Amisulpride, an Unusual “Atypical” Antipsychotic: A Meta-Analysis of Randomized Controlled Trials
Abstract
OBJECTIVE: The “atypical” profile of the new antipsychotics clozapine, olanzapine, quetiapine, and risperidone has been linked to combined antagonism of serotonin 2 (5-HT2) and dopamine 2 (D2) receptors. Although amisulpride is a highly selective D3/D2 receptor antagonist, it is assumed to have atypical properties as well. The purpose of this article was to compare the atypical profile of amisulpride with that of the 5-HT2/D2 antagonists. METHOD: Randomized controlled trials that compared amisulpride with conventional antipsychotics or placebo for patients with schizophrenia were identified and included in a meta-analysis. The mean effect sizes found for amisulpride were compared with those of an updated meta-analysis of the 5-HT2/D2 antagonists. RESULTS: Eighteen randomized controlled trials of amisulpride (N=2,214) were found. In 11 studies of acutely ill patients it proved to be consistently more effective than conventional antipsychotics for global schizophrenic symptoms (measured with the Brief Psychiatric Rating Scale) and negative symptoms. Amisulpride is to date the only atypical antipsychotic for which several studies of patients suffering predominantly from negative symptoms have been published. In four such studies amisulpride was significantly more effective than placebo. Three small studies with conventional antipsychotics as comparators showed only a trend in favor of amisulpride in this regard. Amisulpride was associated with clearly lower use of antiparkinsonian medication and with fewer dropouts due to adverse events than conventional antipsychotics. CONCLUSIONS: These results cast some doubt on the notion that combined 5-HT2/D2 antagonism is the reason that the newer antipsychotic medications are effective for negative symptoms and have fewer extrapyramidal side effects.
The introduction of clozapine, risperidone, olanzapine, and quetiapine in the United States represents an important step forward in the treatment of schizophrenia. The “atypical” profile of these drugs has been linked to a combined antagonism of central serotonin type 2 (5-HT2) and dopamine type 2 (D2) receptors (1, 2). In a meta-analysis (3) we showed that these atypical antipsychotics are at least as effective as conventional drugs. Their main advantage is a lower risk of extrapyramidal side effects. Two of them also showed slight advantages in the treatment of negative symptoms. However, these drugs have been examined only for the treatment of acutely ill patients. It is therefore not clear whether the superiority relates to primary negative symptoms or merely to secondary negative symptoms. Studies with patients suffering predominantly from persistent negative symptoms would be more appropriate for assessing this issue (4). Such studies have been undertaken with amisulpride—a substituted benzamide that has been used as an antipsychotic in France for more than 10 years (5–7). Although this antipsychotic does not block serotonin receptors at all, but is a high-affinity and highly selective D3/D2 receptor antagonist, it is said to have atypical properties as well (8). It is believed that its selective affinity for dopamine receptors in the limbic structures, but not in the striatum, leads to a low risk of extrapyramidal side effects (9, 10). Animal studies have shown that at low doses it preferentially blocks presynaptic dopamine autoreceptors; this blockage facilitates dopaminergic transmission and thus might make amisulpride effective for negative symptoms (10). To further examine the different pharmacological models for atypicality, we performed a meta-analysis of the efficacy and safety of amisulpride. This was compared with an update of our previous meta-analysis of the 5-HT2/D2 antagonists.
Method
The method of Rosenthal (11) was used, as in our previous meta-analysis (3). The results on olanzapine, quetiapine, risperidone, and sertindole were updated by including newly published studies.
Identification of Studies
We searched for randomized controlled trials that compared amisulpride with conventional antipsychotics and/or placebo for the treatment of schizophrenia and schizophrenia-like psychoses. Relevant studies were identified by 1) a MEDLINE search (1966 to April 2000) and a Current Contents search (1997 to April 2000) using the search term “amisulpride,” 2) cross-referencing of reviews and included studies, and 3) contacting the pharmaceutical company that produces the drug. The company was also contacted to obtain data from unpublished trials. Our previous meta-analysis on olanzapine, quetiapine, risperidone, and sertindole was updated by using the search strategy described in our report (3). For this update only the effect sizes derived from the new studies and the resulting mean effect sizes will be presented. All other data can be found in our previous publication (3).
Outcome Measures
To assess the improvement of mental state, we analyzed the mean changes from baseline to endpoint in the total score on the Brief Psychiatric Rating Scale (BPRS) (12) and in the score on the Scale for the Assessment of Negative Symptoms (13). Intent-to-treat, last-observation-carried-forward data sets were used whenever available. To avoid the pitfall of applying parametric tests to nonparametric data, a study was included in the meta-analytic calculation of a specific outcome measure only if normal distribution could be assumed. For this, a request was sent to the statisticians of the manufacturer of amisulpride. As regards the other new antipsychotics, only studies that used parametric statistical tests were included. The number of patients requiring at least one dose of antiparkinsonian medication was used to assess extrapyramidal side effects. Scale-derived data on extrapyramidal side effects were not used, because these were mostly skewed and therefore not suitable for meta-analysis. Finally, we analyzed three types of dropout rates: global, for treatment failure, and for adverse events.
Statistical Method
In this meta-analysis all effect sizes are presented as Pearson’s correlation coefficients (r) according to the method described by Rosenthal (11), since r can be calculated from both continuous and dichotomous data and is easy to interpret. As a rough estimate, r corresponds to the mean percentage difference in treatment effects between the intervention and the control groups, i.e., the absolute risk difference (11, 14). The following calculations were made.
For continuous variables, Student’s t tests were calculated by using means, standard deviations, and numbers of patients in the intervention and control groups. We then calculated r by using the formula where df=N1+N2–2, N1 and N2 are the numbers of patients in the control and intervention groups, and t=result of the t test. For dichotomous data, chi-square tests using 2×2 tables were calculated to obtain phi (φ) according to the formula
where df=N1+N2–2, N1 and N2 are the numbers of patients in the control and intervention groups, and t=result of the t test. For dichotomous data, chi-square tests using 2×2 tables were calculated to obtain phi (φ) according to the formula  where χ2=result of the chi-square test and N=total number of patients in both groups. Phi corresponds to Pearson’s correlation coefficient for continuous data. All statistical calculations involving correlation coefficients (calculation of means, confidence intervals, etc.) were made on the basis of zr, i.e., Fisher’s z transformation for correlation coefficients. Results were transformed back to r. For studies that compared several doses of amisulpride with a control condition, the different dose groups of amisulpride were pooled; i.e., for continuous data, the mean effect r achieved by the different doses was calculated, and for dichotomous data, all patients treated with amisulpride were considered as a single group in the chi-square test. For combining the effect sizes of the single studies, the random-effects model according to DerSimonian and Laird (15) was used. This model is usually more conservative than fixed-effects models because it takes into account variability between studies. For this, a chi-square test of homogeneity was calculated with
where χ2=result of the chi-square test and N=total number of patients in both groups. Phi corresponds to Pearson’s correlation coefficient for continuous data. All statistical calculations involving correlation coefficients (calculation of means, confidence intervals, etc.) were made on the basis of zr, i.e., Fisher’s z transformation for correlation coefficients. Results were transformed back to r. For studies that compared several doses of amisulpride with a control condition, the different dose groups of amisulpride were pooled; i.e., for continuous data, the mean effect r achieved by the different doses was calculated, and for dichotomous data, all patients treated with amisulpride were considered as a single group in the chi-square test. For combining the effect sizes of the single studies, the random-effects model according to DerSimonian and Laird (15) was used. This model is usually more conservative than fixed-effects models because it takes into account variability between studies. For this, a chi-square test of homogeneity was calculated with  where zr=Fisher’s z of r and
where zr=Fisher’s z of r and  r. Then tau2 was estimated by using the formula
r. Then tau2 was estimated by using the formula  with k–1=number of degrees of freedom. Tau2 was used for the weighting of the single studies by w=(N–3)/{1+[(N–3)*tau2]}. In cases where the heterogeneity statistic is less than or equal to k–1, tau2 is zero and the weights are the same as in the fixed-effects model (see the following). The weighted mean z was calculated as [S(zr*w)]/Sw and transformed back to r̄ as the coefficient of the effect size. To maintain comparability with the previous results (3), the mean effect sizes obtained by a fixed-effects model are also presented (thick gray bars in the figures). Here N–3 is used for weighting the individual studies irrespective of heterogeneity among studies. The results are presented as (mean) effect size r along with the 95% confidence interval (CI) calculated as
with k–1=number of degrees of freedom. Tau2 was used for the weighting of the single studies by w=(N–3)/{1+[(N–3)*tau2]}. In cases where the heterogeneity statistic is less than or equal to k–1, tau2 is zero and the weights are the same as in the fixed-effects model (see the following). The weighted mean z was calculated as [S(zr*w)]/Sw and transformed back to r̄ as the coefficient of the effect size. To maintain comparability with the previous results (3), the mean effect sizes obtained by a fixed-effects model are also presented (thick gray bars in the figures). Here N–3 is used for weighting the individual studies irrespective of heterogeneity among studies. The results are presented as (mean) effect size r along with the 95% confidence interval (CI) calculated as  for the single studies and
for the single studies and  for the mean confidence intervals, each limit zr back-transformed to its corresponding r. Thus, positive r values indicate effects favoring the new antipsychotic. Only the updated mean effect sizes for the 5-HT2/D2 antagonists and the results of the new studies on them are presented in the figures. We used z tests to assess the statistical significance of the mean effect sizes. This statistical significance was assumed when p values were less than 0.05 (two-tailed).
for the mean confidence intervals, each limit zr back-transformed to its corresponding r. Thus, positive r values indicate effects favoring the new antipsychotic. Only the updated mean effect sizes for the 5-HT2/D2 antagonists and the results of the new studies on them are presented in the figures. We used z tests to assess the statistical significance of the mean effect sizes. This statistical significance was assumed when p values were less than 0.05 (two-tailed).
Heterogeneity analysis
Results of the just-described chi-square test for homogeneity that were statistically significant will be reported, since in these cases it is likely that the heterogeneity was due to factors other than chance, and therefore reasons for the heterogeneity should be sought (11).
Assessment of publication bias
Studies with negative results are less likely to be published than studies with significant results. The possibility of such publication bias, which can affect the results of a meta-analysis, was examined by using two methods. First, according to the “funnel-plot” method preferred by the Cochrane collaboration (16), the effect sizes of the single studies are plotted against the numbers of subjects. If all studies have been published, a symmetrical figure resembling a funnel should result. Second, the number (x) of unretrieved studies averaging null results that is required to bring the new overall p to the level just significant at p=0.05 was calculated according to the formula  with k=number of studies combined and z̄=mean z obtained for the k studies (11).
with k=number of studies combined and z̄=mean z obtained for the k studies (11).
Sensitivity analyses
In two sensitivity analyses we 1) examined only optimum doses of amisulpride and 2) excluded studies that did not use last-observation-carried-forward data.
Results
Study Characteristics
Eighteen randomized controlled trials including 2,214 patients were identified (5–7, 17–31) (Table 1); details of the studies on the 5-HT2/D2 antagonists have been published elsewhere (3). Apart from the trial of Colonna et al. (27), which was an open randomized study, all were double-blind and used a parallel design. Study durations ranged between 3 weeks and 1 year. There was some variation of diagnostic criteria over time; the earlier studies generally used ICD-9 and DSM-III, whereas the newest study (26) used DSM-IV. Also, the newer studies used intent-to-treat, last-observation-carried-forward analyses, whereas some of the older investigations included only the study completers in their statistical analyses. As in our previous meta-analysis on the 5-HT2/D2 antagonists, the patients were typically in their mid-30s, the majority were male, they showed moderate to severe schizophrenic symptoms at baseline, and their mean duration of illness ranged between 3 and 37 years (median=10). The most common comparators were haloperidol and placebo, but four studies compared amisulpride with flupentixol (24), perazine (17), and fluphenazine (30, 31). One randomized controlled trial (32) had to be excluded, because it used risperidone and not a conventional antipsychotic as a comparator. Eleven trials examined the effectiveness of amisulpride for acutely ill patients, whereas seven studies examined low-dose amisulpride (50–300 mg/day) for patients with persistent, predominantly negative symptoms (with some variation of the criteria used). This symptom profile is different from that in the meta-analysis of the 5-HT2/D2 antagonists, for which only studies of patients with acute exacerbations of schizophrenia have been published, to our knowledge.
Update of Previous Meta-Analysis
Several new randomized controlled trials of the 5-HT2/D2 receptor antagonists have been published since our last meta-analysis (3). Although these did not lead to significant changes of the overall results of our meta-analysis, they filled some important gaps in our knowledge about these compounds: Olanzapine was compared with chlorpromazine for treatment-resistant patients (33) and with haloperidol for cannabis-induced psychoses (34) and for a small group of male schizophrenic patients (35). For quetiapine, results of a comparison with haloperidol have now been fully published (36) but do not provide new data. For risperidone we identified two new comparisons with haloperidol or standard treatment for treatment-resistant patients (37–39). Emsley et al. (40) compared risperidone and haloperidol in a study of antipsychotic-naive patients. One study (41) compared risperidone with haloperidol for cannabis-induced psychosis, and a further study (42) compared risperidone with methotrimeprazine and haloperidol. Two other studies compared risperidone with haloperidol and focused on cognitive functions (43) or changes in plasma norepinephrine levels (44). New studies with sertindole have not been published, to our knowledge, possibly because of the withdrawal of this drug from the market.
Several further studies on the 5-HT2/D2 receptor antagonists have been presented at congresses, but the abstracts did not allow extraction of the necessary data. It was also not possible to obtain these by contacting authors and pharmaceutical companies.
Reduction in BPRS Score
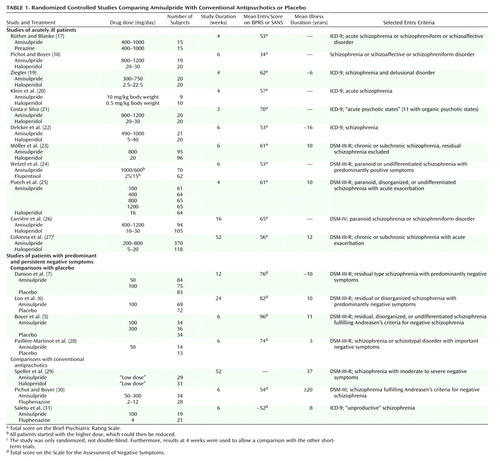
The patients suffering predominantly from negative symptoms showed hardly any positive symptoms and therefore were excluded from our analysis of change in BPRS score from baseline to endpoint. According to 10 studies with acutely ill patients, amisulpride was significantly superior to conventional antipsychotics. The mean effect size of r=0.11 indicates a superiority of amisulpride over conventional antipsychotics of roughly 11 percentage points. The result is remarkably consistent, since in all but one study there was at least a trend in favor of amisulpride (Figure 1).
Negative Symptoms
In the studies of acutely ill patients, for negative symptoms there was again a clear and consistent superiority of amisulpride to conventional compounds (Figure 2, middle). These studies do not, however, permit a conclusion as to whether the superiority affects primary negative symptoms or merely secondary negative symptoms (e.g., negative symptoms secondary to positive symptoms). Studies of patients suffering predominantly from persistent negative symptoms are more appropriate for this. As far as we know, such studies have been performed up to now only with amisulpride. In four studies with patients suffering predominantly from persistent negative symptoms amisulpride was significantly superior to placebo (Figure 3). Olanzapine, quetiapine, risperidone, and sertindole were also significantly superior to placebo for negative symptoms (Figure 3), but these results were derived only from studies with acutely ill patients. Only three small studies compared amisulpride with conventional antipsychotics for patients with predominantly negative symptoms. There was no significant difference between amisulpride and conventional drugs (Figure 2, top). The high variability of the durations of the amisulpride studies on negative symptoms, ranging from 6 weeks to 12 months (29), is notable, although no statistical heterogeneity of their results was found.
Use of Antiparkinsonian Medication
Amisulpride was not associated with significantly more use of antiparkinsonian medication than placebo (Figure 4). It must be noted that in all placebo comparisons only low doses of amisulpride (50–300 mg/day) were used. We know of no comparisons of higher doses of amisulpride with placebo. However, amisulpride induced clearly fewer extrapyramidal side effects than conventional antipsychotics (Figure 5). The mean effect size of r=0.25 lay between the effect sizes of risperidone (r=0.14) on the one hand and olanzapine (r=0.39), quetiapine (r=0.32), and sertindole (r=0.34) on the other. When only optimum doses of risperidone (4–8 mg/day) are considered, its effect size (r=0.17) approaches that of amisulpride. A significant heterogeneity of the amisulpride study results (χ2=19.06, df=11, p=0.06) was resolved when the two studies that used the highest haloperidol doses (20, 21) were excluded.
Dropout Rates
In the studies of acutely ill patients, significantly fewer patients treated with amisulpride dropped out than patients treated with conventional drugs (11 studies, r=0.17, 95% CI=0.08 to 0.26, z=3.68, p=0.0002). This superiority resulted from fewer dropouts due to adverse events (r=0.15, 95% CI=0.07 to 0.25, z=3.02, p=0.003), with the individual studies showing a consistent pattern. Only one study (27) showed a trend in favor of haloperidol in this regard. This flexible-dose study led to significant study heterogeneity (χ2=22.00, df=8, p=0.005), which was no longer apparent after its exclusion. No difference in dropouts due to inefficacy of treatment was found (r=0.01, 95% CI=–0.04 to 0.06, z=0.34, p=0.37).
In the studies of patients with predominantly persistent negative symptoms, fewer patients treated with amisulpride than with placebo left the studies earlier, whether because of ineffective treatment (r=0.17, 95% CI=0.07 to 0.27, z=3.13, p=0.002), because of adverse events (r=0.13, 95% CI=0.03 to 0.23, z=2.56, p=0.008), or for any reason (r=0.20, 95% CI=0.12 to 0.28, z=4.58, p<0.0001). No significant differences in dropout rates between amisulpride and conventional antipsychotics were found in three small studies of negative symptom schizophrenia (all dropouts: r=0.08, 95% CI=–0.08 to 0.23, z=0.96, p=0.34; patients who left because of ineffective treatment: r=0.11, 95% CI=–0.05 to 0.26, z=1.35, p=0.18; patients who left because of adverse events: r=0.04, 95% CI=–0.11 to 0.20, z=0.54, p=0.59). The use of a fixed-effects model did not lead to major changes here. The exact data can be obtained from us on request.
Sensitivity Analyses
The sensitivity analysis of only optimum doses (400–800 mg/day for acutely ill patients and 50–300 mg/day for patients suffering predominantly from persistent negative symptoms) did not lead to important changes. With the exception of dropouts due to adverse events, all effect sizes slightly increased in favor of amisulpride. For BPRS change, r=0.12, 95% CI=0.06 to 0.17; for change in negative symptoms, r=0.15, 95% CI=0.09 to 0.20; for antiparkinsonian medication, r=0.27, 95% CI=0.17 to 0.37; for global dropout rate, r=0.21, 95% CI=0.12 to 0.30; for dropouts due to adverse events, r=0.15, 95% CI=0.06 to 0.26; for dropouts due to ineffective treatment, r=0.02, 95% CI=–0.03 to 0.08. For the studies of patients with predominantly negative symptoms a sensitivity analysis was not necessary because no study used doses outside the optimum range (50–300 mg/day).
No significant changes were noted in a sensitivity analysis in which the earlier studies that used completer analyses were excluded.
Random- Versus Fixed-Effects Model
Compared to the fixed-effects model, the random-effects model sometimes widened the confidence intervals or led to slight changes of the mean effect sizes, but no substantial changes in the amisulpride results were obtained (see Figure 1—Figure 5. Unlike in the fixed-effects model, in the random-effects model the difference between olanzapine and conventional antipsychotics in terms of BPRS change and the borderline difference between quetiapine and conventional antipsychotics in terms of negative symptoms were no longer statistically significant. The reason for the substantially broader confidence interval of the effect size for olanzapine in the random-effects model was a significant heterogeneity among studies (χ2=9.84, df=2, p=0.007). This disappeared when subtherapeutic doses (below 7.5 mg/day) were excluded (r=0.10, 95% CI=0.06 to 0.14, z=5.03, p<0.0001).
Exploration of Publication Bias
A funnel plot of the main outcome variable (mean BPRS score) revealed the potential of publication bias. Some small studies with negative results might not have been published (Figure 6). However, according to the manufacturer, no further studies have been undertaken. According to the method of Rosenthal (11), 62 unpublished studies would be necessary to reverse statistical significance. It is unlikely that so many studies have remained unpublished.
Discussion
Since the discovery that clozapine induces fewer extrapyramidal side effects and is more effective than conventional antipsychotics for the treatment of schizophrenia (45, 46), psychopharmacological research has focused on the development of drugs that block central 5-HT2 receptors more than D2 receptors. Combined 5-HT2/D2 receptor antagonism is the most current explanation for the so-called “atypical” profile of some antipsychotics (2). Although this concept is difficult to define (47) and might be better understood as a continuum (48), the most frequent requirements for atypicality are a low risk of extrapyramidal side effects and greater efficacy for negative symptoms. This meta-analysis showed that a highly selective dopamine antagonist with no effects on serotonergic receptors possesses atypical properties. Amisulpride is a well-studied drug (18 randomized controlled trials) and was consistently superior to conventional antipsychotics for acutely ill patients. Although a funnel plot suggests that publication bias cannot be excluded, further trials do not exist, according to the manufacturer, and would also be unlikely to change the overall effect.
The efficacy of amisulpride for negative symptoms has been examined more than that of the 5-HT2/D2 receptor antagonists. This does not mean that amisulpride is more effective than the other atypicals, but the slight and debatable superiority of the latter over typical antipsychotics has been derived only from studies of acutely ill patients (3). Such studies do not allow a judgment about whether the superiority refers only to secondary or also to primary negative symptoms. Path analysis is a statistical method that has been used to rule out the effects of extrapyramidal side effects, depression, and positive symptoms on negative symptoms (49–51), but studies of patients suffering predominantly from persistent negative symptoms are more appropriate (4). The placebo comparisons clearly showed that amisulpride is an effective treatment for negative symptoms. However, the mean effect size (r=0.26) is relatively small, so it is possible that no dramatic improvements might be seen in many patients. Furthermore, the few comparisons with typical drugs, which had insufficiently large study groups, failed to show a significant superiority of amisulpride. Similar preliminary results have been presented for a trial comparing quetiapine with a conventional antipsychotic (52). Further large randomized controlled trials are indispensable for clarifying whether the new antipsychotics are really more effective for the treatment of primary negative symptoms.
The most consistent characteristic of atypicality is a low risk of extrapyramidal side effects. Like the 5-HT2/D2 receptor antagonists, amisulpride induced clearly less movement disorder than typical antipsychotics. More comparisons with drugs that have fewer extrapyramidal side effects than haloperidol would be desirable for all new antipsychotics. This meta-analysis can help to indirectly compare the new antipsychotics since direct comparisons are scarce. It suggests that amisulpride’s risk for extrapyramidal side effects lies between those of olanzapine, quetiapine, and sertindole on the one hand and risperidone on the other. When only optimum doses of risperidone (4–8 mg/day) are considered, its effect size is similar to that of amisulpride. This was also shown in a multicenter study in which risperidone, 8 mg/day, and amisulpride, 800 mg/day, showed similar occurrences of extrapyramidal side effects (32). More direct comparisons of atypical antipsychotics are needed to clarify their risks of extrapyramidal side effects. Furthermore, similar to that of risperidone, amisulpride’s risk of extrapyramidal side effects is dose related, and in the dose-finding study by Puech et al. (25) there was no significant difference in the rates of patients who received antiparkinsonian medication between the subjects who were taking 16 mg/day of haloperidol and those who were taking 1200 mg/day of amisulpride. We did not evaluate side effects other than extrapyramidal side effects, but a global indicator of amisulpride’s tolerability is the fact that significantly fewer patients left the studies early because of side effects than in the studies with conventional drugs. The main concern with amisulpride is the induction of substantial prolactin increase, although it is unclear whether this leads to higher rates of adverse endocrine events than found with other antipsychotics (53). Weight gain is a problem of the 5-HT2/D2 antagonists. Allison et al. (54) estimated mean weight gains for olanzapine, risperidone, and sertindole of 3.5 kg, 2.0 kg, and 2.5 kg within 10 weeks. Amisulpride was not included in this meta-analysis. Amisulpride was associated with relatively low mean weight gains in short-term studies (4–12 weeks) and long-term studies (6–12 months), i.e., 0.7 kg (SD=3.1) and 1.2 kg (SD=6.5), respectively (Sanofi-Synthélabo, data on file; see also reference 55).
The results of this meta-analysis must be seen in the light of several methodological problems. The small differences in the mean effect sizes for efficacy must not be regarded as real differences. A formal statistical comparison of mean effect sizes was not undertaken. Despite the similarity of studies on new antipsychotics, certain differences in study designs, such as slightly different inclusion criteria, study durations, comparators, or dose regimens (e.g., dose titration versus fixed doses), would make such statistics inappropriate. This points to a general problem of meta-analysis, which is an elegant approach for combining the data of several studies but is not adequate for dealing with the particular features of the individual studies. Further such design differences relate to measurement intervals and analysis methods, what was meant by “intent to treat,” and the way dropouts were handled. The use of continuous outcome measures is problematic because it is impossible to assure normal distribution with absolute certainty. An analysis of response rates was not possible because of a high variability of criteria within the studies of one new antipsychotic and between studies of different new antipsychotics (e.g., 20%, 40%, or 50% reduction in BPRS score) and because it would not have been possible to examine negative symptoms. With the exception of some data in the oldest studies, which could not be obtained owing to changes in ownership, a complete amisulpride data set was available for the meta-analysis, whereas not all data were received for the update of the other drugs, despite our requests. These data might have led to slight changes but would not have vitiated the global results, i.e., that amisulpride leads to clinical effects similar to those of the other atypical antipsychotics. A recent analysis (56) of 163 randomized controlled trials (18,585 patients) included in the Cochrane reviews on conventional and new antipsychotics showed a constant increase in dropout rates from the 1950s to the present. In the short-term studies on amisulpride, risperidone, olanzapine, quetiapine, and sertindole, global dropout rates of 22%, 30%, 43%, 46%, and 47% were found. It should be kept in mind that the higher the dropout rate, the more difficult it is to interpret the data, since assumptions such as the last-observation-carried-forward model must be made. In this model, when a patient leaves the study before completion, data from his or her last assessment are used in the statistics. Given that the conventional antipsychotics are usually less tolerable, patients who take these drugs drop out of the studies earlier. In the end effect this can give the false appearance that the atypical antipsychotic is more effective than the conventional one. In a meta-regression of 52 trials, Geddes et al. (57) showed that the advantages in terms of efficacy and dropouts of the atypical antipsychotics disappear when doses below 12 mg/day of haloperidol (or equivalent) are used. Finally, many of the patients included in the trials had previously had partial responses to conventional antipsychotics, and so many of those in the groups receiving new antipsychotics may just have benefited from a switch to a compound with a different receptor binding profile. These limitations must be kept in mind when considering any superiority in efficacy of the new antipsychotics found in this meta-analysis.
The main conclusion of this meta-analysis is that combined 5-HT2/D2 receptor antagonism is not the only mechanism that makes an antipsychotic atypical. Mesolimbic selectivity of dopamine receptor blockade is another explanation for the low risk of extrapyramidal side effects associated with some antipsychotics. Such a selectivity has been described not only for amisulpride but also for sertindole (58), olanzapine (59), and quetiapine (60). Whereas the high efficacy of low doses of amisulpride for negative symptoms has been explained by its action on presynaptic dopaminergic autoreceptors, the inhibition of 5-HT2 receptors by the other atypicals leads to enhanced dopamine release in the frontal lobe and possibly a reduction of negative symptoms. Some other models currently under examination are a selective affinity to D4 receptors (61), a selective affinity to D3 receptors (62), partial dopamine agonism (63), and multireceptor effects similar to those of clozapine (64).
 |
Presented in part at the 10th Biennial Winter Workshop on Schizophrenia, Davos, Switzerland, Feb. 5–11, 2000, and at the 13th European College of Neuropsychopharmacology Congress, Munich, Sept. 9–13, 2000. Received Aug. 7, 2000; revisions received March 20 and July 30, 2001; accepted Aug. 20, 2001. From the Klinik und Poliklinik für Psychiatrie und Psychotherapie der Technischen Universität München, Klinikum rechts der Isar, Munich; and the Psychiatrische Klinik der Ludwig-Maximilian Universität, Munich. Address reprint requests to Dr. Leucht, Klinik und Poliklinik für Psychiatrie und Psychotherapie der Technischen Universität München, Klinikum rechts der Isar, Ismaningerstrasse 22, 81675 München, Germany; stefan.leucht@ lrz.tu-muenchen.de (e-mail).Supported in part by an educational grant from Sanofi-Synthélabo Germany. No funds have been paid directly to the authors.The authors thank Sanofi-Synthélabo (especially Drs. Eich, Giudicelli, and Rein) for permission to use unpublished data and Profs. Berk, Blin, Liu, and See for sending missing data on their trials.
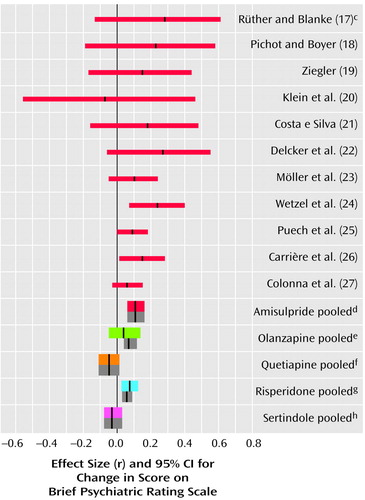
Figure 1. Differences in Mean Change in BPRS Score in Comparisons of Amisulpride or Other New Antipsychotic Drugs With Conventional Antipsychoticsa,b
aThe thin red lines represent individual studies of amisulpride. The thick colored lines represent pooled studies when effect sizes were combined according to a random-effects model. The thick gray bars are the mean effect sizes and their confidence intervals when a fixed-effects model was used.
bA meta-analysis of the other new antipsychotics was published earlier (3). In the three studies of olanzapine published since that meta-analysis the effect sizes were r=0.11 (95% CI=–0.10 to 0.32, N=81) (33), r=–0.02 (95% CI=–0.38 to 0.34, N=30) (34), and r=0.04 (95% CI=–0.40 to 0.44, N=23) (35). Either these were effect sizes derived from BPRS scores at endpoint or a normal distribution could not be assumed, and the scores could not be included in the mean effect size. In the new studies of risperidone the effect sizes were r=–0.19 (95% CI=–0.48 to 0.14, N=38) (43), r=0.04 (95% CI=–0.12 to 0.20, N=182) (40), r=0.26 (95% CI=–0.21 to 0.63, N=20) (44), r=0.27 (95% CI=–0.05 to 0.54, N=41) (reference 42, comparison with haloperidol), and r=0.30 (95% CI=–0.01 to 0.56, N=42) (reference 42, comparison with methotrimeprazine). One study (39) provided data only for response rates (r=0.09, 95% CI=–0.17 to 0.34, N=58), and for the BPRS scores at endpoint in one study (41) a normal distribution could not be assumed (r=0.01, 95% CI=–0.36 to 0.36, N=30).
cEndpoint analysis, not used for the mean effect size for the pooled data.
dr=0.11, 95% CI=0.06 to 0.16, z=4.40, p<0.0001, N=1,654.
er=0.04, 95% CI=–0.05 to 0.13, z=0.86, p=0.39, N=2,994.
fr=–0.05, 95% CI=–0.11 to 0.01, z=–1.50, p=0.13, N=953.
gr=0.08, 95% CI=0.03 to 0.12, z=3.08, p=0.002, N=3,362.
hr=–0.03, 95% CI=–0.08 to 0.03, z=–0.90, p=0.37, N=1,218.
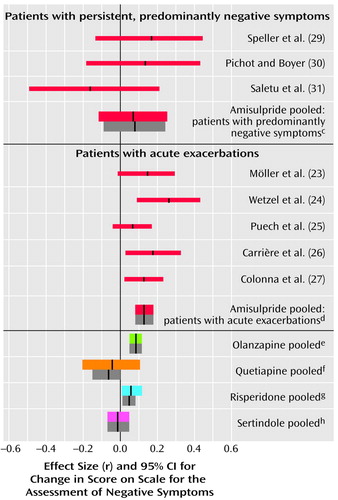
Figure 2. Differences in Mean Change in Negative Symptoms in Comparisons of Amisulpride or Other New Antipsychotic Drugs With Conventional Antipsychotics for Patients With Persistent Negative Symptoms and Patients With Acute Exacerbationsa,b
aThe thin red lines represent individual studies of amisulpride. The thick colored lines represent pooled studies when effect sizes were combined according to a random-effects model. The thick gray bars are the mean effect sizes and their confidence intervals when a fixed-effects model was used.
bA meta-analysis of the other new antipsychotics was published earlier (3). In the two studies of olanzapine published since that meta-analysis the effect sizes were r=0.05 (95% CI=–0.02 to 0.27, N=81) (33) and r=0.06 (95% CI=–0.40 to 0.46, N=23) (35). Both were derived from endpoint data. In the new studies of risperidone the effect sizes were r=0.00 (95% CI=–0.33 to 0.33, N=38) (43), r=0.04 (95% CI=–0.13 to 0.20, N=182) (40), r=0.02 (95% CI=–0.43 to 0.48, N=20) (44), r=0.19 (95% CI=–0.13 to 0.48, N=41) (reference 42, comparison with haloperidol), and r=0.23 (95% CI=–0.09 to 0.51, N=22) (reference 42, comparison with methotrimeprazine).
cr=0.08, 95% CI=–0.12 to 0.26, z=0.77, p=0.44, N=130 (only endpoint values could be used for the calculation of this effect size).
dr=0.14, 95% CI=0.08 to 0.19, z=4.53, p<0.0001, N=1,563. Six early studies (17–22) did not use scales to assess negative symptoms and could therefore not be included.
er=0.08, 95% CI=0.05 to 0.12, z=4.63, p<0.0001, N=2,993.
fr=–0.05, 95% CI=–0.20 to 0.11, z=–0.62, p=0.53, N=685.
gr=0.06, 95% CI=0.01 to 0.12, z=2.29, p=0.02, N=3,340.
hr=–0.01, 95% CI=–0.07 to 0.05, z=–0.23, p=0.81, N=1,125.
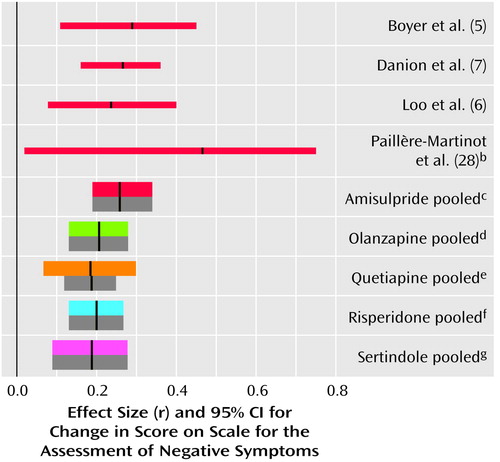
Figure 3. Differences in Mean Change in Negative Symptoms in Comparisons of Amisulpride or Other New Antipsychotic Drugs With Placeboa
aAll of the comparisons of amisulpride with placebo involved patients suffering predominantly from negative symptoms, whereas to our knowledge, all other new antipsychotics were studied only in acutely ill patients. The thin red lines represent individual studies of amisulpride. The thick colored lines represent pooled studies when effect sizes were combined according to a random-effects model. The thick gray bars are the mean effect sizes and their confidence intervals when a fixed-effects model was used.
bThis study was not included in the mean effect size for the pooled data because the data were not normally distributed.
cr=0.26, 95% CI=0.19 to 0.34, z=6.59, p<0.0001, N=624.
dr=0.21, 95% CI=0.13 to 0.28, z=5.02, p<0.0001, N=582.
er=0.19, 95% CI=0.07 to 0.30, z=3.09, p=0.002, N=823.
fr=0.20, 95% CI=0.13 to 0.27, z=5.31, p<0.0001, N=686.
gr=0.19, 95% CI=0.09 to 0.28, z=3.69, p=0.0002, N=392.
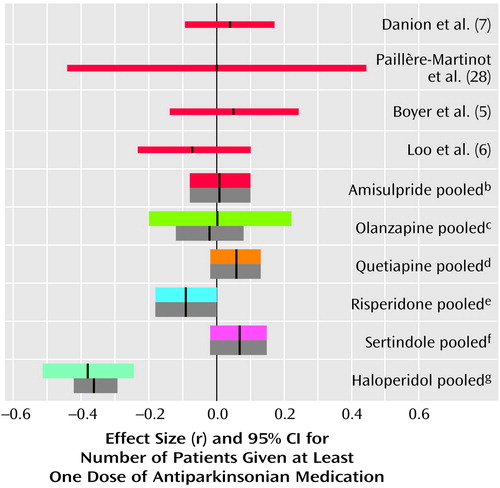
Figure 4. Differences in Use of Antiparkinsonian Medication in Comparisons of Amisulpride or Other New Antipsychotic Drugs With Placeboa
aThe thin red lines represent individual studies of amisulpride. The thick colored lines represent pooled studies when effect sizes were combined according to a random-effects model. The thick gray bars are the mean effect sizes and their confidence intervals when a fixed-effects model was used.
br=0.01, 95% CI=–0.08 to 0.10, z=0.22, p=0.82, N=507.
cr=–0.02, 95% CI=–0.12 to 0.08, z=0.03, p=0.90, N=418.
dr=0.06, 95% CI=–0.02 to 0.08, z=1.50, p=0.13, N=716.
er=–0.09, 95% CI=–0.18 to 0.00, z=–1.87, p=0.06, N=436.
fr=0.07, 95% CI=–0.02 to 0.15, z=1.47, p=0.14, N=494.
gr=–0.36, 95% CI=–0.42 to –0.29, z=–5.04, p<0.0001, N=696 (this effect size was calculated from the comparisons of haloperidol and placebo in studies on olanzapine, quetiapine, and sertindole).
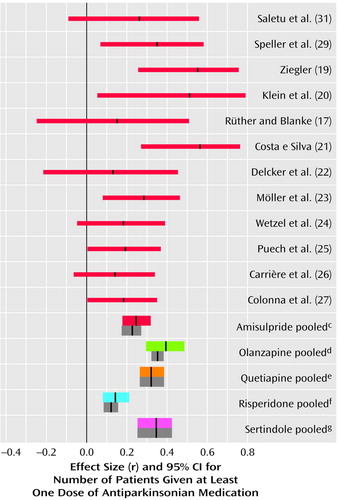
Figure 5. Differences in Use of Antiparkinsonian Medication in Comparisons of Amisulpride or Other New Antipsychotic Drugs With Conventional Antipsychoticsa,b
aThe thin red lines represent individual studies of amisulpride. The thick colored lines represent pooled studies when effect sizes were combined according to a random-effects model. The thick gray bars are the mean effect sizes and their confidence intervals when a fixed-effects model was used.
bA meta-analysis of the other new antipsychotics was published earlier (3). In the studies of risperidone published since that meta-analysis the effect sizes were r=0.25 (95% CI=0.07 to 0.42, N=183) (40), r=0.21 (95% CI=–0.27 to 0.61, N=20) (44), r=0.21 (95% CI=–0.13 to 0.50, N=41) (reference 42, comparison with haloperidol), r=–0.12 (95% CI=–0.43 to 0.21, N=42) (reference 42, comparison with methotrimeprazine), and r=–0.22 (95% CI=–0.57 to 0.19, N=27) (38).
cr=0.25, 95% CI=0.17 to 0.32, z=6.53, p<0.0001, N=1,599. In two studies (18, 30) this outcome was not measured.
dr=0.39, 95% CI=0.30 to 0.48, z=7.56, p<0.0001, N=2,694.
er=0.38, 95% CI=0.32 to 0.44, z=10.90, p<0.0001, N=757.
fr=0.14, 95% CI=0.08 to 0.21, z=4.29, p<0.0001, N=2,421.
gr=0.34, 95% CI=0.25 to 0.42, z=7.27, p<0.0001, N=424.
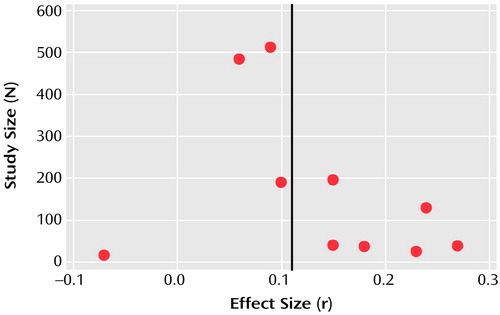
Figure 6. Publication Bias for Studies Comparing Amisulpride With Conventional Antipsychotic Drugs on the Basis of Mean Change in BPRS Scorea
a“Funnel plot” indicates likelihood that small studies with negative results were not published (in the absence of publication bias, a funnel shape is formed by the data). Data points represent the individual studies shown in Figure 1 except that by Rüther and Blanke. The black vertical line represents mean effect size (r=0.11).
1. Roth BL, Meltzer H: The role of serotonin in schizophrenia, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 1215-1227Google Scholar
2. Buckley PF: New dimensions in the pharmacologic treatment of schizophrenia and related psychoses. J Clin Pharmacol 1997; 37:363-378Crossref, Medline, Google Scholar
3. Leucht S, Pitschel-Walz G, Abraham D, Kissling W: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res 1999; 35:51-68Crossref, Medline, Google Scholar
4. Möller HJ, Van Praag HM, Aufdembrinke B (Working Group on Negative Symptoms in Schizophrenia): Negative symptoms in schizophrenia: considerations for clinical trials. Psychopharmacology (Berl) 1994; 115:221-228Crossref, Medline, Google Scholar
5. Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F: Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995; 166:68-72Crossref, Medline, Google Scholar
6. Loo H, Poirier-Littre MF, Theron M, Rein W, Fleurot O: Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry 1997; 170:18-22Crossref, Medline, Google Scholar
7. Danion J-M, Rein W, Fleurot O (Amisulpride Study Group): Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Am J Psychiatry 1999; 156:610-616Abstract, Google Scholar
8. Rein W, Turjanski S: Clinical update on amisulpride in deficit schizophrenia. Int Clin Psychopharmacol 1997; 12(suppl 2):S19-S27Google Scholar
9. Perrault G, Depoortère R, Morel E, Sanger DJ, Scatton B: Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997; 280:73-82Medline, Google Scholar
10. Scatton B, Claustre Y, Cudennec A, Oblin A, Parrault G, Sanger DJ, Schoemaker H: Amisulpride: from animal pharmacology to therapeutic action. Int Clin Psychopharmacol 1997; 12(suppl):S29-S36Google Scholar
11. Rosenthal R: Meta-Analytic Procedures for Social Research, 1st revised ed. New York, Sage Publications, 1991Google Scholar
12. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
13. Andreasen NC: The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 7:49-58Medline, Google Scholar
14. Rosenthal R, Rubin DB: A simple general purpose display of magnitude of experimental effect. J Educ Psychol 1982; 74:166-169Crossref, Google Scholar
15. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177-188Crossref, Medline, Google Scholar
16. Mulrow CD, Oxman AD: Cochrane Collaboration Handbook: Update 21. London, British Medical Journal Publishing Group, 1996Google Scholar
17. Rüther E, Blanke J: Therapievergleich von Aminosultoprid (DAN 2163) und Perazin bei schizophrenen Patienten, in Therapie mit Neuroleptika—Perazin. Edited by Helmchen H, Hippius H, Tölle R. Stuttgart, Germany, Georg Thieme Verlag, 1988, pp 65-71Google Scholar
18. Pichot P, Boyer P: Etude multicentrique controlée en double insu, amisulpride (Solian 200) versus halopéridol à forte dose dans les états psychotiques aigus. Annales de Psychiatrie 1988; 3:326-332Google Scholar
19. Ziegler B: Study of the efficacy of a substituted benzamide amisulpride, versus haloperidol, in productive schizophrenia, in Amisulpride. Paris, Expansion Scientifique Française, 1989, pp 73-82Google Scholar
20. Klein HE, Dieterle D, Rüther E, Eben E, Nedopil N, Hippius H: A double-blind comparison of amisulpride vs haloperidol in acute schizophrenic patients, in Psychiatry: The State of the Art, vol 3: Pharmacopsychiatry. Edited by Pichot P, Berner P, Wolf R, Thau K. Cambridge, Mass, Perseus Books, 1985, pp 687-691Google Scholar
21. Costa e Silva JA: Comparative double-blind study of amisulpride versus haloperidol in the treatment of acute psychotic states, in Amisulpride. Paris, Expansion Scientifique Française, 1989, pp 93-104Google Scholar
22. Delcker A, Schoon ML, Oczkowski B, Gaertner HJ: Amisulpride versus haloperidol in treatment of schizophrenic patients—results of a double-blind study. Pharmacopsychiatry 1990; 23:125-130Crossref, Medline, Google Scholar
23. Möller HJ, Boyer P, Fleurot O, Rein W: Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. Psychopharmacology (Berl) 1997; 132:396-401Crossref, Medline, Google Scholar
24. Wetzel H, Grunder G, Hillert A, Philipp M, Gattaz WF, Sauer H, Adler G, Schroder J, Rein W, Benkert O: Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology—a double-blind controlled study comparing a selective D-2-like antagonist to a mixed D-1-/D-2-like antagonist. Psychopharmacology (Berl) 1998; 137:223-232Crossref, Medline, Google Scholar
25. Puech A, Fleurot O, Rein W: Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs haloperidol. Acta Psychiatr Scand 1998; 98:65-72Crossref, Medline, Google Scholar
26. Carrière P, Bonhomme D, Lempérière T (Amisulpride Study Group): Amisulpride has superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study. Eur Neuropsychopharmacol 2000; 15:321-329Google Scholar
27. Colonna L, Saleem P, Dondey-Nouvel L, Rein W (Amisulpride Study Group): Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Int Clin Psychopharmacol 2000; 15:13-22Crossref, Medline, Google Scholar
28. Paillère-Martinot M-L, Lecrubier Y, Martinot J-L, Aubin F: Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. Am J Psychiatry 1995; 152:130-133Link, Google Scholar
29. Speller JC, Barnes TRE, Curson DA, Pantelis C, Alberts JL: One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms—amisulpride v haloperidol. Br J Psychiatry 1997; 171:564-568Crossref, Medline, Google Scholar
30. Pichot P, Boyer P: Controlled double-blind multi-centre trial of low dose amisulpride versus fluphenazine in the treatment of the negative syndrome of chronic schizophrenia, in Amisulpride. Paris, Expansion Scientifique Française, 1989, pp 125-138Google Scholar
31. Saletu B, Küfferle B, Gruenberger J, Földes P, Topitz A, Anderer P: Clinical, EEG mapping and psychometric studies in negative schizophrenia: comparative trials with amisulpride and fluphenazine. Pharmacopsychiatry 1994; 29:125-135Google Scholar
32. Peuskens J, Bech P, Möller HJ, Bale R, Fleurot O, Rein W (Amisulpride Study Group): Amisulpride vs risperidone in the treatment of acute exacerbations of schizophrenia. Psychiatry Res 1999; 88:107-117Crossref, Medline, Google Scholar
33. Conley RR, Tamminga CA, Bartko JJ, Richardson C, Peszke M, Lingle J, Hegerty J, Love R, Gounaris C, Zaremba S: Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155:914-920Link, Google Scholar
34. Berk M, Brook S, Trandafir AI: A comparison of olanzapine with haloperidol in cannabis-induced psychotic disorder: a double-blind randomized controlled trial. Int Clin Psychopharmacol 1999; 14:177-180Medline, Google Scholar
35. Allan ER, Sison CE, Alpert M, Connolly B, Crichton J: The relationship between negative symptoms of schizophrenia and extrapyramidal side-effects with haloperidol and olanzapine. Psychopharmacol Bull 1998; 34:71-74Medline, Google Scholar
36. Copolov DL, Link CGG, Kowalcyk B: A multicentre, double-blind, randomized comparison of quetiapine (ICI 204,636, “Seroquel”) and haloperidol in schizophrenia. Psychol Med 2000; 30:95-105Crossref, Medline, Google Scholar
37. Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk SR, Marder SR, Mintz J: Risperidone versus haloperidol on secondary memory: can newer medications aid learning? Schizophr Bull 1999; 25:223-232Crossref, Medline, Google Scholar
38. Mercer G, Finlayson A, Johnstone E, Murray C, Owens DGC: A study of enhanced management in patients with treatment-resistant schizophrenia. J Psychopharmacol 1997; 11:349-356Crossref, Medline, Google Scholar
39. Wirshing DA, Marshall BD Jr, Green MF, Mintz J, Marder SR, Wirshing WC: Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999; 156:1374-1379Abstract, Google Scholar
40. Emsley RA (Risperidone Working Group): Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull 1999; 25:721-729Crossref, Medline, Google Scholar
41. Berk M, Brook S, Nur F: Risperidone compared to haloperidol in cannabis-induced psychotic disorder: a double-blind randomized controlled trial. Int J Psychiatry in Clin Practice 2000; 4:139-142Crossref, Google Scholar
42. Blin O, Azorin JM, Bouhours P: Antipsychotic properties of risperidone, haloperidol, and methotrimeprazine in schizophrenic patients. J Clin Psychopharmacol 1996; 16:38-44Crossref, Medline, Google Scholar
43. Liu SK, Chen WJ, Chang C-J, Lin H-N: Effects of atypical neuroleptics on sustained attention deficits in schizophrenia: a trial of risperidone versus haloperidol. Neuropsychopharmacology 2000; 22:311-319Crossref, Medline, Google Scholar
44. See RE, Fido AA, Maurice M, Ibrahim MM, Salama GMS: Risperidone-induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Biol Psychiatry 1999; 45:1653-1656Crossref, Medline, Google Scholar
45. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789-796Crossref, Medline, Google Scholar
46. Wahlbeck K, Cheine M, Essali A, Adams C: Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry 1999; 156:990-999Abstract, Google Scholar
47. Reynolds GP: What is an atypical antipsychotic? J Psychopharmacol 1997; 11:195-199Crossref, Medline, Google Scholar
48. Möller HJ: Aktuelle Bewertung neuer/atypischer Neuroleptika. Nervenarzt 2000; 71:329-344Crossref, Medline, Google Scholar
49. Möller HJ, Müller H, Borison RL, Schooler NR: A path-analytical approach to differentiate between direct and indirect effects on negative symptoms in schizophrenia: a re-evaluation of the North American risperidone study. Eur Arch Psychiatry Clin Neurosci 1995; 245:45-49Crossref, Medline, Google Scholar
50. Tollefson GD, Sanger TM: Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry 1997; 154:466-474Link, Google Scholar
51. Wehnert A, Mack R, Stilwell C, Rasmussen C, Silber D: Direct effect of sertindole on the primary negative symptoms of schizophrenia: a PATH analysis. Biol Psychiatry 1997; 42(suppl 1):188SGoogle Scholar
52. Kudo Y, Nomura J, Ikawa G, Nakajima T, Saito M, Sakai T, Yishimasu F, Hanada M, Kuroda S, Watanabe S, Yamawaki S, Nobutada T, Nakane Y, Tanaka M (ICI 204 636 Clinical Evaluation Group): Clinical trial of quetiapine in schizophrenia—efficacy and tolerability of quetiapine: a comparative double-blind study with mosapramine in schizophrenic patients, in Psychiatry on New Thresholds: Abstracts of the XI World Congress of Psychiatry, vol 2. New York, World Psychiatric Association, 1999, p 250Google Scholar
53. Coulouvrat C, Dondey-Nouvel L: Safety of amisulpride (Solian): a review of 11 clinical studies. J Clin Psychopharmacol 1999; 14:209-218Crossref, Google Scholar
54. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686-1696Abstract, Google Scholar
55. Taylor DM, McAskill R: Atypical antipsychotics and weight gain—a systematic review. Acta Psychiatr Scand 2000; 101:416-432Crossref, Medline, Google Scholar
56. Wahlbeck K, Tuunainen A, Ahokas A, Leucht S: Dropout rates in randomised antipsychotic drug trials. Psychopharmacology (Berl) 2001; 155:230-233Crossref, Medline, Google Scholar
57. Geddes J, Freemantle N, Harrison P, Bebbington P: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Br Med J 2000; 321:1371-1376Crossref, Medline, Google Scholar
58. Arnt J, Skarsfeldt T: Do novel antipsychotics have similar pharmacological characteristics? a review of the evidence. Neuropsychopharmacology 1998; 18:63-101Crossref, Medline, Google Scholar
59. Bymaster F, Perry KW, Nelson DL, Wong DT, Rasmussen K, Moore NA, Calligaro DO: Olanzapine: a basic science update. Br J Psychiatry 1999; 174(suppl 37):36-40Google Scholar
60. Goldstein JM, Litwin LC, Sutton EB, Malick JB: Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology (Berl) 1993; 112:293-298Crossref, Medline, Google Scholar
61. Kramer MS, Last B, Getson A, Reines SA: D4 dopamine antagonist group: the effects of a selective D4 dopamine receptor antagonist (L-745,870) in acutely psychotic inpatients with schizophrenia. Arch Gen Psychiatry 1997; 54:567-572Crossref, Medline, Google Scholar
62. Taubes G: Will new dopamine receptors offer a key to schizophrenia? Science 1994; 265:1034-1035Crossref, Medline, Google Scholar
63. Lahti AC, Weiler MA, Corey PK, Lahti RA, Carlsson A, Tamminga CA: Antipsychotic properties of the partial dopamine agonist (-)-3-(3-hydroxyphenyl)-N-n-propylpiperidine (preclamol) in schizophrenia. Biol Psychiatry 1998; 43:2-11Crossref, Medline, Google Scholar
64. Volonté M, Monferine E, Cerutti M, Fodritto F, Borsini FRA: BIMG 80, a novel potential antipsychotic drug: evidence for multireceptor actions and preferential release of dopamine in prefrontal cortex. J Neurochem 1997; 69:182-190Crossref, Medline, Google Scholar



