transaxial slices by using a Shepp radial filter with cutoff frequency of 0.35 and a ramp axial filter with cutoff frequency of 0.5. Attenuation was measured by a 10-minute 68Ge/68Ga transmission scan acquired immediately after the hour of image acquisition.
MRI Scans
MRI scans for volumetric analysis and anatomically based region of interest definition were acquired with a T1-weighted axial sequence, slices parallel to the anterior commissure-posterior commissure line, by using three-dimensional spoiled gradient recalled acquisition in the steady state. These produced thin-section images of the whole brain with the following gradient-echo parameters: TR=34 msec, TE=5 msec, flip angle=45°, slice thickness=1.5 mm with zero gap, voxels=1.5 mm × 0.78 mm × 0.78 mm.
Image Analysis
Three methods of PET image analysis were used to test the hypothesis of a decreased rate of regional cerebral glucose metabolism in the ventromedial prefrontal cortex in the patient group. All image analysis was performed by using UNIX workstations (Sun Microsystems, Inc., Palo Alto, Calif.) with the MEDx software system (Sensor Systems, Inc., Sterling, Va.) or with Statistical Parametric Mapping, Version 96 (SPM 96).
The image analysis methods were as described below.
1. Anatomically defined region of interest
MRI-based anatomically defined regions of interest allow evaluation of function in regions specified by brain structure and by anatomical landmarks. The fact that the design of our study was, in part, a replication of the study of Drevets et al. (4) led to the use of this method, since that paper specified anatomical landmarks. In this method, the subgenual cingulum was manually traced within the ventromedial prefrontal cortex by using anatomic criteria on the MRI scan of each subject by an operator blind to placebo or fenfluramine condition and to diagnosis. The subgenual cingulum as defined here included the Drevets subgenual cingulum, or Brodmann’s areas 33 and 24 (termed “subgenual prefrontal cortex” by Drevets et al. [4]), plus the subgenual portion of Brodmann’s area 32. The criteria included the agranular and granular cortex (4, 23) bounded anteriorly by the most anterior extent of the genu of the corpus callosum and posteriorly by the most anterior plane where the internal capsule no longer divides the striatum. Superior-inferior extent ranged from the inferior surface of the corpus callosum to the inferior boundary of the second gyrus ventral to the corpus callosum. Gray matter within the manually traced region was defined in this approach by an MRI tissue segmentation method that used a triple-Gaussian curve fit to the histogram of voxel intensities from the whole brain to accommodate peaks for cerebrospinal fluid, gray matter, and white matter (24). Interrater reliability was evaluated for four raters who measured volumes of prefrontal cortical gray matter regions that included the subgenual cingulum for nine cases, using the above tissue segmentation method for gray matter selection. Intraclass correlation coefficient ranged from 0.91 to 0.92 across these cases. The corresponding regional signal from the individually coregistered PET images was computed, with intermodality image coregistration performed by using the AIR algorithm (25) as incorporated in the MEDx software system. PET images were rescaled to a gray matter mean metabolic rate of 53 mmol/100 gm tissue/minute (26).
2. Statistical parametric mapping
The second analytic approach was a functional, pixel-based method (27) that used SPM 96 as incorporated into the MEDx system to test differences in the mean metabolism between groups, in the a priori defined ventromedial prefrontal cortical region. The statistical parametric mapping method allows functional activity differences to determine regions of comparison. These procedures were as previously described (3) except for the use of a newer version of statistical parametric mapping. One-tailed t tests of the differences of the means between groups at each voxel under each of the two conditions were converted to z scores for graphical display as parametric maps. For the a priori specified ventromedial prefrontal cortical region, protection against type I error accumulation in multiple comparisons using the theory of Gaussian random fields in statistical parametric mapping exploratory analyses was not needed (28). The statistical parametric mapping analysis identifies the voxels of significantly lower cerebral glucose metabolism in the patient group as computed by the aforementioned methods.
3. Functionally defined region of interest
The third approach was a functionally defined region of interest method, used in prior SPECT, PET, and fMRI studies (29–31). When a functionally defined region of interest is determined by the result of a statistical parametric mapping analysis, as in the current study, the resulting region of interest data are not independent of the statistical parametric mapping data, and the image data analysis results are not additional to the statistical parametric mapping results but merely corroborative. The advantage, however, of generating functionally defined region of interest data is the availability of these data for comparison with other variables that are not image-related (plasma data, clinical ratings).
The z threshold value of 2.88 was selected to define a localized region in the ventromedial prefrontal cortex on the statistical parametric map of the placebo condition group difference. The method does not provide independent testing of the placebo condition deficit but does yield new data for further statistical analysis of the challenge condition (whose data play no role in the functional region definition). This region of interest was then applied to each individual PET image in the study, both pre- and postchallenge, in the stereotaxic-atlas-transformed space. Gray matter (defined by using the statistical parametric mapping criterion as all voxels of intensity greater than 0.8 of the image mean) signal intensity was rescaled for all images to a mean of 53 mmol/100 g tissue/minute (26). Statistical analyses were then applied to the mean PET region of interest values.
Statistical Analysis
Plasma levels of prolactin, fenfluramine, and norfenfluramine were assessed for group effects by two-tailed t tests. Two-tailed t tests were used to evaluate volume differences between the diagnostic groups, supplemented by analysis of covariance (ANCOVA) to control for whole brain volume. Repeated-measures analysis of variance (ANOVA) was applied to both the anatomically defined and functionally defined regional metabolism data. In the statistical parametric mapping analysis, significance testing was performed with one-tailed t tests of differences between the adjusted means at each voxel, which were then converted to z scores as implemented in SPM 96. Simple regression analysis with Spearman’s test of significance was used to study the relation of the metabolic changes to the POMS depression scores. A mixed-effects model (32) was used to assess the potential confounding effects of plasma fenfluramine and norfenfluramine levels on the brain metabolism data derived from the functionally defined region of interest method. This model contained metabolic activity as the response and diagnosis, fenfluramine plus norfenfluramine plasma levels (0 on day 1, and 3- and 4-hour postchallenge average on day 2), the interaction between diagnosis and fenfluramine plus norfenfluramine plasma levels, and subject as the random effect in the analysis.
Results
Plasma Levels
Prolactin levels at the time of injection of FDG (3- and 4-hour postchallenge average) were not different between patients and healthy volunteers (Table 2). However, fenfluramine plus norfenfluramine levels (3- and 4-hour postchallenge average) showed a group difference that approached significance, with mean plasma level 26% higher in healthy volunteers than in depressed subjects. This difference was not attributable to group mean body weight difference (Table 2). Therefore, a mixed-effects model was used to covary fenfluramine plus norfenfluramine levels and compare region of interest metabolism changes controlling for this plasma level difference.
MRI Anatomically Defined Region of Interest
Volumes and PET metabolic activity measures showed no group differences for the MRI-based subgenual cingulum regions. Volumes of the right subgenual cingulum region were not different between the patients (mean=445.5 mm3, SD=116.3) and the healthy volunteers (mean=509.8 mm3, SD=171.1) (t=1.20, df=27, p=0.24). Results were similar for the left subgenual cingulum region (patients: mean=518.9 mm3, SD=190.0; healthy volunteers: mean=535.0 mm3, SD=172.9) (t=0.22, df=27, p=0.83). Subgenual cingulum region of interest volumes were also not different after we controlled for total brain volume (right: F=1.47, df=1, 25, p=0.24; left: F=0.281, df=1, 25, p=0.60). Metabolic activity (PET signal intensity in the subgenual cingulum region of interest per unit volume) was not different between patients and healthy volunteers within the right subgenual cingulum region of interest on placebo day (patients: mean=48.2, SD=5.57; healthy volunteers: mean=47.8, SD=6.78) (t=–0.15, df=27, p=0.88) or on fenfluramine challenge day (patients: mean=49.0, SD=6.06; healthy volunteers: mean=47.7, SD=6.99) (t=–0.52, df=27, p=0.61). Results were similar for the left subgenual cingulum region of interest on placebo day (patients: mean=48.1, SD=5.03; healthy volunteers: mean=48.8, SD=4.61) (t=0.39, df=27, p=0.70) and on challenge day (patients: mean=48.2, SD=5.76; healthy volunteers: mean=49.6, SD=5.00) (t=0.67, df=27, p=0.51).
Statistical Parametric Mapping Voxel-Level Analyses
Figure 1 depicts placebo scan day comparisons by voxel and shows three brain regions with lower cerebral glucose metabolism in the patient group compared with the healthy volunteers. The z score threshold was chosen to be z0=2.88, corresponding to p<0.002 (one-tailed t test of patients lower than healthy volunteers) unprotected for multiple comparisons. These regions are seen to include a ventromedial prefrontal cortical region, slightly to the right of midline (z=3.52, p<0.0003) in Brodmann’s area 32 (with Talairach-Tournoux atlas [33] coordinates for the epicenter voxel of x=4, y=34, z=–12); a region in the left putamen (z=3.55, p<0.0002) with coordinates (x=–26, y=8, z=–4); and a right occipital cortical region (z=3.59, p<0.0002) with coordinates (x=26, y=–64, z=4) in Brodmann’s area 18. The unprotected one-tailed t test was highly significant (p<0.0003) for the a priori specified ventromedial prefrontal cortical region. The fenfluramine day group difference was also significant for this region at the z=3.06 (p<0.002) level. Figure 1 also shows the anatomical location of the regions where statistical parametric mapping identified lower cerebral glucose metabolism in patients relative to healthy comparison subjects under placebo conditions. The figure is restricted to a near-midsagittal slice overlaid on an MRI in Talairach space.
Functionally Defined Region of Interest
Repeated-measures ANOVA showed significantly lower cerebral glucose metabolism in the functionally defined region of interest in patients compared with healthy volunteers. Main effects of diagnosis and challenge condition were significant (Figure 2), confirming the statistical parametric mapping findings. Unipolar subjects did not differ from bipolar subjects in cerebral glucose metabolism at baseline or under fenfluramine challenge nor did comorbid axis I diagnoses affect metabolic response to fenfluramine. Patients receiving no benzodiazepine medication for at least 24 hours before scan time (N=12) showed no difference in their fenfluramine-induced change in metabolism from those receiving benzodiazepines at scan time (N=7) (t=0.63, df=17, p=0.54). No significant group-by-condition interaction was found, i.e., the patients and healthy volunteers suppressed metabolism similarly with fenfluramine challenge, even when fenfluramine levels were controlled for (mixed-effects model, z=–0.42, p=0.68).
Clinical Ratings
These analyses are presented for the patients only. Acute mood improvement assessed by the percent decrease in the POMS total score from placebo to fenfluramine challenge condition correlated with fenfluramine-induced percent decrease in functionally defined region of interest metabolism. This relation is shown in Figure 3 for the nine subjects with unipolar depression who were rated. Nonsignificant relations in the same hypothesized direction (mood improvement correlating with metabolic suppression) were found with the POMS total score when all patients rated (N=12) were analyzed (data not shown). There was no effect of concurrent (N=6) compared with more than 24-hour prior administration of benzodiazepine medication (N=6) on acute mood ratings (t=0.35, df=10, p=0.73).
Discussion
The functionally defined region of interest and the statistical parametric mapping methods both found a ventromedial prefrontal cortical metabolic deficit in the patient group. This deficient area suppressed further with serotonergic challenge. The results of our functional analyses are consistent with the finding of Drevets et al. (4) of a region of lower cerebral glucose metabolism in the subgenual cingulum in subjects with familial mood disorders at baseline. It is important to note that the brain region in which we found a functional difference was slightly anterior and inferior to the subgenual region identified by Drevets et al. (4), although these regions are seen to overlap when z thresholding is chosen to correspond to p=0.05 rather than p<0.0003 as displayed.
The failure of the MRI-based region of interest approach to detect a metabolic or volume deficit may be correct and differ from the functional data because of lack of exact anatomic correspondence between the definitions. The MRI-based region of interest was defined as inferior to the genu of the corpus callosum to retain comparability to published studies, whereas the region found by the functional methods was inferior but anterior to the genu. The regions partly overlapped in the second gyrus (granular cortex) inferior to the genu. Specification of reliable anatomic criteria for this more anterior functionally defined region might enable MRI-based methods to detect the metabolic difference reported here. Our MRI-defined gray matter volume for the subgenual cingulum in normal volunteers was 2.3-fold greater than that of Drevets et al. (4), who measured the subgenual agranular cortex only. When we measured a larger region of interest (the “subgenual prefrontal cortex”) within the ventromedial prefrontal cortex defined as the subgenual cingulum plus Brodmann’s area 25 (the subcallosal gyrus) and the gyrus rectus, the volume was even larger and about 4.5-fold greater than that of Drevets et al. (4). The volume also did not differ between the depressed subjects and the healthy volunteers. Thus, with neither of our two MRI region definitions could we confirm the volume reduction reported by Drevets et al. (4).
A limitation of our study may have been the pooling of bipolar and unipolar depressed subjects into one group, although this was justifiable based on the absence of patient subgroup differences. A larger subgroup of bipolar subjects will be needed for definitive study of this issue.
Our study found that a further decrease in metabolism in the fenfluramine condition appeared to correlate with acute mood improvement. The study of Mayberg et al. (5) assessed depressed subjects’ metabolism pre- and postrecovery from depression but not relative to comparison subjects. Our study design permitted the additional comparison with healthy subjects undergoing the same pharmacological challenge, revealing that relative regional metabolism was below normal at baseline in the functionally defined region of interest in patients with depression and was suppressed further with acute serotonergic challenge. Since fenfluramine-induced serotonin release is not activity-dependent and therefore not affected by autoreceptor activation, its acute effects may resemble late-phase treatment when autoreceptors are thought to become desensitized. If we therefore conjecture that acute metabolic response to fenfluramine is similar to metabolic change seen in recovery from depression, then our finding of a correlation with acute mood improvement suggests consistency between the prior results of Drevets et al. (4) and of Mayberg et al. (5). The ventromedial prefrontal cortex is hypoactive in depression and becomes more so with mood improvement. This apparently paradoxical suppression with recovery from depression has been found in both blood flow and metabolism in the frontal cortex in studies of antidepressant response to electroconvulsive therapy (9, 10). How this metabolic inhibition might contribute to mood improvement remains to be elucidated.
A remaining question is the mechanism of the baseline functionally defined region of interest metabolic deficit itself. An important conclusion from the fenfluramine data is that the deficit appears not to be related to serotonergic transmission. That is, the absence of a difference in the magnitude of the fenfluramine challenge response between depressed and healthy subjects suggests normal serotonin function in this region. Instead, deficient metabolism, both pre- and postchallenge, may involve a deficit of glial (34, 35) or pyramidal cells that might interfere with glutamate transmission, the major mechanism of neuronal energy consumption (36, 37).
Future work should aim to determine the predictive value of the magnitude of the functionally defined region of interest metabolic deficit and its responsivity to serotonergic challenge as a potential predictor of response to serotonergic antidepressant medication (compare with reference 38) or other treatment.
 |
 |
Presented in part at the 54th annual meeting of the Society of Biological Psychiatry, Washington, D.C., May 12–15, 1999, and the 46th annual meeting of the Society of Nuclear Medicine, Los Angeles, June 6–10, 1999. Received Oct. 4, 2001; revision received Aug. 9, 2002; accepted Aug. 15, 2002. From the Departments of Psychiatry and Radiology, Columbia University, New York; and the Department of Neuroscience, New York State Psychiatric Institute. Address reprint requests to Dr. Kegeles, New York State Psychiatric Institute, 1051 Riverside Dr., Box 31, New York, NY 10032; [email protected] (e-mail). Supported by NIH grants for the Study of Suicidal Behavior (MH-46745) and the Irving Center for Clinical Research (RR-00645) and NIMH grant MH-40695. The authors thank Victoria Arango, Ph.D., for discussions on neuroanatomy; Satish Anjilvel, Ph.D., for guidance in image analysis; and Joseph Grun, Melissa Nau, and Manuel De La Nuez for technical support.
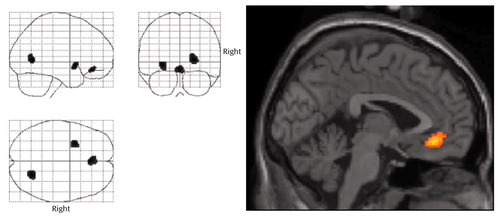
Figure 1. Regions of Significantly Lower Cerebral Glucose Metabolism Rates in 19 Depressed Subjects Relative to 10 Healthy Comparison Subjects Following Placebo Challengea
aThe statistical parametric mapping on the left depicts regions of greater metabolic activity in the healthy subjects than in the depressed subjects. The most anterior region, shown in the image on the right, was in Brodmann’s area 32, inferior and anterior to the genu of the corpus callosum (Talairach-Tournoux coordinates for the epicenter voxel: x=4, y=34, z=–12). The z height threshold displayed is z0=2.88, corresponding to p<0.002 (one-tailed t test). The highest z score for the anterior cingulum region was 3.52 (p<0.0003).
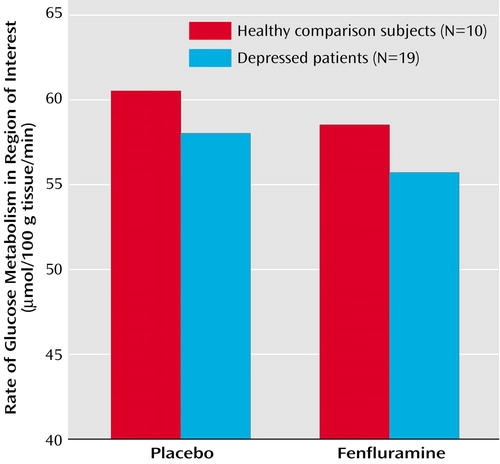
Figure 2. Metabolism in a Functionally Defined Region of the Ventromedial Prefrontal Cortex in Healthy Comparison Subjects and Depressed Subjects Following Placebo and Fenfluramine Challengea
aRepeated-measures ANOVA showed significant main effects of diagnosis (F=13.90, df=1, 27, p=0.001) and challenge condition (F=43.16, df=1, 27, p<0.0001).
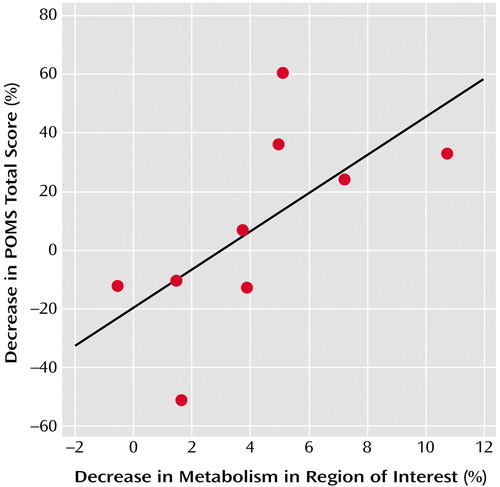
Figure 3. Relationship Between Acute Mood Improvement and Metabolic Suppression in a Functionally Defined Region of the Ventromedial Prefrontal Cortex in Subjects With Unipolar Depression Following Fenfluramine Challengea
ars=0.68, N=9, p=0.053.
1. Soares JC, Mann JJ: The functional neuroanatomy of mood disorders. J Psychiatr Res 1997; 31:393-432Crossref, Medline, Google Scholar
2. Soares JC, Mann JJ: The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86-106Crossref, Medline, Google Scholar
3. Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA: Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry 1996; 153:174-182; correction, 153:588Link, Google Scholar
4. Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824-827Crossref, Medline, Google Scholar
5. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675-682Abstract, Google Scholar
6. Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD: Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 2002; 51:342-344Crossref, Medline, Google Scholar
7. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS: Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 2002; 51:273-279Crossref, Medline, Google Scholar
8. Brambilla P, Nicoletti MA, Sassi RB, Frank E, Kupfer DJ, Keshavan MS, Soares JC: Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar disorder patients (abstract). Biol Psychiatry 2002; 51:138SGoogle Scholar
9. Nobler MS, Sackeim HA, Prohovnik I, Moeller JR, Mukherjee S, Schnur DB, Prudic J, Devanand DP: Regional cerebral blood flow in mood disorders, III: treatment and clinical response. Arch Gen Psychiatry 1994; 51:884-897Crossref, Medline, Google Scholar
10. Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell C, Sackeim HA, Mann JJ: Decreased regional brain metabolism after ECT. Am J Psychiatry 2001; 158:305-308Link, Google Scholar
11. George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341-351Link, Google Scholar
12. Gemar MC, Kapur S, Segal ZV, Brown GM, Houle S: Effects of self-generated sad mood on regional cerebral activity: a PET study in normal subjects. Depression 1996; 4:81-88Crossref, Medline, Google Scholar
13. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ: Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 1997; 154:926-933Link, Google Scholar
14. Bechara A, Tranel D, Damasio H, Damasio AR: Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 1996; 6:215-225Crossref, Medline, Google Scholar
15. Damasio AR, Tranel D, Damasio H: Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 1990; 41:81-94Crossref, Medline, Google Scholar
16. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
18. McBride PA, Tierney H, DeMeo M, Chen JS, Mann JJ: Effects of age and gender on CNS serotonergic responsivity in normal adults. Biol Psychiatry 1990; 27:1143-1155Crossref, Medline, Google Scholar
19. Mann JJ, Malone KM, Diehl DJ, Perel J, Nichols TE, Mintun MA: Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. J Cereb Blood Flow Metab 1996; 16:418-426Crossref, Medline, Google Scholar
20. Krebs HA, Cheng LK, Wright GJ: Determination of fenfluramine and norfenfluramine in plasma using a nitrogen-sensitive detector. J Chromatogr 1984; 310:412-417Crossref, Medline, Google Scholar
21. Myers JE, Mieczkowski T, Perel J, Abbondanza D, Cooper TB, Mann JJ: Abnormal behavioral responses to fenfluramine in patients with affective and personality disorders: correlation with increased serotonergic responsivity. Biol Psychiatry 1994; 35:112-120Crossref, Medline, Google Scholar
22. McNair DM, Lorr M, Droppleman LF: Manual for the Profile of Mood States. San Diego, Educational and Industrial Testing Service, 1981Google Scholar
23. Carmichael ST, Price JL: Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol 1994; 346:366-402Crossref, Medline, Google Scholar
24. Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA: Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res Neuroimaging 2000; 98:163-175Crossref, Medline, Google Scholar
25. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620-633Crossref, Medline, Google Scholar
26. Tyler JL, Strother SC, Zatorre RJ, Alivisatos B, Worsley KJ, Diksic M, Yamamoto YL: Stability of regional cerebral glucose metabolism in the normal brain measured by positron emission tomography. J Nucl Med 1988; 29:631-642Medline, Google Scholar
27. Friston KJ, Frith CD, Liddle PF, Frackowiak RS: Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991; 11:690-699Crossref, Medline, Google Scholar
28. Friston KJ: Testing for anatomically specified regional effects. Hum Brain Mapp 1997; 5:133-136Crossref, Medline, Google Scholar
29. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943-958Crossref, Medline, Google Scholar
30. Lahti AC, Holcomb HH, Medoff DR, Weiler MA, Tamminga CA, Carpenter WT Jr: Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry 2001; 158:1797-1808Link, Google Scholar
31. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC: Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 2002; 15:247-262Crossref, Medline, Google Scholar
32. Searle SR, Casella G, McCulloch CE: Variance Components. New York, John Wiley & Sons, 1992Google Scholar
33. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
34. Drevets WC, Ongur D, Price JL: Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry 1998; 3:220-226Crossref, Medline, Google Scholar
35. Ongur D, Drevets WC, Price JL: Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 1998; 95:13290-13295Crossref, Medline, Google Scholar
36. Magistretti PJ, Pellerin L, Rothman DL, Shulman RG: Energy on demand. Science 1999; 283:496-497Crossref, Medline, Google Scholar
37. Shulman RG: Functional imaging studies: linking mind and basic neuroscience. Am J Psychiatry 2001; 158:11-20Link, Google Scholar
38. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT: Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8:1057-1061Crossref, Medline, Google Scholar



