Does the Definition of Borders of the Planum Temporale Influence the Results in Schizophrenia?
Abstract
OBJECTIVE: The planum temporale, a highly asymmetric neocortical area of the temporal lobe, has a possible role in schizophrenia. The authors used three different anatomical definitions of the planum temporale to examine the anterior, posterior, and total planum temporale gray matter volumes simultaneously. METHOD: Magnetic resonance imaging was used to examine 30 male schizophrenic patients and 30 healthy male comparison subjects. The total planum temporale was identical in all three anatomical definitions applied to determine the border between the anterior and posterior planum temporale regions. RESULTS: No significant differences between men with and without schizophrenia were detected with regard to planum temporale volumes and asymmetry coefficients for any of the three definitions. CONCLUSIONS: The authors could not prove the hypothesis that the definition of planum temporale borders influences the results concerning possible disturbances of planum temporale asymmetry in patients with schizophrenia.
Several studies have used in vivo and postmortem methods to examine whether the lateralization of the planum temporale is disturbed in schizophrenia (1–6). Methodological differences between studies, such as the application of different definitions of planum temporale and planum temporale borders (1–5), may have contributed to the conflicting results. However, this has not been clarified. Barta et al. (6) found that different methods of surface rendering may yield different results with regard to planum temporale asymmetry and that the examination of planum temporale surface and planum temporale volume may produce different results in the same subjects. All volumetric planum temporale studies that detected asymmetry disturbances in patients with schizophrenia examined the total planum temporale (1, 4, 5), and studies that examined the anterior planum temporale failed to detect significant differences between patients and comparison subjects (2, 3).
To test the hypothesis that different definitions of planum temporale borders may influence the results with regard to a possible disturbance of planum temporale asymmetry in schizophrenia, we attempted to examine the anterior, posterior, and total planum temporale volumes simultaneously. Three frequently used definitions of the posterior border of the planum temporale proper were applied (4).
Method
Subjects
The study group comprised 30 right-handed, medicated male inpatients with schizophrenia (mean age=29.37 years, SD=7.97, range=18–47). Demographic information and clinical history were obtained by using a semistructured interview. The comparison group comprised 30 right-handed, healthy male comparison subjects who were recruited from the general population. Their mean age was 29.17 years (SD=8.03, range=18–47); they were matched to the patients in a one-to-one fashion for age (within ±3 years) and educational achievement. Handedness was evaluated by using the Edinburgh Inventory (7). Neither healthy subjects nor their first-degree relatives had a history of neurological or mental illness. Exclusion criteria for both groups were a history of serious neurological disorder, use of benzodiazepine or cortisol medication in the last 3 months, any mental disorder other than schizophrenia, and previous ECT. All patients and comparison subjects provided written informed consent after the procedures had been fully explained.
Measures
Magnetic resonance imaging (MRI) data were acquired by using coronal T2-weighted and proton-density-weighted dual-echo sequences and three-dimensional magnetization-prepared rapid-gradient-echo sequences, described in detail elsewhere (8). Volumetric measurements of planum temporale volumes and total intracranial content were performed on the MRI data sets by using the software program BRAINS (9).
Details of the definition of the planum temporale borders and interrater and intrarater reliability are available elsewhere (8). Briefly, interactive measurements included a planum temporale total for each hemisphere, which was separated into anterior and posterior planum temporale by using three separate definitions.
Definition 1 was the region lateral to Heschl’s gyrus (planum temporale lateral to Heschl’s gyrus for the anterior definition and planum temporale posterior to Heschl’s gyrus for the posterior definition).
Definition 2 was the knife-cut method (the horizontal planum temporale anterior to the knife-cut for the anterior definition and the ascending planum temporale posterior to the knife-cut for the posterior definition).
Definition 3 was the bifurcation of Sylvian fissure (the region anterior to the branching point of the horizontal ramus of the Sylvian fissure into an ascending and descending ramus for the anterior definition and the region posterior to the branching point for the posterior definition).
The planum temporale total (anterior plus posterior) was identical in all definitions. Asymmetry coefficients were calculated according to the equation of Galaburda et al. (10).
Statistical Analysis
Possible confounding variables were assessed by using t tests for independent samples. Morphometric data were normally distributed. Relative volumes ([absolute region of interest volumes/intracranial content] × 100) were subjected to a two-factor analysis of variance (ANOVA) to assess the main and interaction effects of the between-subject factor diagnosis (patients, comparison subjects) and the within-subject factor (left hemisphere, right hemisphere). Unpaired Student’s t tests were used to assess the significance of differences in asymmetry coefficients.
Results
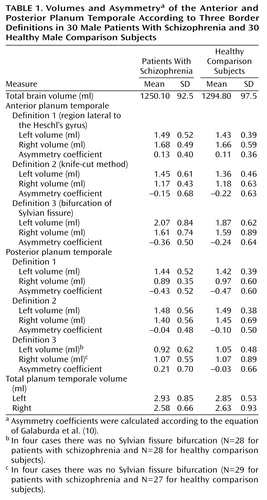
An overview of the results is given in Table 1. The groups did not differ significantly with respect to age (t=–0.97, df=58, p=0.92).
Regarding the total planum temporale, the two-factor ANOVA revealed no significant differences between the groups (F=0.69, df=1, 58, p=0.41). The total left planum temporale was significantly larger than the total right planum temporale (F=5.7, df=1, 58, p=0.02), without significant interaction between groups and hemispheres (F=0.26, df=1, 58, p=0.61).
Regarding the anterior planum temporale, the two-factor ANOVA found no significant volume differences between patients and healthy subjects for any of the three definitions (definition 1: F=1.0, df=1, 58, p=0.32; definition 2: F=1.1, df=1, 58, p=0.30; definition 3: F=1.3, df=1, 58, p=0.29).
Regarding the posterior planum temporale, the two-factor ANOVA found no significant volume differences between patients and healthy subjects for any of the three definitions (definition 1: F=0.02, df=1, 58, p=0.89; definition 2: F=0.05, df=1, 58, p=0.82; definition 3: F=0.19, df=1, 54, p=0.66).
No significant differences between men with and without schizophrenia emerged regarding asymmetry coefficients for any of the three definitions.
Discussion
To our knowledge, this investigation is the largest study to date on planum temporale volumes of male patients with schizophrenia. Our data suggest that the definition of planum temporale borders does not influence the results concerning possible disturbances of planum temporale asymmetry in schizophrenia. Therefore, results may reflect real morphological brain differences between our group of patients and those examined by others. There are four studies that examined the volume of the planum temporale in schizophrenia in vivo by MRI (2–5) and one that performed an in vitro examination of postmortem tissue (1).
Falkai et al. (1), who detected planum temporale asymmetry disturbances, examined the brains of chronically ill hospitalized patients. In contrast, patients in the Maudsley family study (2), which did not find significant differences in the asymmetry of the planum temporale between patients and control subjects, had a shorter illness duration than those examined by Kwon et al. (4) and Falkai et al. (1). These findings are consistent with the assumption that patients with a more chronic course of disease may show more brain abnormalities.
In conclusion, our results may support the neurodevelopmental hypothesis of more severe brain alterations in a subgroup of more severely ill patients with schizophrenia. Possible correlations between factors of chronicity, severity of the schizophrenic disturbances, and planum temporale measurements need to be evaluated in further studies.
 |
Received June 20, 2001; revision received Feb. 19, 2002; accepted Feb. 25, 2002. From the Department of Psychiatry and the Department of Radiology (Klinikum Innenstadt), Ludwig-Maximilians-University Munich. Address reprint requests to Dr. Meisenzahl, Department of Psychiatry, Ludwig-Maximilians-University Munich, Nußbaumstr. 7, 80336 München, Germany; [email protected] (e-mail).
1. Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, Gonsiorzcyk C, Majtenyi C, Ovary I: Disturbed planum temporale asymmetry in schizophrenia: a quantitative post-mortem study. Schizophr Res 1995; 14:161-176Crossref, Medline, Google Scholar
2. Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM: The Maudsley Family Study, 4: normal planum temporale asymmetry in familial schizophrenia: a volumetric MRI study. Br J Psychiatry 1997; 170:328-333Crossref, Medline, Google Scholar
3. Barta PE, Pearlson GD, Brill LB II, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG: Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry 1997; 154:661-667Link, Google Scholar
4. Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton M: Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry 1999; 56:142-148Crossref, Medline, Google Scholar
5. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry 2000; 57:692-699Crossref, Medline, Google Scholar
6. Barta PE, Petty RG, McGilchrist IK, Lewis RW, Jerram M, Casanova MF, Powers RE, Brill LB, Pearlson GD: Asymmetry of the planum temporale: methodological considerations and clinical associations. Psychiatr Res Neuroimaging 1995; 61:137-150Crossref, Medline, Google Scholar
7. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
8. Zetzsche T, Meisenzahl EM, Preuss UW, Holder JJ, Leinsinger G, Hahn H, Hegerl U, Möller HJ: In-vivo analysis of the human planum temporale: does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? J Psychiatr Res Neuroimaging 2001; 107:99-115Crossref, Medline, Google Scholar
9. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125-133Crossref, Medline, Google Scholar
10. Galaburda AM, Corsiglia J, Rosen G, Sherman GF: Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia 1987; 25:853-868Crossref, Google Scholar



