Catechol O-Methyltransferase Genetic Polymorphism in Panic Disorder
Abstract
OBJECTIVE: The authors examined the distribution of catechol O-methyltransferase (COMT) genotypes in patients with panic disorder as well as the relationship between a COMT polymorphism and the clinical characteristics of these patients. METHOD: Fifty-one patients with panic disorder and 45 healthy comparison subjects were tested for a genetic polymorphism of COMT. Clinical variables were assessed for the patients with panic disorder. RESULTS: The frequency of the L/L genotype was significantly higher in the patients with panic disorder than in the healthy subjects (19.6% versus 2.2%). Panic disorder was significantly associated with the L allele and L/L genotype. Patients with panic disorder who had the L/L genotype showed poorer treatment response than those with other genotypes. CONCLUSIONS: These results suggest that the L/L genotype of the COMT gene may be related to the development and treatment outcome of panic disorder in some patients.
Catechol O-methyltransferase (COMT) is a ubiquitous enzyme that is involved in the inactivation of catecholamines (1). Lachman et al. (2) showed that a genetic locus modulating COMT activity exhibited allelic variation: the COMTH(H) allele, associated with high activity, encodes for valine, and the COMTL(L) allele, associated with low activity, encodes for methionine at position 108/158 in the cytosolic/membrane bound form of the protein. The difference in COMT activity resulting from this genetic polymorphism is substantial (threefold to fourfold). In view of the fact that somatic symptoms of anxiety and panic disorder are closely related to the activation of catecholamines and sympathetic pathways, the COMT gene may have a role in the etiology of panic disorder (3).
The main goal of the current study was to determine the association between COMT genotypes and panic disorder. We also examined the association between COMT genotypes and clinical characteristics of panic disorder, including age at onset, presence of agoraphobia, and treatment response.
Method
Fifty-one unrelated patients who met DSM-IV criteria for panic disorder (26 men, 25 women, mean age=40 years, SD=8) participated in the study, and 45 healthy, sex-matched subjects (23 men, 22 women, mean age=37 years, SD=5) were included for comparison. All patients were recruited from Samsung Medical Center in Seoul, Korea; comparison subjects were recruited from workers at the same hospital through advertisement. After a complete description of the study was given to the subjects, written informed consent was obtained. This study was approved by the Institutional Review Board of Samsung Medical Center.
The patients had no comorbid major medical illnesses and no psychiatric illnesses other than panic disorder. They were treated with paroxetine, 20–40 mg/day, for more than 3 months. Comparison subjects were carefully assessed by two physicians to exclude any illnesses that may be related to abnormalities in catecholamine levels.
Genomic DNA was extracted from peripheral blood leukocytes by using a standard phenol-chloroform method. The Val158Met polymorphism of the COMT gene (gene map locus: chromosome 22q11.2, G/A1947 polymorphism in HSCOMT 2 gene, gene bank accession number z26491, dbSNP: rs165388) was tested from a 217-bp polymerase chain reaction product amplified by using the oligonucleotide primers 5′-TCG TGG ACG CCG TGA TTC AGG-3′ and 5′-AGG TCT GAC AAC GGG TCA GGC-3′ (Bioneer, Seoul, Korea) as described elsewhere (4). The polymerase chain reaction product was digested by the restriction enzyme Nla III (New England Biolabs, Beverly, Mass.) for 6 hours at 37°C. After electrophoresis with 100 V for 35 minutes on 2% Metaphor agarose gels (FMC, Rockland, Maine) containing 0.5% ethidium bromide, the gels were photographed under ultraviolet light. Restriction fragments of 114, 83, and 20 bp revealed the COMTH allele, and 96, 83, 20, and 18 bp revealed the COMTL allele.
We used the Clinical Global Impression to assess clinical variables, including treatment response, in the patients with panic disorder. For statistical analysis we used the chi-square test for categorical measures and analysis of variance for comparing continuous measures. Odds ratios were estimated to evaluate an association between COMT genotypes and panic disorder. Analyses were run on SPSS software, version 10.0 (SPSS, Chicago).
Results
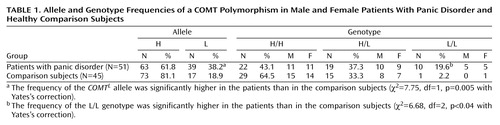
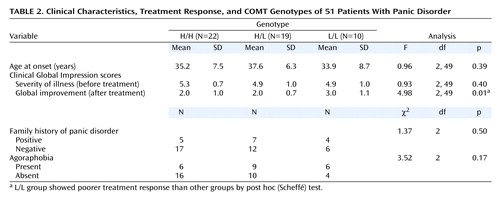
The frequency of the COMTL allele was significantly higher in patients with panic disorder than in healthy comparison subjects, and the frequency of the L/L genotype was also higher in patients than in comparison subjects (Table 1). There was a marked dose-related effect, in that the odds ratio for panic disorder was 7.9 for H/L and 13.2 for L/L. Patients with panic disorder who had the L/L genotype showed poorer treatment response to pharmacotherapy than those with H/L and L/L genotypes (Table 2). We did not find any significant difference in gender, age at onset, family history of panic disorder, presence of agoraphobia, and pretreatment severity of illness among the three different genotypes in patients with panic disorder.
Discussion
This study revealed that COMT activity might be related to susceptibility to panic disorder in that significantly higher proportions of the COMTL allele and L/L genotype were observed in patients with panic disorder than in healthy comparison subjects. The genotype distribution was significantly different, with evidence of a dose-dependent effect of the low activity allele.
Although the possibility that the COMT gene might have a role in panic disorder is intriguing, the results of previous reports on this issue have not been consistent (5, 6), possibly because of ethnic differences or different sample sizes. The allelic frequencies of COMTL were about 50% for Caucasians, 18% for Han Chinese, and 29% for Japanese, and the frequencies of L/L genotype were about 25% for Caucasians, 3% for Han Chinese, and 6% for Japanese (4, 5, 7). The relatively low COMTL frequency in Asian subjects may suggest a possible role of a COMT polymorphism in panic disorder. Lifetime prevalence rates of panic disorder in Taiwan, Japan, and Korea are as low as 0.4%, 1.0%, and 1.7%, respectively, but the rate in the U.S. population is 3.5%, according to estimates from the National Comorbidity Study (8–11). Thus the low frequency of the COMTL genotype in Asian populations may explain this ethnic difference in the prevalence of panic disorder.
It should be noted that patients with panic disorder who have the L/L genotype showed poorer treatment response to pharmacotherapy than patients with other genotypes. Different COMT genotypes led to different levels of catecholaminergic activity in patients with panic disorder, which might have an effect on the response to pharmacotherapy. Thus our results suggest that a COMT genetic polymorphism may be an important factor in the development and treatment response of panic disorder.
 |
 |
Received Oct. 15, 2001; revision received May 14, 2002; accepted May 23, 2002. From the Department of Neuropsychiatry, Seoul Paik Hospital, Inje University, Seoul, Korea; the Department of Psychiatry, Samsung Medical Center, Sungkyunkwan University School of Medicine; and the Department of Biochemistry, School of Medicine, Eulji University, Taejon, Korea. Address reprint requests to Dr. Yu, Department of Psychiatry, Samsung Medical Center, 50 Ilwon-Dong, Gangnam-Gu, Seoul, Korea, 135-710; [email protected] (e-mail). This research was supported by Korea Research Foundation grant KRF-2000-003-F00158.
1. Axelrod J, Tomchick R: Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem 1958; 233:702-705Medline, Google Scholar
2. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM: Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996; 6:243-250Crossref, Medline, Google Scholar
3. Wilkinson DJC, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD: Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry 1998; 55:511-520Crossref, Medline, Google Scholar
4. Li T, Vallada H, Curtis D, Arranz M, Xu K, Cai G, Deng H, Liu J, Murray R, Liu X, Collier DA: Catechol-O-methyltransferase Val158Met polymorphism: frequency analysis in Han Chinese subjects and allelic association of the low activity allele with bipolar affective disorder. Pharmacogenetics 1997; 7:349-353Crossref, Medline, Google Scholar
5. Ohara K, Nagai M, Suzuki Y, Ochiai M, Ohara K: No association between anxiety disorders and catechol-O-methyltransferase polymorphism. Psychiatry Res 1998; 80:145-148Crossref, Medline, Google Scholar
6. Hamilton SP, Slager SL, Heiman GA, Deng Z, Haghighi F, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA: Evidence for a susceptibility locus for panic disorder near the catechol-O-methyltransferase gene on chromosome 22. Biol Psychiatry 2002; 51:591-601Crossref, Medline, Google Scholar
7. Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, McGuffin P, Owen MJ: No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry 1996; 153:268-270Link, Google Scholar
8. Hwu HG, Yeh EK, Chang LY: Prevalence of psychiatric disorders in Taiwan defined by the Chinese Diagnostic Interview Schedule. Acta Psychiatr Scand 1989; 79:136-147Crossref, Medline, Google Scholar
9. Aoki Y, Fujihara S, Kitamura T: Panic attacks and panic disorder in Japanese non-patient population: epidemiology and psychosocial correlates. J Affect Disord 1994; 32:51-59Crossref, Medline, Google Scholar
10. Lee CK, Kwak YS, Yamamoto J, Rhee H, Kim YS, Han JH, Choi J, Lee YH: Psychiatric epidemiology in Korea, I: gender and age differences in Seoul. J Nerv Ment Dis 1990; 178:242-246Crossref, Medline, Google Scholar
11. Eaton WW, Kessler RC, Wittchen HU, Magee WJ: Panic and panic disorder in the United States. Am J Psychiatry 1994; 151:413-420Link, Google Scholar



