In Vivo Determination of Muscarinic Acetylcholine Receptor Availability in Schizophrenia
Abstract
OBJECTIVE: Postmortem studies have implicated the central muscarinic acetylcholine system in schizophrenia. However, central muscarinic receptor availability has not previously been studied in vivo. Using [I-123]iodoquinuclidinyl benzilate ([123I]IQNB) single photon emission computed tomography (SPECT), the authors sought to compare the muscarinic receptor availability in vivo in unmedicated patients with schizophrenia and normal subjects. METHOD: Twelve medication-free patients with schizophrenia underwent an [123I]IQNB SPECT scan during approximate-equilibrium conditions. A group of 10 age- and gender-matched normal comparison subjects were given the same kind of scan under similar conditions. Regions of interest were analyzed in the cortex, basal ganglia, thalamus, and pons. Binding data were analyzed as nCi/ml tissue per mCi injected dose. RESULTS: Muscarinic receptor availability was significantly less in patients with schizophrenia than in normal subjects in all regions of interest except the pons. Reductions ranged from –33% in the caudate to –20% in the occipital cortex. Positive symptoms of schizophrenia correlated negatively with muscarinic receptor availability in the striatum and the frontal cortex. CONCLUSIONS: These results indicate a reduction in muscarinic acetylcholine receptor availability in vivo in unmedicated patients with schizophrenia, confirming results from postmortem studies and adding further evidence that the muscarinic system is involved in the pathophysiology of schizophrenia.
Biological investigations of schizophrenia have tended to focus on the function of several neurotransmitter systems. Dopamine has been the most often studied and the most broadly implicated (for review see Carlsson [1]). However, because the dopamine hypothesis of schizophrenia fails to explain many aspects of schizophrenia, other neurotransmitters, including glutamate, serotonin, and acetylcholine, have increasingly been investigated as potentially contributing to the pathophysiology of the disorder.
Diverse pharmacological, endocrinological, and neuropathological observations suggest that the muscarinic system may be altered in schizophrenia. Treatment of schizophrenia with antimuscarinic drugs may result in a worsening of psychosis (2, 3). Sleep studies (4, 5) and endocrinological studies (6) suggest greater-than-normal cholinergic tone in schizophrenia. Studies in postmortem tissue have found relatively consistent alterations in the muscarinic system in schizophrenia. The first study of brain tissue of patients with schizophrenia found that patients had less muscarinic receptor binding in the frontal cortex than normal comparison subjects (7). More recent studies have revealed significant reductions of various subtypes of muscarinic receptors in the caudate-putamen as well as in the hippocampus of patients with schizophrenia (8–11). These postmortem studies, however, have involved patients who were relatively elderly and had been chronically ill and treated with antipsychotic medications for many years. In vivo studies of muscarinic cholinergic receptor availability have not been performed in unmedicated patients with schizophrenia.
Iodoquinuclidinyl benzilate (IQNB), the iodinated form of quinuclidinyl benzilate (QNB), binds specifically and with subnanomolar affinity to all five muscarinic receptor subtypes (12). IQNB can be used as a single photon emission computed tomography (SPECT) ligand to study muscarinic receptors in vivo (13–15). Using [I-123]IQNB ([123I]IQNB) SPECT, our group has previously demonstrated reduced in vivo muscarinic receptor availability in patients treated with the atypical antipsychotics olanzapine and clozapine (16; our work, submitted 2001). On the basis of the cited evidence suggesting an alteration of the muscarinic cholinergic system in schizophrenia, we performed a [123I]IQNB SPECT study to compare the in vivo muscarinic cholinergic receptor availability in unmedicated patients with schizophrenia and normal subjects.
Method
Subjects
Twelve patients with schizophrenia were recruited from the inpatient service at the National Institute of Mental Health (NIMH) Neuropsychiatric Research Hospital at St. Elizabeths in Washington, D.C. Eight of the patients were men, and four were women; their mean age was 34.6 years (SD=6.8, range=24–46), and their mean illness duration was 12.2 years (SD=8.0, range=2–25). Before the start of the medication-free period, each patient gave written informed consent to participate in a [123I]IQNB SPECT study according to a protocol approved by the Institutional Review Board of NIMH and the Radiation Safety Committee and the Radioactive Drug Research Committee of the NIMH Neuroscience Center at St. Elizabeths. Each patient also gave separate written informed consent to participate in medication-free studies according to a separate protocol approved by the Institutional Review Board of NIMH.
All inpatients received a thorough medical, neurological, and psychiatric evaluation before enrollment in this study. The clinical workup included structural brain magnetic resonance imaging (MRI) to rule out structural lesions as well as for coregistration with the SPECT scans. Patients were free of active medical problems or substance abuse during the 6 months preceding this study. All patients were chronically ill and were diagnosed with schizophrenia (N=11) or schizoaffective disorder (N=1) according to the Structured Clinical Interview for DSM-IV (SCID). Patients with other concomitant axis I or axis II diagnoses were excluded from volunteering for this study, as were patients with concomitant physical illnesses or medications that might interfere with the study.
Except for one patient who had not received treatment with antipsychotic medication for nearly half a year, all patients had received regular treatment with typical (N=7) or atypical (N=4) antipsychotics before this study. Five patients had also received adjunctive treatment with anticholinergic medication (benztropine). All antipsychotic and anticholinergic drugs as well as all other medications were stopped before the medication-free studies. The median medication-free period before the [123I]IQNB SPECT study was 17.5 days (range=7–180). Medication-free studies were done in a crossover design that used identical-appearing active and inactive preparations of antipsychotics and anticholinergics. Inpatients and nursing staff were not aware of whether patients were receiving active or inactive preparations. Demographic data as well as previous medications of the patients with schizophrenia are listed in Table 1.
A group of 12 unmedicated healthy volunteers without a history of medical or psychiatric symptoms were studied under identical conditions and served as a comparison group. Eight of the comparison subjects were men, and four were women; their mean age was 29.1 years (SD=9.9, range=23–55). Before inclusion in this study, comparison subjects were evaluated with a medical, neurological, and psychiatric examination. As in the case of the patients, all comparison subjects were administered a structural MRI scan to rule out structural abnormalities and for coregistration with their SPECT images. The structural MRI scans for each individual in both groups were also examined for structural differences that might confound analysis of the SPECT data, such as overall brain size, gyrification, sulcal widths, and cortical integrity. No significant structural differences were noted. Prospective comparison subjects were administered the nonpatient version of the SCID, and those with any axis I or axis II diagnosis were excluded from volunteering for this study.
The comparison group and the patient group did not differ significantly in mean age (t=1.53, df=20, p=0.14) or gender ratio (χ2=0.48, df=1, p=0.48). However, the two groups did differ significantly with respect to cigarette smoking: two of the 10 comparison subjects were smokers, compared with eight of the 12 patients (χ2=4.79, df=1, p=0.03). Therefore, we explored smoking as a possible confound among the patients; the within-group smoker-to-nonsmoker ratio (8:4) in the group of patients provides better statistics than that among the comparison subjects (2:8).
Clinical and Neurological Ratings
Clinical and neurological ratings were obtained for all patients with schizophrenia on the day of the [123I]IQNB SPECT scan. Clinical ratings included the Positive and Negative Syndrome Scale (17). Extrapyramidal signs were assessed with the modified Abnormal Involuntary Movement Scale (AIMS) (18).
[123I]IQNB SPECT Procedure
All subjects received five drops of a nonradioactive iodine solution (Lugol’s solution) on the evening before the injection of [123I]IQNB and for three evenings following the study to minimize thyroid uptake of radioactive iodine. Isomerically pure (R,S)-[123I]IQNB was prepared as previously described (19). Each subject received an intravenous injection of approximately 7 mCi of [123I]IQNB at approximately 1:00 p.m. on the day before SPECT imaging. The overall study group mean injection of [123I]IQNB was 7.0 mCi (SD=1.4); the patient group mean was 6.5 mCi (SD=1.5, range=4.0–10.0), and the comparison group mean was 7.3 mCi (SD=1.5, range=4.5–9.4). The injected dose of [123I]IQNB did not differ significantly between the two groups of subjects (t=1.18, df=20, p=0.25).
Subjects returned to the SPECT laboratory for a 60-minute SPECT scan 21 hours after the injection. We have previously shown that at this time nonspecific binding of [123I]IQNB is reduced to near background levels of radiation and the distribution of specific binding reflects the distribution of muscarinic receptors in the human brain (14, 15, 19). The injected dose of IQNB is a trace amount, generally much less than 10 mg. On the basis of the [123I]IQNB binding data and a typical specific activity of about 8000 Ci/mmol, peak [123I]IQNB concentrations in brain are estimated to be less than 100 pM, which is less than 0.1% of the expected total muscarinic receptor concentration in these tissues. Because of the trace amounts of [123I]IQNB used, pharmacological effects are very unlikely; no effects were observed.
During imaging, subjects reclined comfortably in the chair of the CERASPECT camera (Digital Scintigraphics, Waltham, Mass.). SPECT data were acquired with a high-resolution collimator (7.5 mm full width at half maximum) in 120-projection step-and-shoot mode. Windows below (127–143 keV) and above (175–191 keV) the photopeak window (143–175 keV) were acquired and subtracted from the photopeak window to correct for scatter and septal penetration. The data were reconstructed by filtered back-projection with a Butterworth filter (cutoff=1 cm, power factor=10) and stored as 64 slices, each 128×128 and composed of isotropic 1.67-mm voxels.
Image Analysis
Image analysis was performed exactly as previously described (16). In brief, all [123I]IQNB SPECT images were analyzed by a single investigator (R.A.U.), who was blind to the subjects’ clinical status. First, the SPECT image volumes were coregistered with a structural MRI volume for each individual. Alignment regions of interest circumscribing gross anatomical features in the SPECT data were drawn manually on three orthogonal planes through a central point in the cerebrum. These alignment regions of interest were then superimposed onto the MRI image volume and reoriented visually to best conform to corresponding features of the MRI. Finally, the reoriented regions of interest were superimposed back onto the SPECT image volume, and the SPECT image volume was reoriented to fit back into the region of interest shell originally created from it. If necessary, this procedure was repeated until optimal fit was obtained.
With the aid of standard anatomical atlases (20–22), measurement regions of interest were drawn on five consecutive transverse slices of the MRI to define a volume of interest for each of the following brain regions: caudate, putamen, thalamus, pons, medial frontal cortex, lateral frontal cortex, posterior temporal cortex, occipital cortex, and cerebellum. These measurement regions of interest were then transferred onto the corresponding slices of the coregistered SPECT scan for analysis of [123I]IQNB binding. Raw volume of interest data were expressed as counts per minute per milliliter of tissue (cpm/ml tissue) by using the volume of each individualized volume of interest to estimate the tissue volume. After cerebellar (background) activity was subtracted, the data were corrected for decay and converted to absolute units (nCi/ml tissue) on the basis of calibration data acquired with a uniform flood phantom on each day of SPECT imaging. Finally, the volume of interest data were normalized to the injected dose of [123I]IQNB to yield nCi/ml tissue per mCi injected dose as the outcome measure for subsequent statistical analysis. In the specific case of IQNB, the combination of a very low on-rate and a very long persistence at nearly constant levels of the free-parent compound in plasma causes this outcome measure to be simply proportional to the binding potential at extended times. We have previously shown that this measure is valid for analysis of [123I]IQNB SPECT images (14–16, 19).
Statistical Analyses
Statistical analyses of the data were performed with Statistica for Windows 5.1 (StatSoft, Inc., Tulsa, Okla.) and Microsoft Excel 97 (Microsoft Corp., Redmond, Wash.). Hotelling’s T2 and Student’s t test for independent samples were applied to compare data from unmedicated patients with schizophrenia and normal subjects. Correlations with [123I]IQNB binding data were assessed with Pearson’s product moment correlation coefficient (r) and Spearman’s rank order correlation coefficient (rs) with the requirement of significance on both tests to reduce the influence of outliers and spurious associations. Chi-square tests were used for analyses of two-by-two frequency tables.
Results
Comparisons of Muscarinic Receptor Availability
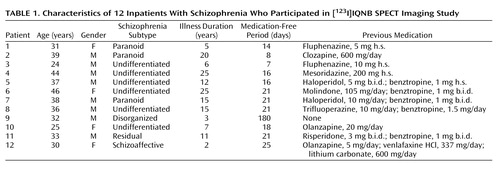
Figure 1 shows typical SPECT images of a normal comparison subject and a patient with schizophrenia depicted on a common global color scale. The images are typical in the sense that for each group the individual nearest the mean of their group was chosen for this figure. As expected, muscarinic receptor availability differed between the regions of interest analyzed. High muscarinic receptor availability was found in the cortical volumes of interest; the highest binding indexes were in the temporal and occipital cortex. The muscarinic receptor availability in the putamen was comparable to that in the cortex, and the binding indexes in the caudate were somewhat lower. Muscarinic receptor availability in the thalamus was about half of that found in the cortex and basal ganglia. Substantially lower muscarinic receptor availability was found in the pons. Virtually no binding was seen in the cerebellum.
This anatomical distribution of muscarinic receptor availability was seen in both normal comparison subjects and unmedicated patients with schizophrenia. It is consistent with earlier imaging reports (13, 14, 19) and data from postmortem tissue (for example, the comparison in Weinberger et al. [14]). lists the muscarinic receptor availability for the different regions of interest.
Overall, muscarinic receptor availability differed significantly between patients and comparison subjects. Post hoc comparisons showed that the values for the unmedicated patients were significantly lower in all regions of interest except the pons (Table 2).
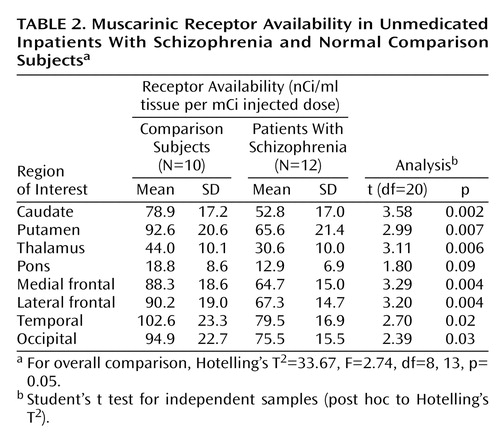
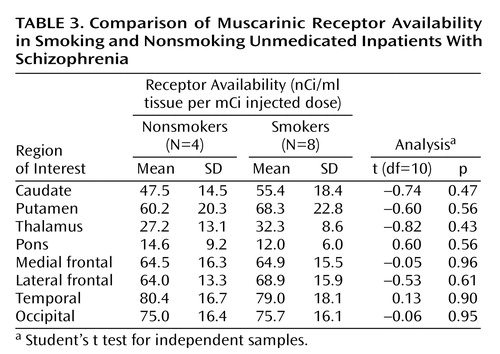
The global nature of the reduction in muscarinic receptor availability becomes more apparent when the data for patients with schizophrenia are expressed as percentages of the corresponding mean value of comparison subjects on a region-by-region basis. Figure 2 displays this comparison; it is apparent in this figure that the reductions for all regions fall into a relatively narrow range (20.5% to 33.2%; mean=27.4%). This is an indication of substantial correlations among the different anatomical regions for the percent of reduction relative to comparison subjects. Since the range of variation from region to region is considerably less than the standard deviations within regions, it seems reasonable to characterize the global reduction in muscarinic receptor availability by a single value: the mean value across regions, 27.4%. If one sets a retrospective “diagnostic” criterion at half this mean reduction value, i.e., midway between the mean of the comparison subjects and the mean of the schizophrenia subjects, then seven of 10 comparison subjects were correctly classified with three false positives and 10 of 12 schizophrenia subjects were classified correctly with two false negatives (Figure 3). Thus, in this small study group, the imaging data achieve a retrospective “diagnostic” sensitivity of 83% and a retrospective “diagnostic” specificity of 70% by using this single global reduction estimate.
Smoking and Previous Anticholinergic Medication as Possible Confounding Factors
The group of unmedicated patients with schizophrenia was further divided into smokers (N=8) and nonsmokers (N=4), and muscarinic receptor availability was examined for differences between these two groups. Using Student’s t test for independent samples, we found that the muscarinic receptor availability did not differ significantly in any of the regions of interest studied (all t<–0.82, p>0.43). In fact, muscarinic receptor availability was reduced in nonsmoking patients with schizophrenia in most regions of interest (Table 3). Because of the small number of smokers among the healthy comparison subjects, it was not possible to perform similar comparisons in that group.
The group of unmedicated schizophrenic subjects was also divided into those who had been given anticholinergic medication (benztropine) before the medication-free period (N=5) and those who had not (N=7). Again using Student’s t test for independent samples, we found that muscarinic receptor availability did not differ significantly in any of the regions of interest (all t<–0.50, p>0.63) (Table 4).
Correlations Between Muscarinic Receptor Availability and Clinical Measures
In the patient group, [123I]IQNB binding indexes did not correlate significantly with age (–0.27<r<0.31, p>0.32), with medication-free interval (–0.28<r<0.12, p>0.37), or with injected dose of radioligand (0.04<r<0.39, p>0.21) in any region of interest. The muscarinic receptor availability in the pons had a nonsignificant correlation with illness duration of r=–0.54 (p=0.07), but for all other regions the correlations with illness duration were far from significant (–0.26<r<0.26, p>0.42). Additionally, in the group of healthy comparison subjects, there were no significant correlations between [123I]IQNB binding indexes and age in any region of interest (–0.19<r<0.06, p>0.60).
We found no significant correlations between muscarinic receptor availability and extrapyramidal signs in the medication-free patients with schizophrenia. The pons had a nonsignificant correlation of r=–0.51 (p=0.09) with the parkinsonism scale of the AIMS; correlations with extrapyramidal signs for all other regions were farther from significance (–0.10<r<0.41, p>0.18).
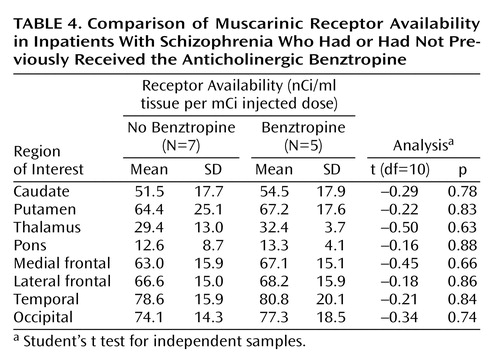
As an exploratory pilot analysis, we assessed whether ratings of schizophrenia symptoms were associated with reduction in muscarinic receptor availability on a region-by-region basis. Our post hoc hypothesis for these correlation analyses was that regions of the brain generally implicated in the pathophysiology of schizophrenia might have reductions that are associated with symptom severity. Using our criterion of significance on both Pearson’s r and Spearman’s r, we found significant correlations between muscarinic receptor availability and Positive and Negative Syndrome Scale symptom ratings only for the frontal cortex (r=–0.64, p=0.03; rs=–0.67, p=0.02) and the striatum (r=–0.63, p=0.03; rs=–0.68, p=0.02) (Figure 4). Furthermore, these significant correlations were restricted to the positive symptom score; we found no significant correlations with the negative symptom score. Individual regions exhibiting significant correlations with the positive symptom score of the Positive and Negative Syndrome Scale were the lateral frontal cortex (r=–0.63, p=0.03; rs=–0.66, p=0.02), medial frontal cortex (r=–0.63, p=0.03; rs=–0.71, p=0.009), caudate (r=–0.63, p=0.03; rs=–0.69, p=0.01), and putamen (r=–0.58, p=0.05; rs=–0.66, p=0.02).
Discussion
To our knowledge, this is the first study to compare in vivo muscarinic receptor availability between unmedicated patients with schizophrenia and age- and gender-matched normal subjects. We found that the muscarinic receptor availability in patients was significantly less (20%–33% less) than that of normal subjects in the cortex, basal ganglia, and thalamus.
The Muscarinic Cholinergic System in Schizophrenia
Our data are consistent with diverse evidence from earlier pharmacological, clinical, and endocrinological observations that implicated the muscarinic cholinergic system in schizophrenia. Several clinical studies have looked at the treatment with anticholinergic agents of patients with schizophrenia. In unmedicated patients with schizophrenia, biperiden, a commonly used anticholinergic agent, led to significant decreases in negative symptoms as well as significant increases in positive symptoms (2, 3). These findings were interpreted as reflecting greater cholinergic activity in schizophrenia. Similar findings of a worsening effect of anticholinergics on positive symptoms were also reported in patients with schizophrenia who were medicated with antipsychotics (23). These earlier findings are compatible with our observation of a negative correlation between muscarinic receptor availability and positive symptoms because treatment with anticholinergics should further reduce muscarinic receptor availability.
In addition to these behavioral observations, sleep studies and endocrinological challenge studies suggest an alteration of the muscarinic system in schizophrenia. In sleep studies, patients with schizophrenia show reduced REM latency, which has been linked to “muscarinic supersensitivity” (4, 5). Endocrinological challenge studies in patients with schizophrenia have reported a greater growth hormone response to pyridostigmine, a cholinesterase inhibitor, suggestive of greater cholinergic tone (6). Anticholinergic treatment may even have an effect on mortality: the lack of treatment with anticholinergics has been associated with reduced survival in elderly patients with schizophrenia (24).
Our finding of reduced muscarinic receptor availability is compatible with the results of neuropathological studies that have examined the muscarinic system in schizophrenia. Analyzing the receptor availability for different neurotransmitters in the frontal cortex in two samples of patients with schizophrenia and normal comparison subjects, Bennett et al. (7) found significantly lower 3H-QNB binding in patients with schizophrenia. Dean et al. (8) reported a significant reduction of M1 receptor density in the caudate-putamen of patients with schizophrenia compared with normal subjects. Although patients with schizophrenia previously treated with anticholinergics had even lower binding, the M1 receptor density was also significantly reduced in subjects who had not received anticholinergics. A similar reduction was also reported for the M2 and M4 subtypes of the muscarinic receptors in the caudate and putamen (9). In other studies from the same group, M1 and M4 receptor density was reduced in patients with schizophrenia in regions of the hippocampal formation (10) and in the prefrontal cortex (11). The results of these studies contrast with two other studies that found greater muscarinic receptor density in patients with schizophrenia in the orbitofrontal and medial frontal cortex (25) as well as the putamen (26). Another study counted cholinergic neurons in the pedunculopontine nucleus and reported significantly more in patients with schizophrenia than in normal comparison subjects but no difference between groups in noradrenergic neurons in the locus ceruleus (27).
Several enzymes involved in the regulation of acetylcholine metabolism also have been investigated. Concentrations of choline acetyltransferase, the enzyme-synthesizing acetylcholine, were significantly smaller in the pons of patients with schizophrenia than normal comparison subjects, but there were no differences between these groups in other brain regions (28). In contrast to this finding, greater levels of choline acetyltransferase in the hippocampus, caudate, putamen, and nucleus accumbens in patients with schizophrenia have also been described (29). In another study (30), cholinergic markers (choline acetyltransferase and acetylcholinesterase) were diminished in the brains of subjects with Alzheimer’s disease but not different in the brains of patients with schizophrenia compared with normal subjects. Although the reasons for these inconsistencies in the postmortem literature are unclear, the possibilities of uncontrolled effects of chronic illness and treatment are difficult to address in such studies.
It is of interest to speculate on the relationship of the cholinergic system to other neurotransmitter systems implicated in schizophrenia. The dopaminergic and cholinergic systems in the brain interact directly and indirectly in the striatum and in the cortex. Functional muscarinic receptors have been shown to exist on dopaminergic neurons in single unit recordings (31) as well as in microdialysis experiments (32). In the substantia nigra, cholinergic fibers have synaptic contact with dopaminergic neurons (33). Similarly, it has been suggested that ventral tegmental area dopamine cells have functional muscarinic receptors and that the activation of these receptors stimulates the release of dopamine (34). In synaptosomes, acetylcholine potentiates the release of dopamine. This effect can be counteracted by atropine (35). In a positron emission tomography (PET) study of human volunteers, the application of muscarinic cholinergic antagonists resulted in increased striatal dopamine release (36). The activation of dopaminergic cells in the midbrain by muscarinic agonists involves M1-like receptors (37). The effects of the application of muscarinic agonists on dopaminergic neurons depends on the pattern of activation and ranges from hyperpolarization after brief activation of muscarinic receptors to desensitization after prolonged activation (38). Acetylcholine itself exerts very little effect on dopaminergic neurons, but muscarinic and nicotinic agonists increased their firing rate (31). On the basis of these observations, Yeomans (39) has speculated that schizophrenia may be caused by an overactivation of cholinergic neurons in the pedunculopontine and the laterodorsal tegmental nucleus, resulting in activation of dopaminergic neurons. Such speculation would suggest that our findings represent a secondary down-regulation of postsynaptic cholinergic receptors. However, it is difficult to arrive at a simple scheme to explain the basic pharmacological data implicating an overactive cholinergic system, evidence that anticholinergics increase positive symptoms in patients, and the results in schizophrenic brain tissue (both the postmortem and our in vivo data).
Other neurotransmitter systems have been studied in vivo in schizophrenia. Most of these studies failed to find significant differences in baseline receptor availability between unmedicated patients with schizophrenia and normal comparison subjects. Although one study found an increase in dopamine D2 receptor availability in medication-free patients with schizophrenia (40), subsequent studies failed to replicate significant differences in dopamine D2 receptor availability (41–43). Similarly, no significant changes have been found for the in vivo availability of the dopamine transporter (44, 45). However, unmedicated patients with schizophrenia showed significantly higher dopamine release after an amphetamine challenge than normal comparison subjects, suggesting that the dopaminergic neurons may be more responsive in schizophrenia (46–48).
Methodological Considerations
In the interpretation of our data, the potential role of previous medication treatment needs to be taken into consideration. Although all subjects had been medication free for at least 7 days before the SPECT scan (and most subjects considerably longer), they had taken antipsychotics before this period. Some subjects had also taken anticholinergics or antipsychotics with known anticholinergic properties. Although little is known about the effects of antipsychotic and antimuscarinic treatment on muscarinic cholinergic receptors in humans, this issue has been studied in animals (49–58). The results have been inconsistent, ranging from a decrease in muscarinic receptor density to no change to an increase. However, most of the data suggest an up-regulation of muscarinic receptors after pharmacological treatment with antagonists, in contrast to our results. Carryover effects from previous pharmacological treatments seem unlikely because muscarinic receptor availability did not correlate with the duration of the medication-free period. Reduced muscarinic receptor availability was found in all subjects regardless of their previous pharmacological treatment (typical versus atypical antipsychotics, use of anticholinergics versus no anticholinergics, and antipsychotics with anticholinergic properties versus no anticholinergic properties).
In clinical practice it is widely recognized that the prevalence of smoking among patients with schizophrenia far exceeds the prevalence of smoking in the general population. Consistent with this observation, smokers are overrepresented among our small group of patients with schizophrenia. However, when comparing muscarinic receptor availability among smokers and nonsmokers in the patient sample, we found even lower muscarinic receptor availability among the nonsmoking schizophrenic subjects. The small number of subjects does not allow firm conclusions, but these results suggest that smoking per se is not responsible for the low muscarinic receptor availabilities found in our patients.
Similarly, it seems unlikely that age-related effects could account for our observations. Our group of comparison subjects did not differ significantly in mean age from the group of patients, and muscarinic receptor availability did not significantly correlate with age in either group. This observation of no significant decline with age in muscarinic receptor availability is consistent with our findings of no significant decline with age in a larger group of 20 healthy comparison subjects ranging in age from 23 to 75 years (59).
Other potential confounds that might lead to erroneously low SPECT region of interest measurements are structural differences in the brain tissue between groups. However, our a priori examination of the MRI scan for each individual in both groups revealed no gross structural differences that might account for the reductions we have observed. Although subtle differences in brain structure or tissue composition might account partially for our findings, it should be noted that the reductions in muscarinic receptor availability that we have determined in vivo are quantitatively in good agreement with corresponding in vitro studies of postmortem tissue (7–11). Thus, we feel it is unlikely that structural differences had a significant effect on our measurements.
SPECT and PET imaging of neurotransmitter receptors uses specific tracers labeled with radioactivity. In our previous [123I]IQNB SPECT studies in patients with schizophrenia treated with olanzapine or clozapine (16), we were able to show that [123I]IQNB SPECT is sensitive to receptor occupancy by medication. Reduced receptor availability in unmedicated subjects can be attributable to either reduced numbers of receptors or increased occupancy by endogenous neurotransmitter, in this case acetylcholine. Although a hypercholinergic state cannot be excluded on the basis of our SPECT study, the neuropathological reports of reduced muscarinic receptor density suggest that our findings reflect a reduction in receptor density. The two effects, however, are not mutually exclusive, and a hypercholinergic state would be expected to be associated with a corresponding down-regulation of muscarinic receptors (see discussion above).
Muscarinic Receptor Selectivity and Treatment Implications
[123I]IQNB binds very selectively and with high affinity to muscarinic receptors (12). [123I]IQNB does not allow discrimination between the different subtypes of the muscarinic receptors. So far, five genetically distinct subtypes of the muscarinic receptor (M1, M2, M3, M4, and M5) are known to be expressed in the brain with different anatomical distributions. One of the unexpected findings in our study is the relatively widespread and regionally nonspecific pattern of reduction in muscarinic binding. This observation suggests that the availability of multiple muscarinic receptor subtypes is reduced in schizophrenia. This is also compatible with the results of neuropathological studies, which have shown decreases in schizophrenia in the different muscarinic receptor subtypes (e.g., M1 and M2/M4). The genes for the different subtypes of the muscarinic receptor subtypes are located on different chromosomes. Although a common transcription factor for all muscarinic receptor genes might explain the reduction in the density of different muscarinic receptor subtypes, little is known about the regulation of the expression of the muscarinic receptor genes, and there is no evidence that they share a common transcription factor (60, 61).
The role of the muscarinic system in schizophrenia recently has been evaluated as a potential novel pharmacological approach for the treatment of psychosis. (5R,6R)6-(3-Propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane (PTAC) is a muscarinic receptor ligand with partial agonist effects at muscarinic M2 and M4 receptors and antagonist effects at M1, M3, and M5 receptors. PTAC selectively inhibits dopamine cell firing as well as the number of spontaneously active dopamine cells. Since this substance proved to have functional dopamine receptor antagonistic properties in animals, it may be further investigated as a novel approach for the treatment of schizophrenia (62). Treatment with other muscarinic agonists, including xanomeline, resulted in behavioral responses similar to treatment with traditional antipsychotics in animal models that may be applicable to humans (63).
In conclusion, there is mounting evidence that the muscarinic cholinergic system is altered in schizophrenia. Our study shows a significant reduction of muscarinic receptor availability in unmedicated schizophrenic subjects. We cannot address whether this finding represents a primary pathophysiological phenomenon or a secondary effect of other factors. These results need to be replicated in additional groups of patients, especially those without previous medication exposure and without chronic illness. The availability of a noninvasive neuroimaging method for measurement of muscarinic binding in vivo makes this possible. These latter replication studies might apply the retrospective “diagnostic” criteria that we found useful in this study to test their utility as a diagnostic aid in a prospective manner.
 |
 |
 |
 |
Received Dec. 21, 2001; revision received Sept. 18, 2002; accepted Sept. 19, 2002. From the Clinical Brain Disorders Branch, Division of Intramural Research Programs, NIMH; the Department of Psychiatry, University of Hamburg, Hamburg, Germany; and the Stanley Research Foundation, Bethesda, Md. Address reprint requests to Dr. Weinberger, Clinical Brain Disorders Branch, NIMH, National Institutes of Health, 10 Center Dr., 4S-235, MSC 1379, Bethesda, MD 20892-1379; [email protected] (e-mail).
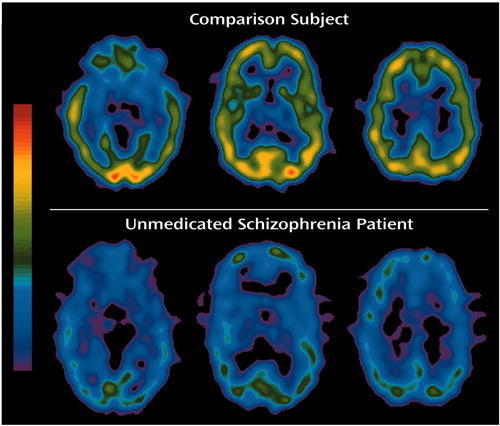
Figure 1. Typical [123I]IQNB SPECT Images of a Normal Comparison Subject and an Unmedicated Inpatient With Schizophreniaa
aThe data are depicted on a single common color scale. The images are typical in the sense that the comparison subject chosen for this figure was the individual with values nearest to the mean values of the comparison group and the schizophrenia patient was the one with values nearest to the mean values of that group.
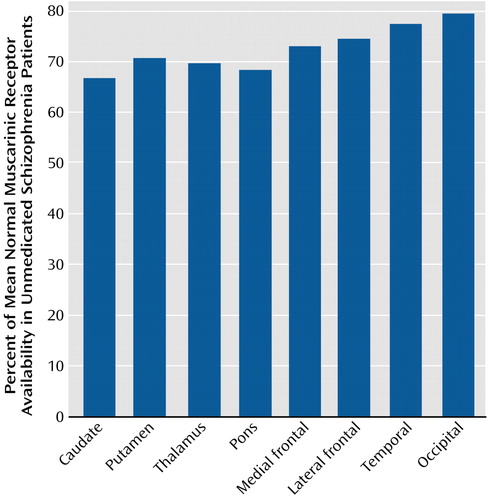
Figure 2. Muscarinic Receptor Availability in Inpatients With Schizophrenia for Individual Volumes of Interest Normalized Volume-by-Volume to Mean Data From Comparison Subjects
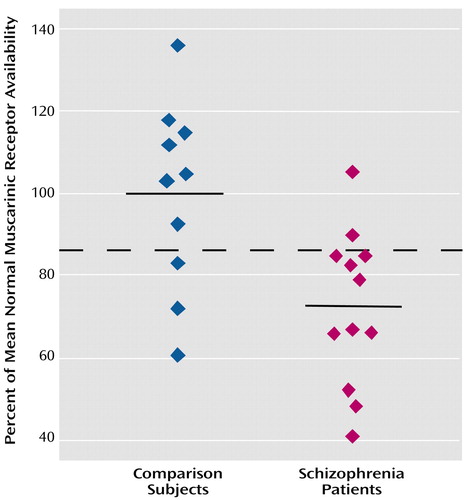
Figure 3. Normalized Muscarinic Receptor Availability Averaged Across Anatomical Regions for Individual Inpatients With Schizophrenia and Normal Comparison Subjectsa
aThe dashed line is positioned midway between the group mean values as an example of a simple “diagnostic” criterion, in this case retrospective, with 83% sensitivity and 70% specificity.
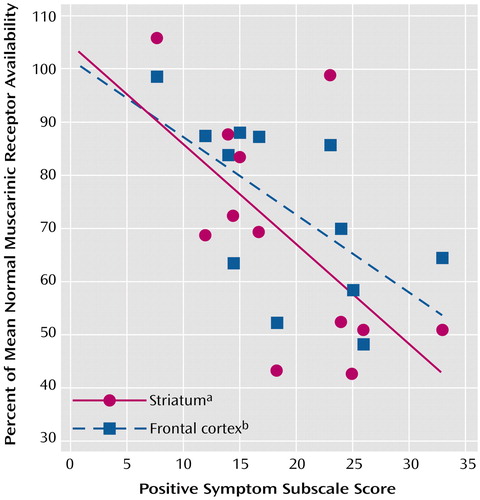
Figure 4. Correlations Between Positive Symptom Subscale Score on the Positive and Negative Syndrome Scale and Muscarinic Receptor Availability in the Striatum and Frontal Cortex of 12 Inpatients With Schizophrenia
aAverage of caudate and putamen volumes (r2=0.39, p<0.03).
bAverage of medial frontal cortex and lateral frontal cortex volumes (r2=0.40, p<0.03).
1. Carlsson A: The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988; 1:179-186Crossref, Medline, Google Scholar
2. Tandon R, Shipley JE, Greden JF, Mann NA, Eisner WH, Goodson JA: Muscarinic cholinergic hyperactivity in schizophrenia: relationship to positive and negative symptoms. Schizophr Res 1991; 4:23-30Crossref, Medline, Google Scholar
3. Tandon R, DeQuardo JR, Goodson J, Mann NA, Greden JF: Effect of anticholinergics on positive and negative symptoms in schizophrenia. Psychopharmacol Bull 1992; 28:297-302Medline, Google Scholar
4. Riemann D, Hohagen F, Krieger S, Gann H, Muller WE, Olbrich R, Wark HJ, Bohus M, Low H, Berger M: Cholinergic REM induction test: muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res 1994; 28:195-210Crossref, Medline, Google Scholar
5. Maixner S, Tandon R, Eiser A, Taylor S, DeQuardo JR, Shipley J: Effects of antipsychotic treatment on polysomnographic measures in schizophrenia: a replication and extension. Am J Psychiatry 1998; 155:1600-1602Link, Google Scholar
6. O’Keane V, Abel K, Murray RM: Growth hormone responses to pyridostigmine in schizophrenia: evidence for cholinergic dysfunction. Biol Psychiatry 1994; 36:582-588Crossref, Medline, Google Scholar
7. Bennett JP Jr, Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH: Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 1979; 36:927-934Crossref, Medline, Google Scholar
8. Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL: The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry 1996; 1:54-58Medline, Google Scholar
9. Crook JM, Dean B, Pavey G, Copolov D: The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci 1999; 64:1761-1771Crossref, Medline, Google Scholar
10. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B: Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000; 48:381-388Crossref, Medline, Google Scholar
11. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B: Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry 2001; 158:918-925Link, Google Scholar
12. Bolden C, Cusack B, Richelson E: Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther 1992; 260:576-580Medline, Google Scholar
13. Eckelman WC, Reba RC, Rzeszotarski WJ, Gibson RE, Hill T, Holman BL, Budinger T, Conklin JJ, Eng R, Grissom MP: External imaging of cerebral muscarinic acetylcholine receptors. Science 1984; 223:291-293Crossref, Medline, Google Scholar
14. Weinberger DR, Gibson R, Coppola R, Jones DW, Molchan S, Sunderland T, Berman KF, Reba RC: The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia: a controlled study with 123IQNB and single photon emission computed tomography. Arch Neurol 1991; 48:169-176Crossref, Medline, Google Scholar
15. Sunderland T, Esposito G, Molchan SE, Coppola R, Jones DW, Gorey J, Little JT, Bahro M, Weinberger DR: Differential cholinergic regulation in Alzheimer’s patients compared to controls following chronic blockade with scopolamine: a SPECT study. Psychopharmacology (Berl) 1995; 121:231-241Crossref, Medline, Google Scholar
16. Raedler TJ, Knable MB, Jones DW, Lafargue T, Urbina RA, Egan MF, Pickar D, Weinberger DR: In vivo olanzapine occupancy of muscarinic acetylcholine receptors in patients with schizophrenia. Neuropsychopharmacology 2000; 23:56-68Crossref, Medline, Google Scholar
17. Kay SR, Opler LA, Fiszbein A: Positive and Negative Syndrome Scale (PANSS). North Tonawanda, NY, Multi-Health Systems, 1986Google Scholar
18. Wyatt RJ: Practical Psychiatric Practice: Forms and Protocols for Clinical Use. Washington, DC, American Psychiatric Press, 1993Google Scholar
19. Lee KS, He XS, Jones DW, Coppola R, Gorey JG, Knable MB, deCosta BR, Rice KC, Weinberger DR: An improved method for rapid and efficient radioiodination of iodine-123-IQNB. J Nucl Med 1996; 37:2021-2024Medline, Google Scholar
20. Aquilonius S-M, Eckernas S-A: A Colour Atlas of the Human Brain. New York, Raven Press, 1980Google Scholar
21. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
22. Duvernoy HM: The Human Brain: Three-Dimensional Sectional Anatomy and MRI. Vienna, Springer-Verlag, 1991Google Scholar
23. Johnstone EC, Crow TJ, Ferrier IN, Frith CD, Owens DG, Bourne RC, Gamble SJ: Adverse effects of anticholinergic medication on positive schizophrenic symptoms. Psychol Med 1983; 13:513-527Crossref, Medline, Google Scholar
24. Waddington JL, Youssef HA, Kinsella A: Mortality in schizophrenia: antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry 1998; 173:325-329Crossref, Medline, Google Scholar
25. Watanabe S, Nishikawa T, Takashima M, Toru M: Increased muscarinic cholinergic receptors in prefrontal cortices of medicated schizophrenics. Life Sci 1983; 33:2187-2196Crossref, Medline, Google Scholar
26. Owen F, Cross AJ, Crow TJ, Lofthouse R, Poulter M: Neurotransmitter receptors in brain in schizophrenia. Acta Psychiatr Scand Suppl 1981; 291:20-28Crossref, Medline, Google Scholar
27. Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, Karson CN: Mesopontine neurons in schizophrenia. Neuroscience 1995; 66:321-335Crossref, Medline, Google Scholar
28. Karson CN, Casanova MF, Kleinman JE, Griffin WS: Choline acetyltransferase in schizophrenia. Am J Psychiatry 1993; 150:454-459Link, Google Scholar
29. McGeer PL, McGeer EG: Possible changes in striatal and limbic cholinergic systems in schizophrenia. Arch Gen Psychiatry 1977; 34:1319-1323Crossref, Medline, Google Scholar
30. Haroutunian V, Davidson M, Kanof PD, Perl DP, Powchik P, Losonczy M, McCrystal J, Purohit DP, Bierer LM, Davis KL: Cortical cholinergic markers in schizophrenia. Schizophr Res 1994; 12:137-144Crossref, Medline, Google Scholar
31. Gronier B, Rasmussen K: Activation of midbrain presumed dopaminergic neurones by muscarinic cholinergic receptors: an in vivo electrophysiological study in the rat. Br J Pharmacol 1998; 124:455-464Crossref, Medline, Google Scholar
32. De Klippel N, Sarre S, Ebinger G, Michotte Y: Effect of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism: an in vivo microdialysis study comparing normal and 6-hydroxydopamine-lesioned rats. Brain Res 1993; 630:57-64Crossref, Medline, Google Scholar
33. Bolam JP, Francis CM, Henderson Z: Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience 1991; 41:483-494Crossref, Medline, Google Scholar
34. Gronier B, Perry KW, Rasmussen K: Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000; 147:347-355Crossref, Medline, Google Scholar
35. Marchi M, Raiteri M: On the presence in the cerebral cortex of muscarinic receptor subtypes which differ in neuronal localization, function and pharmacological properties. J Pharmacol Exp Ther 1985; 235:230-233Medline, Google Scholar
36. Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MacGregor RR, Fowler JS, Volkow ND, Wolf AP: Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci USA 1993; 90:11816-11820Crossref, Medline, Google Scholar
37. Gronier B, Rasmussen K: Pertussis toxin treatment differentially affects cholinergic and dopaminergic receptor stimulation of midbrain dopaminergic neurons. Neuropharmacology 1999; 38:1903-1912Crossref, Medline, Google Scholar
38. Fiorillo CD, Williams JT: Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci 2000; 20:7855-7860Crossref, Medline, Google Scholar
39. Yeomans JS: Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology 1995; 12:3-16Crossref, Medline, Google Scholar
40. Wong DF, Wagner HN Jr, Tune LE, Dannals RF, Pearlson GD, Links JM, Tamminga CA, Broussolle EP, Ravert HT, Wilson AA, Toung JKT, Malat J, Williams JA, O’Tuama LA, Snyder SH, Kuhar MJ, Gjedde A: Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science 1986; 234:1558-1563Crossref, Medline, Google Scholar
41. Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordstrom AL, Hall H, Sedvall G: D2 dopamine receptors in neuroleptic-naive schizophrenic patients: a positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry 1990; 47:213-219Crossref, Medline, Google Scholar
42. Pilowsky LS, Costa DC, Ell PJ, Verhoeff NP, Murray RM, Kerwin RW: D2 dopamine receptor binding in the basal ganglia of antipsychotic-free schizophrenic patients: an 123I-IBZM single photon emission computerised tomography study. Br J Psychiatry 1994; 164:16-26Crossref, Medline, Google Scholar
43. Knable MB, Egan MF, Heinz A, Gorey J, Lee KS, Coppola R, Weinberger DR: Altered dopaminergic function and negative symptoms in drug-free patients with schizophrenia: [123I]-iodobenzamide SPECT study. Br J Psychiatry 1997; 171:574-577Crossref, Medline, Google Scholar
44. Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, Seibyl J, Baldwin R, Innis R: Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [123I]beta-CIT. Biol Psychiatry 2000; 47:371-379Crossref, Medline, Google Scholar
45. Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J: Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry 2000; 157:269-271Link, Google Scholar
46. Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB: Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93:9235-9240Crossref, Medline, Google Scholar
47. Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, deBartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D: Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94:2569-2574Crossref, Medline, Google Scholar
48. Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M: Increased baseline occupancy of D-2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000; 97:8104-8109Crossref, Medline, Google Scholar
49. Muller P, Seeman P: Brain neurotransmitter receptors after long-term haloperidol: dopamine, acetylcholine, serotonin, alpha-noradrenergic and naloxone receptors. Life Sci 1977; 21:1751-1758Crossref, Medline, Google Scholar
50. Ben-Barak J, Gazit H, Silman I, Dudai Y: In vivo modulation of the number of muscarinic receptors in rat brain by cholinergic ligands. Eur J Pharmacol 1981; 74:73-81Crossref, Medline, Google Scholar
51. Misra CH, Shelat H, Smith RC: Influence of age on the effects of chronic fluphenazine on receptor binding in rat brain. Eur J Pharmacol 1981; 76:317-324Crossref, Medline, Google Scholar
52. Theodorou A, Gommeren W, Clow A, Leysen J, Jenner P, Marsden CD: Chronic neuroleptic treatment specifically alters the number of dopamine receptors in rat brain. Life Sci 1981; 28:1621-1627Crossref, Medline, Google Scholar
53. Friedman E, Gianutsos G, Kuster J: Chronic fluphenazine and clozapine elicit opposite changes in brain muscarinic receptor binding: implications for understanding tardive dyskinesia. J Pharmacol Exp Ther 1983; 226:7-12Medline, Google Scholar
54. Akiyama K, Sato M, Yamada N, Otsuki S: Effect of chronic administration of haloperidol (intermittently) and haloperidol-decanoate (continuously) on D2 dopamine and muscarinic cholinergic receptors and on carbachol-stimulated phosphoinositide hydrolysis in the rat striatum. Jpn J Psychiatry Neurol 1987; 41:311-320Medline, Google Scholar
55. Boyson SJ, McGonigle P, Luthin GR, Wolfe BB, Molinoff PB: Effects of chronic administration of neuroleptic and anticholinergic agents on densities of D2 dopamine and muscarinic cholinergic receptors in rat striatum. J Pharmacol Exp Ther 1988; 244:987-993Medline, Google Scholar
56. Hietala J, Syvalahti E, Roytta M: Dopamine D2 and muscarinic receptor binding characteristics in rat brain after withdrawal of subchronic fluphenazine and sulpiride treatment. Prog Neuropsychopharmacol Biol Psychiatry 1989; 13:247-258Crossref, Medline, Google Scholar
57. See RE, Toga AW, Ellison G: Autoradiographic analysis of regional alterations in brain receptors following chronic administration and withdrawal of typical and atypical neuroleptics in rats. J Neural Transm Gen Sect 1990; 82:93-109Crossref, Medline, Google Scholar
58. Cawley TA Jr, Shickley TJ, Ruggieri MR, Luthin GR: Effect of chronic neuroleptic treatment on central and peripheral muscarinic receptors. J Pharmacol Exp Ther 1993; 267:134-139Medline, Google Scholar
59. Raedler TJ, Knable MB, Jones DW, Gorey JG, Lee KS, Sunderland T, Weinberger DR: Age-related changes in central muscarinic receptor availability in vivo (abstract). J Nucl Med 2000; 41(suppl):92Google Scholar
60. Saffen D, Mieda M, Okamura M, Haga T: Control elements of muscarinic receptor gene expression. Life Sci 1999; 64:479-486Crossref, Medline, Google Scholar
61. Buckley NJ, Bachfischer U, Canut M, Mistry M, Pepitoni S, Roopra A, Sharling L, Wood IC: Repression and activation of muscarinic receptor genes. Life Sci 1999; 64:495-499Crossref, Medline, Google Scholar
62. Bymaster FP, Shannon HE, Rasmussen K, Delapp NW, Mitch CH, Ward JS, Calligaro DO, Ludvigsen TS, Sheardown MJ, Olesen PH, Swedberg MD, Sauerberg P, Fink-Jensen A: Unexpected antipsychotic-like activity with the muscarinic receptor ligand (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane. Eur J Pharmacol 1998; 356:109-119Crossref, Medline, Google Scholar
63. Shannon HE, Hart JC, Bymaster FP, Calligaro DO, DeLapp NW, Mitch CH, Ward JS, Fink-Jensen A, Sauerberg P, Jeppesen L, Sheardown MJ, Swedberg MD: Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats. J Pharmacol Exp Ther 1999; 290:901-907Medline, Google Scholar



