Clinical and Cognitive Factors Associated With Verbal Memory Task Performance in Patients With Schizophrenia
Abstract
OBJECTIVE: The authors have previously shown the role of depression, slowing of processing speed, and selective attention deficit in verbal memory task performance in schizophrenia. They wished to determine the specific contribution of each of these factors to various types of memory impairment. METHOD: The negative symptom score from the Positive and Negative Syndrome Scale, the Hamilton Depression Rating Scale score, a measure of processing speed, and a measure of selective attention were entered as predictors in regression analyses. Furthermore, analyses of covariance were conducted on the memory measures to test the significance of the differences between schizophrenic patients and healthy comparison subjects after control for processing speed and selective attention. RESULTS: Depression was associated only with deep encoding reflected by semantic clustering. Selective attention was associated only with superficial encoding reflected by serial recall. Slowing of processing speed was associated with both deep and superficial encoding. Negative symptoms were not associated with memory impairment except for the avolition item from the Scale for the Assessment of Negative Symptoms. Processing speed accounted for all the group differences on the memory measures that reflected superficial encoding. In addition, a subgroup of patients with no or minor depression was not significantly impaired on deep encoding relative to the healthy comparison group. CONCLUSIONS: The authors suggest that verbal memory impairment in schizophrenia is a consequence of depression and slowness, rather than a primary feature of the disease.
Memory impairment has been well documented in schizophrenia (1). However, whether this deficit is a primary feature of the disease or a consequence of other factors is unclear. Our group has carried out a series of independently published studies to investigate the extent to which various clinical and cognitive factors were associated with memory deficits in patients with schizophrenia.
Negative symptoms are generally thought to be a factor in cognitive impairment in schizophrenia, and an association between negative symptoms score and memory deficit has been revealed by a recent meta-analysis (1). However, this association has not been consistently observed. One reason for these discrepancies may be differences in the scales used for rating negative symptoms. Indeed, there is no consensus about which symptoms should be included in a negative symptom scale. A previous study by our group (2) showed that only the avolition item from the Scale for the Assessment of Negative Symptoms (SANS) (3) was correlated with global verbal memory score. On the other hand, no item from the negative symptom score from the Positive and Negative Syndrome Scale (4) was correlated with it. This suggests that studies that use the SANS are more likely to reveal correlations with memory efficiency.
Another factor that has been shown to affect memory in various populations is depression (5), which impedes effortful but not automatic processes (6). Correlations between memory impairment and depression severity have been observed in depressed patients (7). Depression is commonly observed in patients with schizophrenia (8). However, its role in cognitive functioning has not been generally studied in this population, in which positive and negative symptoms are most often targeted. Our group has shown that depression severity is correlated with various verbal memory measures in a group of schizophrenic patients (9). In particular, it is correlated with the measures that rely on effortful encoding. However, depression has several components (including psychomotor slowing, lack of motivation, and fatigue), and it would be interesting to investigate which are mainly responsible for this association. In our study group, data from SANS scores were available. This enabled us to investigate further the association of avolition with various memory measures and therefore to examine whether the pattern of association was the same as for depression. Our reasoning was that if depression and avolition led to similar patterns of impairment, it would suggest that the role of depression on memory performance is mediated by a motivational deficit.
Our group has also demonstrated the role of the slowing of processing speed on verbal memory impairment in schizophrenia. Processing speed refers to the rate at which elementary operations can be performed. Slowing in this function has been the object of a growing body of research in elderly subjects and is now considered a main factor of their memory deficits relative to young subjects (10, 11). Slowing has also been shown to play a role in memory performance in other populations, such as children (12) and persons with depression (7, 13). However, its role in schizophrenia has been the object of few studies. Our group has shown that the slowing of processing speed is related to various verbal memory measures in these patients, including a deficit at the encoding stage (14). Two other groups have reported a relationship between slowing and cognitive impairment in schizophrenia (15, 16). Slowing of processing speed was correlated with depression in our patient group (2). Therefore, it is possible that the just-referenced role of depression on memory impairment is mediated by psychomotor slowing, as assessed by a behavioral measure of processing speed. This question may be addressed by studying these two factors together in a regression analysis that would reveal the specific contribution of each. If the effects of depression are accounted for by its psychomotor slowing component, then the contribution of depression should no longer be significant after control for the measure of processing speed.
Finally, several studies have shown that patients with schizophrenia, or people at risk for schizophrenia, were disproportionately disturbed during serial recall by the presence of distractors (17–20). This suggests that in these patients, higher distractibility, which is equivalent to a deficit in selective attention, interferes with serial learning. This was confirmed in our study (21).
In the current study, in which we combined all these published data, we investigated further the role of these factors in verbal memory. Our study involved free recall and recognition tasks. We distinguished two levels of encoding (22) that corresponded to effortful versus more automatic processes. Superficial encoding was assessed in both short-term and long-term memory by the ability to learn series of items by means of rehearsal. Deep encoding, assumed to be more effortful, was assessed by the ability to organize the information in semantic clusters. The four just-mentioned factors—negative symptoms, depression, processing speed, and selective attention—were entered as predictors in regression analyses so that the specific contribution of each to various memory measures could be assessed. Furthermore, we studied the specific association of the avolition item from the SANS with the memory measures. Finally, we compared our patient group with a healthy comparison group before and after controlling for the factors that were related to memory task performance. The purpose of those analyses was to address the following questions:
1. Is the role of depression severity on memory impairment accounted for by the psychomotor slowing commonly observed in depression?
2. Is the role of depression severity on memory impairment accounted for by lack of motivation?
3. To what extent would memory functions be restored if schizophrenic patients were neither depressed, distractible, nor slow?
Method
Participants
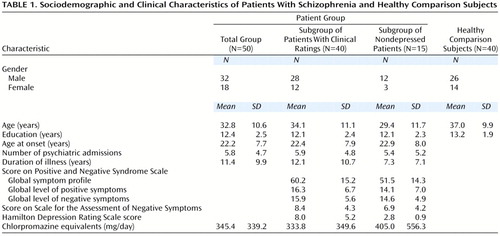
Fifty inpatients who met the DSM-IV criteria for schizophrenia or schizoaffective disorder, screened to rule out history of neurological or other nonpsychiatric disorders and recent alcohol abuse or drug addiction, were included in our patient group. Their demographic and clinical characteristics are presented in Table 1. They were recruited from the Schizophrenia Research Unit at the New York State Psychiatric Institute and Creedmoor Psychiatric Center. All patients were taking antipsychotic medication, either typical (haloperidol, fluphenazine, or chlorpromazine) or atypical (clozapine or risperidone), and were taking fixed doses at testing. These patients and healthy comparison subjects were also the subjects in our previously mentioned reports. Forty of these patients were assigned clinical ratings. The characteristics of this subgroup are presented in Table 1.
Forty healthy comparison subjects were recruited from a pool of normal volunteers from the Mental Health Clinical Research Center at the New York State Psychiatric Institute and from advertising (Table 1). They were screened to rule out any current or recent psychiatric or neurologic history, alcohol abuse, or drug addiction. The healthy comparison group and the total schizophrenic group were not significantly different with regard to age, education level, or gender distribution. Written informed consent was obtained from each patient and comparison subject after the procedures had been fully explained.
Material and Procedure
Memory tasks
Six equivalent lists of 16 concrete, highly common words were constructed. Two of the lists were organizable into four different semantic categories, with four words in each category. The 16 words of these organizable lists were randomized. One of the nonorganizable lists and one of the organizable lists were used for the immediate free recall task. One of each was also used for delayed free recall. The two remaining nonorganizable lists were used for the recognition task. Two recognition sheets were prepared, each including the 16 words of the recognition list mixed with 16 distractors that were equivalent in length and frequency.
Subjects were given a sheet displaying a list of 16 words and told that they had 30 seconds to learn it. Then they were required to write down as many words as they could remember, in any order and without any time limit. This free recall was required immediately after the learning phase for two of the lists and after a 1-minute delay for the two other lists. In the delayed condition, subjects were asked to read a passage of a book out loud for 1 minute. All list presentation and recall conditions were counterbalanced. Subjects were not warned of the possible organization of the lists into semantic categories during the learning stage.
The total number of words recalled in the four lists was used as a measure of global memory efficiency in free recall. Superficial encoding was assessed in each of the four lists by a sequence index, which was defined as the proportion of words recalled in their order of presentation out of the total number of recalled words. This index reflected the propensity to learn the words of the list by heart through rehearsal, without using their possible semantic organization. The number of words recalled in sequence was computed for each list, reflecting the efficiency of this superficial encoding strategy. An averaged sequence index and an averaged number of words recalled in sequence were computed across the four lists.
Deep encoding was assessed by a categorization index, which was computed from the results of the recall of the two semantically organizable lists. It was defined as the number of words following a word of the same semantic category in the recalled list, divided by the number of such possible associations. This index reflected the propensity to encode words according to their semantic properties. The efficiency of this strategy was then assessed by two indices: the number of categories recalled and the number of recalled words within each category. These indices were averaged across the two organizable lists. Storage of the information over time was assessed by the difference between the total number of words recalled in immediate versus delayed recall.
The last two nonorganizable lists were learned in the same conditions as in the free recall task. After the learning phase, subjects were given the recognition sheet and required to circle the words that they recognized from the previously learned list. One list was followed by immediate recognition and the other one by 1-minute delayed recognition. All conditions were counterbalanced. The discrimination accuracy index Pr from the two-high threshold theory (23), which reflects the ability to discriminate target words from distractors, was computed for both lists. An average Pr index was derived.
The forward and backward digit span tests of the WAIS-R (24) were administered to assess short-term memory. Increasingly long series of digits had to be repeated, either in the same or in reverse order. The total number of correct trials was tallied.
Predictors
One of three clinicians who was blind to the cognitive scores completed symptom evaluations for 40 of the patients. Raters were trained to high reliability with each other (kappa>0.80). The experimenter (G.B.) was blind to all ratings of symptom profiles throughout patient testing. Scores from the Positive and Negative Syndrome Scale were used. Negative symptoms from this scale are the following: blunted affect, emotional withdrawal, poor rapport, passive or apathetic social withdrawal, difficulty with abstract thinking, lack of spontaneity in flow of conversation, and stereotypical thinking. In addition, scores from the SANS were available for 33 patients. That measure includes the following items: alogia, affective flattening, avolition, anhedonia or asociality, and attentional impairment. Scores from the Hamilton Depression Rating Scale (25) were also used.
The digit symbol substitution test of the WAIS-R was administered to assess processing speed. Several rows of empty boxes labeled with a digit were presented on a sheet. Subjects were required to write a symbol in the empty boxes as quickly as they could according to a digit/symbol code that was permanently displayed. The number of boxes that had been correctly filled in after 1.5 minutes constituted a measure of processing speed. The color-naming time from the Stroop Color and Word Test (26) was used as a second measure. These two measures were highly correlated with each other in both schizophrenic and healthy comparison groups. Therefore, they were averaged after z transformation to create a global measure of processing speed.
The Stroop Color and Word Test was also used to assess selective attention. Subjects first had to call out names of blocks of colors displayed on a sheet as quickly as they could, which constituted the color-naming condition. Then they were given a sheet displaying names of colors printed in a different color (e.g., “RED” printed in blue) and were required to name the color of the ink as quickly as they could while inhibiting the reading of the words. This constituted the color-word-naming condition. The times for both conditions (color-naming time and color-word-naming time) were recorded. A measure of selective attention was computed by the percentage of the increment of time spent during the Stroop color-word naming condition relative to during the color-naming condition. One subject—who did not belong to the subgroup with clinical ratings—was not administered the Stroop test because he was color-blind.
Results
Intercorrelations Among Predictors
The Positive and Negative Syndrome Scale negative symptom score was significantly correlated with the avolition item score from the SANS (r=0.49, df=31, p<0.005). On the other hand, the Positive and Negative Syndrome Scale negative symptom score was not significantly correlated with the Hamilton depression scale score, the global measure of processing speed, or the measure of selective attention. The depression score was significantly correlated with the avolition score (r=0.34, df=31, p<0.05) and processing speed (r=–0.50, df=38, p<0.001), but not with the selective attention score. Processing speed was not significantly correlated with the avolition or selective attention scores.
Associations Between Predictors and Memory Measures
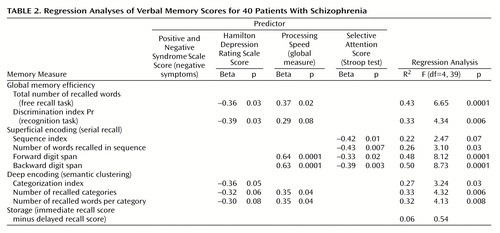
Regression analyses were conducted on all memory measures to assess the specific contribution of each predictor. The Positive and Negative Syndrome Scale negative symptom score, the Hamilton depression scale score, the global measure of processing speed, and the selective attention measure were entered as predictors for each regression analysis.
The results are presented in Table 2. Depression was a significant predictor of the two measures of global memory efficiency and of the categorization index, reflecting the propensity to use deep encoding. This suggests that the role of depression on memory is not mediated by psychomotor slowing. By contrast, depression was not a predictor of any measure of superficial encoding. Processing speed was a significant predictor of the total number of recalled words, the two indices of efficiency of deep encoding, and two of the measures of superficial encoding. Selective attention was a significant predictor of all the measures of superficial encoding but none of the measures of deep encoding. The measure of storage of information over time was not related to any of the predictors.
The avolition rating was not entered as a predictor since it was not available for all the patients with clinical ratings. Furthermore, it was measured by one item from a scale rather than by a full scale, thus its range of variability was narrower than that of the other variables. Therefore, our approach was to study its correlation with each of the memory measures. The only significant correlation was with the total number of recalled words (r=–0.36, df=31, p<0.05). Contrary to the depression score, the avolition rating was not correlated at all with the indices of deep encoding. (The correlations of the categorization index, number of recalled categories, and number of recalled words per category with the avolition score were all nil. With the depression score, these correlations were –0.45, –0.46, and –0.47, respectively).
Role of Predictors on Differences Between Groups
The complete pattern of memory impairment in these schizophrenic patients has been described previously (27). All the memory measures on which the schizophrenic patients were significantly impaired relative to the healthy comparison group were submitted to three independent analyses of covariance (ANCOVAs), each involving only one covariate. In the first ANCOVA, the global measure of processing speed was entered as a covariate. In the second, the Stroop color time was entered to study the role of a simple motor speed measure on memory. In the last ANCOVA, the measure of selective attention was entered. The scores obtained by schizophrenic patients and comparison subjects on the memory measures and the significance of the differences before (t tests) and after (ANCOVAs) control for each factor are presented in Table 3.
It appears that the highly significant group difference on global memory efficiency in the free recall task remained unchanged after the global measure of processing speed had been controlled. By contrast, the highly significant difference on discrimination efficiency in recognition was no longer significant. Furthermore, the significance of the group difference in serial recall in both long-term and short-term memory was eliminated after control for only the motor speed measure. However, the two measures of efficiency in deep encoding remained significant after control for processing speed. As regards selective attention, its statistical control barely affected the significance of the group difference on any measure.
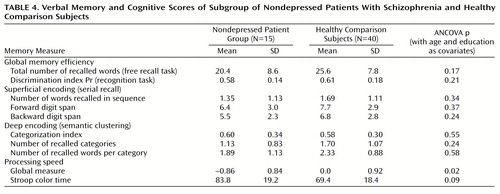
Depression score could not be entered as a covariate since no clinical rating was available for the healthy comparison subjects. We thus considered a subgroup of 15 patients with no significant depression (Hamilton depression scale score 5 or less). Their demographic and clinical characteristics are presented in Table 1. These patients were younger and less educated on average than the healthy comparison group; therefore, age and education levels were entered as covariates in all analyses.
The subgroup of 15 nondepressed patients and the healthy comparison group were compared on each memory measure by means of ANCOVAs. The results are presented in Table 4. It appears that these patients were not significantly impaired on any of the memory measures. In particular, their categorization index, reflecting effortful encoding, was similar to that of the comparison subjects. However, these patients were significantly slower than the comparison group.
Discussion
Our results suggest that the main factor affecting verbal memory task efficiency in patients with schizophrenia is depression. Indeed, depression severity was a significant predictor of the measures of global memory efficiency in both the free recall and recognition tasks and of the categorization index, which reflects the propensity to use effortful encoding. Furthermore, a subgroup of patients with no or very low depression was not significantly impaired on these measures when compared to the healthy comparison group. On the other hand, depression was not a predictor of any of the measures reflecting superficial encoding, and the subgroup of nondepressed patients was not different from the total patient group on these measures. Depression thus appears to affect the effortful processes of encoding while sparing the superficial processes that are assumed to be more automatic. This agrees with results observed in depressed populations.
Depression was significantly correlated with the slowing of processing speed, an association commonly reported in persons with depression (28, 29) and also observed in a schizophrenic sample (30). However, with our data, regression analyses suggested that the role of depression on memory performance is not mediated by the psychomotor slowing component of depression, since depression severity remained a significant predictor when the measure of processing speed was entered into the analyses. It does not seem to be mediated by a motivational deficit either, since no association between avolition rating and any measure of deep encoding was revealed. However, it should be noted that the avolition measure had a low variability; therefore, negative results should be interpreted with caution. The association between motivation and memory deficits in this population should be investigated with more detailed measures.
By contrast, the negative symptom score from the Positive and Negative Syndrome Scale, which was unrelated to depression, was not a significant predictor of any of the memory measures. This may be partly due to the lack of statistical power or differential reliability across the variables. However, avolition appears to be different from the other negative symptoms (2). It was correlated with the Positive and Negative Syndrome Scale negative symptom score, which suggests that avolition is actually a part of the negative symptom profile. However, unlike the other negative symptoms from either the Positive and Negative Syndrome Scale or the SANS, it was also correlated with the depression score. Although there is the possibility of spurious significance, our results are compatible with those of other studies that showed that depression scores were associated with the SANS score but not with the negative symptom score from the Positive and Negative Syndrome Scale or from another scale (2). The observed correlations with the SANS may have been mostly triggered by the avolition item, which is not included in the other scales. In addition to its correlation with depression, avolition was the only negative symptom that was significantly correlated with the global verbal memory score. However, avolition seems to affect memory in a different way than depression, which impedes effortful encoding.
Another main factor affecting verbal memory is the slowing of processing speed, whose contribution to the impairment seems to be in addition to that of depression. Indeed, slowing may be caused by factors other than being depressed. The association between the slowing of processing speed and memory impairment corroborates that observed in other populations, especially elderly subjects. Processing speed seems to be related to both superficial encoding and the efficiency of deep encoding, a pattern of associations different from that of depression. It is likely that the fastest subjects had time to rehearse the material more often at the encoding phase, hence enhancing the mnemonic trace (31).
Our results suggest that if schizophrenic patients were not slow, they would not be impaired in serial learning. Furthermore, they would not be impaired in the recognition task, which is easier than the free recall task and probably does not rely so heavily on deep encoding. Indeed, control for only a measure of motor speed was enough to reduce considerably the high significance of the group difference in recognition scores and to eliminate the significance of the difference between the serial learning scores. This is compatible with another study (15) showing that the deficits in serial recall and other not very demanding cognitive tasks in a sample of patients with schizophrenia were entirely accounted for by a slowing in articulation rate.
However, with control for processing speed, the differences between patients and comparison subjects on the measures that reflect the efficiency of categorization at encoding were still significant, as was the difference on global free recall efficiency. This suggests that even if the patients were not slower than comparison subjects, they would still show a deficit in effortful encoding, probably due to depression.
It should be noted that both depression and the slowing of processing speed affected the encoding phase of memory and not the retention of information over time—at least with the short delay studied. Besides, no impairment in retention ability was observed in our schizophrenic group (27). This enhances our point that depression and the slowing of processing speed are the main factors that affect verbal memory performance in schizophrenia and that the memory functions unrelated to them are not impaired. The finding that the slowing of processing speed mostly acts by impeding the ability to efficiently encode information is consistent with what has been observed in an elderly population (32).
As regards distractibility assessed by a deficit in selective attention, it appears to be consistently associated with serial learning. In other words, distractibility impedes the holding and rote rehearsal of sequences of items, a finding that is in agreement with those of other studies. However, distractibility does not seem to be a major factor in memory impairment in schizophrenia, since group differences remained the same when it was controlled. Finally, we have reported previously (2, 14, 21) that chlorpromazine-equivalent doses and type of neuroleptic medication (typical versus atypical) were unrelated to any of the studied cognitive or clinical measures in the patient group.
Conclusions
Our results suggest that verbal memory impairment in schizophrenia is not a primary deficit of the disease but, rather, is secondary to other clinical and cognitive factors. This pattern of associations must not be specific to schizophrenia. It is likely to be observed in other populations in which depression, avolition, psychomotor retardation, and/or greater distractibility are observed.
 |
 |
 |
 |
Received June 1, 2000; revision received Oct. 17, 2000; accepted Oct. 20, 2000. From the Schizophrenia Research Unit, New York State Psychiatric Institute, New York; Creedmoor Psychiatric Center, New York; and the Department of Psychiatry, Columbia University, New York. Address reprint requests to Dr. Brébion, Institute of Psychiatry, Department of Psychological Medicine, 103 Denmark Hill, London SE5 8AZ, U.K.; [email protected] (e-mail). Supported by a Fulbright grant and a Schizophrenia Research grant to Dr. Brébion and by NIMH Developing Center grant MH-50727. The authors thank Ivan Amodt, Henry Kronengold, Lynn Marcinko, and Scott Yale for their assistance.
1. Aleman A, Hijman R, de Haan EHF, Kahn RS: Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999; 156:1358–1366Google Scholar
2. Brébion G, Amador X, Smith M, Malaspina D, Sharif Z, Gorman J: Depression, psychomotor retardation, negative symptoms, and memory in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 2000; 13:177–183Medline, Google Scholar
3. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
4. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
5. Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern and specificity. Psychol Bull 1995; 117:285–305Crossref, Medline, Google Scholar
6. Hartlage S, Alloy LB, Vasquez C, Dykman B: Automatic and effortful processing in depression. Psychol Bull 1993; 113:247–278Crossref, Medline, Google Scholar
7. Smith MJ, Brébion G, Banquet J-P, Allilaire J-F: Experimental evidence for two dimensions of cognitive disorders in depressives. J Psychiatr Res 1994; 28:401–411Crossref, Medline, Google Scholar
8. Zisook S, McAdams LA, Kuck J, Harris MJ, Bailey A, Patterson TL, Judd LL, Jeste DV: Depressive symptoms in schizophrenia. Am J Psychiatry 1999; 156:1736–1743Google Scholar
9. Brébion G, Smith MJ, Amador X, Malaspina D, Gorman JM: Clinical correlates of memory in schizophrenia: differential links between depression, positive and negative symptoms, and two types of memory impairment. Am J Psychiatry 1997; 154:1538–1543Google Scholar
10. Nettelbeck T, Rabbitt PMA, Wilson C, Batt R: Uncoupling learning from initial recall: the relationship between speed and memory deficits in old age. Br J Psychol 1996; 87:593–607Crossref, Medline, Google Scholar
11. Salthouse TA: The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103:403–428Crossref, Medline, Google Scholar
12. Fry AF, Hale S: Processing speed, working memory, and fluid intelligence: evidence for a developmental cascade. Psychol Sci 1996; 7:237–241Crossref, Google Scholar
13. Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF: Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000; 30:679–691Crossref, Medline, Google Scholar
14. Brébion G, Amador X, Smith M, Gorman J: Memory impairment and schizophrenia: the role of processing speed. Schizophr Res 1998; 30:31–39Crossref, Medline, Google Scholar
15. Salamé P, Danion J-M, Peretti S, Cuervo C: The state of functioning of working memory in schizophrenia. Schizophr Res 1998; 30:11–29Crossref, Medline, Google Scholar
16. Schatz J: Cognitive processing efficiency in schizophrenia: generalized vs domain specific deficits. Schizophr Res 1998; 30:41–49Crossref, Medline, Google Scholar
17. Corrigan PW, Green MF: Signal detection analysis of short-term recall in schizophrenia. J Nerv Ment Dis 1991; 179:495–498Crossref, Medline, Google Scholar
18. Harvey PD, Serper MR: Linguistic and cognitive failures in schizophrenia: a multivariate analysis. J Nerv Ment Dis 1990; 178:487–494Crossref, Medline, Google Scholar
19. Harvey P, Winters K, Weintraub S, Neale JM: Distractibility in children vulnerable to psychopathology. J Abnorm Psychol 1981; 90:298–304Crossref, Medline, Google Scholar
20. Oltmanns TF, Neale JM: Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol 1975; 84:205–209Google Scholar
21. Brébion G, Smith M, Gorman J, Malaspina D, Sharif Z, Amador X: Memory and schizophrenia: differential link of processing speed and selective attention with two levels of encoding. J Psychiatr Res 2000; 34:121–127Crossref, Medline, Google Scholar
22. Craik FIM, Lockart RS: Levels of processing: a framework for memory research. J Verbal Learning Verbal Behavior 1972; 11:671–684Crossref, Google Scholar
23. Corwin J: On measuring discrimination and response bias: unequal numbers of targets and distractors and two classes of distractors. Neuropsychology 1994; 1:110–117Crossref, Google Scholar
24. Wechsler D: Manual for the Wechsler Adult Intelligence Scale. New York, Psychological Corp, 1981Google Scholar
25. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
26. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale, Ill, Stoelting Co, 1978Google Scholar
27. Brébion G, Amador X, Smith M, Gorman J: Mechanisms underlying memory impairment in schizophrenia. Psychol Med 1997; 27:383–393Crossref, Medline, Google Scholar
28. White DA, Myerson J, Hale S: How cognitive is psychomotor slowing in depression? evidence from a meta-analysis. Aging, Neuropsychology and Cognition 1997; 4:166–174Crossref, Google Scholar
29. Caligiuri MP, Ellwanger J: Motor and cognitive aspects of motor retardation in depression. J Affect Disord 2000; 57:83–93Crossref, Medline, Google Scholar
30. Holthausen EAE, Wiersma D, Knegtering RH, Van den Bosch RJ: Psychopathology and cognition in schizophrenia spectrum disorders: the role of depressive symptoms. Schizophr Res 1999; 39:65–71Crossref, Medline, Google Scholar
31. Burgess N, Hitch GJ: Toward a network model of the articulatory loop. J Memory and Language 1992; 31:429–460Crossref, Google Scholar
32. Salthouse TA: The aging of working memory. Neuropsychology 1994; 8:535–543Crossref, Google Scholar



