Posttraumatic Stress Symptoms and Salivary Cortisol Levels
Abstract
OBJECTIVE: This study assessed the relationship between posttraumatic stress symptoms and salivary cortisol levels after a severe ice storm. METHOD: Posttraumatic stress symptoms (Impact of Event Scale scores) and salivary cortisol levels were determined in 115 victims of an ice storm and in 27 healthy comparison subjects 1 month and approximately 1 year after the ice storm. RESULTS: One month after the storm, Impact of Event Scale scores for the victims (mean=20.31, SD=15.23) exceeded those of the comparison subjects (mean=5.30, SD=9.78) but were reduced approximately 1 year later (mean=14.01, SD=13.68). A quadratic relation was found to exist between Impact of Event Scale scores and cortisol levels. CONCLUSIONS: One month after the storm, cortisol levels were found to be elevated among the victims but were diminished among those with the highest Impact of Event Scale scores. This relationship was found not to exist approximately 1 year later.
Increasing attention has focused on hypothalamic-pituitary-adrenal functioning associated with posttraumatic stress disorder (PTSD) (1). Although stressors ordinarily increase circulating cortisol levels (2), PTSD has been associated with reduced levels of plasma, urinary, and salivary cortisol (1). As PTSD is also associated with enhanced dexamethasone-induced suppression of cortisol, elevated corticotropin-releasing hormone (CRH) levels in CSF, and a blunted ACTH response to challenges with CRH (3, 4), it is likely that with a sufficiently intense or protracted trauma, enhanced negative feedback involving hypothalamic or pituitary mechanisms may culminate in reduced cortisol secretion (1, 2).
Typically, analysis of cortisol levels has been conducted by comparing individuals with or without posttraumatic stress symptoms. The present investigation assessed salivary cortisol levels among individuals differentially exposed to a common stressor (a severe and debilitating ice storm) and among those who exhibited varying degrees of posttraumatic stress symptoms and determined whether disturbances in cortisol levels were evident both 1 month and approximately 1 year after the stressor occurred.
Method
The victims of the ice storm were 37 men (mean age=51.10 years, SD=2.09) and 78 women (mean age=9.49 years, SD=1.50) who were recruited by means of media presentations or by referral from the volunteers (their neighbors). The individuals most affected by the ice storm came from the regions surrounding Ottawa, Canada, including farming areas and towns with populations of fewer than 10,000 people. Of this group of 115, 61 (53.0%) reported financial losses, and 92 (80.0%) reported having to leave their homes because of loss of electricity and/or water, ranging from 3 to 21 days (mean=11.27, SD=4.29). The participants experienced significant self-reported sleep disturbances and anxiety regarding their personal safety and possessions. The comparison participants were 10 men (mean age=47.50 years, SD=3.91) and 17 women (mean age=48.17 years, SD=3.34) residing within Ottawa who experienced only transient electrical outages (of less than 6 hours).
After written informed consent was obtained, the participants completed the revised Impact of Event Scale (5) and the Mini International Neuropsychiatric Interview (6) to confirm past or present depression. Exclusion criteria included viral illness during the preceding 2 weeks or any medical disorder requiring drug treatment that may have affected glucocorticoid activity. None was using psychotropic medication. The participants provided a morning saliva sample (from 9:00 a.m. to 11:00 a.m.), using dental cotton inserted into their cheek for about 2 minutes. The saliva was extracted by centrifugation and stored at –70°C.
Approximately 1 year after the storm, 80 participants were recontacted, and 77 agreed to a follow-up session. The initial Impact of Event Scale scores of those who continued in the study (mean=20.17, SD=14.12) were comparable to the scores of those who did not (mean=20.05, SD=17.56). The participants completed the Impact of Event Scale and provided a saliva sample approximately 30 minutes after awakening (before 11:00 a.m.). Salivary cortisol levels were determined in duplicate by means of a solid-phase radioimmunoassay by using iodine 125 kits obtained from ICN Biomedicals Inc. (Costa Mesa, Calif.). The intra- and extraassay variability of these kits was less than 10%.
Two- (victims versus comparison subjects) by-two (1 month versus 1 year) mixed-model analyses of variance were conducted to examine differences in Impact of Event Scale scores and salivary cortisol levels. Independent t tests assessed sex differences between the victims. Effect sizes were reported as η2, indicating the proportion of variance accounted for (7). Polynomial regression analyses were used to predict cortisol levels from posttraumatic stress symptom profiles.
Results
The victims had higher total Impact of Event Scale scores (mean=20.31, SD=15.23) than did the comparison subjects (mean=5.30, SD=9.78) (F=23.01, df=1, 89, p<0.001, η2=0.21), which was evident with respect to both the intrusiveness subscale score (mean=11.80, SD=8.94; mean=2.81, SD=5.21, respectively) and the avoidance subscale score (mean=8.31, SD=7.02; mean=2.49, SD=5.29). Although Impact of Event Scale scores declined over the 1-year period of the study (mean=14.01, SD=13.68) (F=11.66, df=1, 89, p<0.001, η2=0.12), the group-by-time interaction was not significant (F=0.20, df=1, 89, p=0.66). Impact of Event Scale scores 1 year later were related to the scores seen 1 month after the ice storm (r=0.62, df=78, p<0.001).
Although the male (mean=17.79, SD=14.58) and female (mean=21.62, SD=15.96) victims’ Impact of Event Scale scores did not differ (t=–1.22, df=109, p=0.22), of those scoring above 40 at the initial testing, 13 were women (16.7% of the 78 women tested) and only one was a man (2.7% of the 37 men tested). At the 1-year follow-up, all four participants who scored above 40 were women. Mean scores at 1 year were 12.02 (SD=13.01) for women and 10.46 (SD=14.75) for men.
Because of attrition of the participants providing saliva samples by the 1-year follow-up, simple comparisons between the victim and comparison groups were conducted at each time point. One month after the storm, the victims’ cortisol levels (mean=0.17 μg/dl, SD=0.08) exceeded those of the comparison subjects (mean=0.13 μg/dl, SD=0.06) (F=6.12, df=1, 117, p<0.05, η2=0.03). Among the victims, the cortisol levels of the men (mean=0.17 μg/dl, SD=0.01) and the women (mean=0.17 μg/dl, SD=0.01) did not differ (t=–0.56, df=102, p=0.58). One year later, the cortisol levels were comparable in the victims (mean=0.15 μg/dl, SD=0.14) and the comparison subjects (mean=0.12 μg/dl, SD=0.05) (F=0.54, df=1, 91, p=0.47). The cortisol levels 1 month after the ice storm were not significantly related to the cortisol levels 1 year later (r=–0.09, df=75, p=0.45).
A polynomial regression using the victims’ responses did not reveal a linear relation between Impact of Event Scale scores and cortisol levels 1 month after the ice storm (B=0.05, t=0.52, df=100, p=0.60). In contrast, the addition of the quadratic term significantly predicted cortisol activity 1 month after the ice storm (R2=0.07, F=3.65, df=2, 99, p<0.05). As shown in Figure 1, the participants who experienced the highest Impact of Event Scale scores 1 month after the storm had relatively low cortisol levels. Neither linear nor quadratic Impact of Event Scale scores predicted cortisol levels 1 year after the ice storm (F=0.52, df=2, 69, p=0.59).
Discussion
The ice storm increased Impact of Event Scale scores among the victims, with 14 (12.2%) of the participants reporting scores in excess of 40 after 1 month. Consistent with the findings of a previous report (8), the women were more prone to develop posttraumatic stress symptoms than the men. Although the Impact of Event Scale scores were still elevated 1 year after the storm, symptom severity was reduced, as only four of 77 (5.2%) of the participants still scored in excess of 40. However, posttraumatic stress symptoms were evaluated only by the Impact of Event Scale score, and an important feature of PTSD—namely, hyperarousal—was not assessed by the version of the Impact of Event Scale that was used. Thus, despite high Impact of Event Scale scores, it cannot be assumed that the participants met DSM-IV criteria for PTSD.
One month after the ice storm, the relation between posttraumatic symptoms and cortisol levels was not linear but, instead, quadratic. Although cortisol levels were generally increased among the ice storm victims, among the participants with the highest Impact of Event Scale scores, cortisol levels were lower than among moderately stressed individuals. It has been suggested that activation of neuroendocrine and neural mechanisms may be of adaptive significance, but excessive or protracted strain on these systems (allostatic load) may result in behavioral or physical pathology (2). From this perspective, posttraumatic stress symptoms evolve over time after the traumatic event, as might the changes in cortisol levels.
One year after the ice storm, the cortisol levels among the victims did not differ from those of the comparison subjects, and the relation between Impact of Event Scale scores and cortisol levels was no longer evident. The saliva samples were collected over several morning hours; hence, it is possible that circadian variations in cortisol levels may have precluded detection of group differences. Nevertheless, the low levels of cortisol that were initially evident among the individuals exhibiting posttraumatic stress symptoms normalized, with a diminution of symptoms at lengthier poststress intervals.
Presented at the 15th annual meeting of the International Society for Traumatic Stress Studies, Miami, Nov. 14–17, 1999. Received Sept. 22, 2000; revisions received Jan. 31 and March 27, 2001; accepted April 19, 2001. From the Institute of Neuroscience, Carleton University; the Department of Psychiatry, Royal Ottawa Hospital, Ottawa, Ontario, Canada; the School of Psychology and the Institute of Cellular and Molecular Medicine, University of Ottawa, Ottawa, Ontario, Canada. Address reprint requests to Dr. Anisman, Institute of Neuroscience, Life Sciences Research Bldg., Carleton University, Ottawa, Ont. K1S 5B6 Canada; [email protected] (e-mail). Funded by the Natural Science and Research Council of Canada. Dr. Anisman is an Ontario Mental Health Senior Research Fellow. The authors thank Marilee Zaharia, Shawn Haley, Sandra Lefebvre, Jerzy Kulczycki, Judy McIntosh, Tania Bedard, Kelly Brennan, Sam Khan, and Dave Michaud for their help.
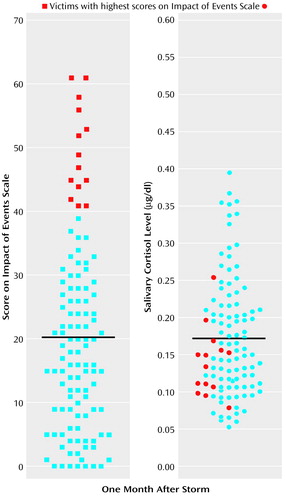
Figure 1. Impact of Event Scale Scores and Salivary Cortisol Levels for Victims of an Ice Storm Approximately 1 Month After the Eventa
aVictims with the highest Impact of Event Scale scores exhibited relatively low levels of salivary cortisol. Means are designated by black lines.
1. Yehuda R: Linking the neuroendocrinology of post-traumatic stress disorder with recent neuroanatomic findings. Semin Clin Neuropsychiatry 1999; 4:256-265Medline, Google Scholar
2. McEwen BS: Stress, adaptation, and disease: allostasis and allostatic load. Ann NY Acad Sci 1998; 840:33-44Crossref, Medline, Google Scholar
3. Yehuda R: Biology of posttraumatic stress disorder. J Clin Psychiatry 2000; 61:14-21Medline, Google Scholar
4. Newport DJ, Nemeroff CB: Neurobiology of posttraumatic stress disorder. Curr Opin Neurobiol 2000; 10:211-218Crossref, Medline, Google Scholar
5. Horowitz MJ, Wilner N, Alvarez W: Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41:209-218Crossref, Medline, Google Scholar
6. Sheehan DV, Lecrubier Y, Janavs J, Knapp E, Weiller E, Hergueta T, Bonora LI, Amorim P, Lepine JP, Sheehan MF, Baker RR, Sheehan KH: Mini International Neuropsychiatric Interview (MINI). Tampa, University of South Florida Institute for Research in Psychiatry, 1998Google Scholar
7. Hays WL: Statistics for Psychologists. New York, Holt, Rinehart & Winston, 1963Google Scholar
8. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048-1060Google Scholar



