Effectiveness of Second-Generation Antipsychotics in Patients With Treatment-Resistant Schizophrenia: A Review and Meta-Analysis of Randomized Trials
Abstract
OBJECTIVE: The authors conducted a review and meta-analysis of studies that compared the efficacy and tolerability of typical and second-generation antipsychotics for patients with treatment-resistant schizophrenia. METHOD: A systematic search revealed 12 controlled studies (involving 1,916 independent patients), which were included in the review. For the seven studies that compared clozapine to a typical antipsychotic, a meta-analysis was performed to examine clozapine’s effects on overall psychopathology, response rate, extrapyramidal symptoms, and tardive dyskinesia. RESULTS: The meta-analysis confirmed that treatment-resistant schizophrenic patients have more favorable outcomes when treated with clozapine rather than a typical antipsychotic, as reflected by Brief Psychiatric Rating Scale total score, categorical response rate, Scale for the Assessment of Negative Symptoms score, Simpson-Angus Rating Scale score, and compliance rate. Clozapine also conferred benefits on the sickest treatment-resistant schizophrenic patients. Patients treated with olanzapine also had more favorable outcomes with regard to categorical response and compliance rates. CONCLUSIONS: In the aggregate, the results of a meta-analysis indicated that clozapine exhibits superiority over typical antipsychotics in terms of both efficacy (as measured by improvement in overall psychopathology) and safety (in terms of reduced extrapyramidal side effects). However, the magnitude of the clozapine treatment effect was not consistently robust. Efficacy data for other second-generation antipsychotics in the treatment of patients with refractory schizophrenia were inconclusive. There is, therefore, a growing need to consider new and different treatment strategies, whether they be adjunctive or monotherapeutic, for schizophrenia that continues to be resistant or only partially responsive to treatment.
The advent of second-generation antipsychotic drugs has been hailed as a breakthrough in the pharmacotherapy of schizophrenia and other psychotic disorders. As a class, these drugs are believed to be more effective and safer than conventional antipsychotics (1–9). Claims of their superior efficacy have been based, in large part, on studies of clozapine, which has demonstrated its superior efficacy in patients that were either predominantly refractory or only partially responsive to conventional antipsychotics (2–6, 9). Other second-generation antipsychotic medications introduced subsequent to clozapine such as risperidone, olanzapine, and quetiapine are also widely believed to have superior efficacy to conventional antipsychotic drugs, although the evidentiary basis for this is variable. Understandably, these medications, along with clozapine, have been preferentially used in patients whose symptoms are either predominantly refractory or only partially responsive to conventional medications (i.e., treatment-resistant patients). Because these other drugs do not have the same risk of agranulocytosis as clozapine, they are used as “screening” medications for clozapine in treatment algorithms and practice patterns (10).
It is important to know definitively and precisely the comparative efficacy of the new medications vis-à-vis the previous standards. Among other reasons is the fact that the new medications are much more costly. Moreover, to switch a patient who may be persistently symptomatic but stable to a new atypical drug with the promise of an improved response is not without the risk of destabilization and all of the attendant consequences. In addition, there is the potential loss of credibility with our patients and public policy makers. The introduction of the phenothiazines and other classes of neuroleptics in the period between 1953 and 1980 both raised expectations that were never fully realized and prompted policies of deinstitutionalization that were not very successful and have had considerable adverse social ramifications.
The purpose of conducting this review and meta-analysis was to examine the comparative value of the second-generation antipsychotic medications. As most of these studies (seven of 12) compared the effectiveness of clozapine versus a typical antipsychotic in patients with treatment-resistant schizophrenia, the primary focus of the meta-analysis was on these seven studies. Meta-analysis is a statistical analytic method that combines data from several sources, thereby increasing the sample size and overcoming the power limitations of undersized studies or small treatment effects. It can also potentially give greater weight to the more reliable studies (11).
Method
Characteristics of Reviewed Studies
For our review, we sought randomized, controlled clinical trials that compared the effectiveness of typical and second-generation antipsychotics in the treatment of chronic refractory schizophrenia. Relevant studies were identified through 1) MEDLINE (1966–August 1999) and Current Contents (1996–August 1999) searches; 2) cross-referencing of original studies; and 3) obtaining information from studies presented at scientific meetings that were pending publication.
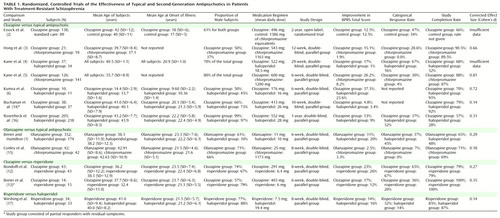
We found a total of 12 randomized, controlled clinical trials (1–6, 12μ/cross-ref>17) that examined the efficacy of second-generation antipsychotics in patients with treatment-resistant schizophrenia (N=1,916 subjects). Ten studies (N=1,801) compared a typical antipsychotic with a second-generation antipsychotic (clozapine [2–6, 14, 16; N=1,124], olanzapine [1, 15; N=610], or risperidone [17; N=67]). Two studies (12, 13) compared risperidone to clozapine (N=115). Subject characteristics and response and completion rates for the individual studies are summarized in Table 1.
All patients met DSM-III-R criteria for chronic schizophrenia with the exception of 15% (N=79) of the patients in the study by Breier and Hamilton (1), who met criteria for chronic schizoaffective disorder. However, from the clinical description of the population, the subjects were largely chronically ill and residually symptomatic. The 1,916 subjects in the 12 studies under review came from Veterans Administration (VA) hospitals (N=489), state hospitals (N=261), academic medical centers (N=196), and from a VA/state hospital combination (N=67); the patient origin was not reported for 903 subjects. Approximately 76% of the subjects were men. All studies used reliable diagnostic criteria, well-defined inclusion and exclusion criteria, and well-established scales for the measurement of outcome. All presented treatment efficacy by using an intent-to-treat, last-observation-carried-forward analysis. All studies involved randomly assigning subjects into parallel treatment groups and were double-blind with the exception of the study by Essock et al. (2), which was an open-label study with random treatment assignment.
Most subjects (N=1,812) were classified as treatment resistant, although the stringency of this definition varied across studies. The most stringent criteria for treatment resistance were those used by Kane et al. (5), who required 1) evidence of adequate previous medication trials (a minimum of three 6-week trials in the preceding 5 years with typical antipsychotics from at least two chemical classes at doses equivalent to at least 1000 mg/day of chlorpromazine) without significant symptom relief; 2) persistent positive psychotic symptoms, with rating scores of moderate or worse on at least two of four positive symptom items on the Brief Psychiatric Rating Scale (BPRS) (18); 3) the presence of at least moderately severe illness, defined as a minimum total BPRS score of 45 and a score of at least moderate on the Clinical Global Impression (19); and 4) no period of good social or occupational functioning in the preceding 5 years. Included in our analysis were subjects from two studies (13, 14) of chronic schizophrenic patients who were “partially responsive to neuroleptics” with persistent residual symptoms (N=104). These subjects had a history of residual positive or negative symptoms after at least a 6-week trial of a typical antipsychotic medication at a therapeutic dose and a score of at least 8 on four positive symptom items of the BPRS or a score of at least 20 on the Scale for the Assessment of Negative Symptoms (SANS) (20) at the time of study evaluation and after at least 2 weeks of prospective treatment with fluphenazine.
Outcome Measures
The principal outcomes of interest were BPRS total and positive symptom scores (18) as well as scores from the Clinical Global Impression (19), SANS (20), Simpson-Angus Rating Scale (21), and the Abnormal Involuntary Movement Scale (AIMS) (22). For cases in which the BPRS total and positive symptom scores were not available, the total and positive symptom scores from the Positive and Negative Syndrome Scale (23) were converted. Additional measures of outcome were adverse events and completion rates. Assessment scale scores across studies for each separate treatment by time point and the percent improvement in outcome measures are summarized in Table 2.
Statistical Methods
Four approaches were undertaken for the comparison of typical and second-generation antipsychotics: analysis of covariance (ANCOVA), weighted least squares analysis, categorical data analysis of responders, and calculation of a treatment effect size.
For the seven studies that compared clozapine with a typical antipsychotic, a meta-analysis with an ANCOVA model was used to examine improvement in scores on the BPRS (both total and positive symptom score), SANS, AIMS, and the Simpson-Angus Rating Scale. The data used for the meta-analysis were from the intent-to-treat analyses, since data from the studies in which the patients completed the protocol were sparse. The primary hypothesis of interest for the meta-analysis was that clozapine was more effective or was better tolerated than typical antipsychotic medication in patients with treatment-resistant schizophrenia. Although some of the studies were of different duration or had different dose levels, the studies were deemed to be similar in design, purpose, and study population.
The ANCOVA used the endpoint score as the outcome measure, with treatment and study as factors and baseline score and the interaction between treatment and the baseline score as covariates. If the interaction was not significant at the 0.05 level, then it was deleted from the model. The ANCOVA method gives equal weight to each study, with no indication of precision of the mean in the analysis.
The weighted least squares analysis used the change score as the outcome measure, with dummy variables for the different treatments and studies; the weight was a function of the sample size. Because of sample size considerations, weighted least squares analysis was only done for BPRS total score.
The categorical analysis used the Cochran-Mantel-Haenszel approach to examine treatment response in patients who met an a priori definition of response. The Cochran-Mantel-Haenszel approach is based on weighted averages of the proportion of responders across the studies, so this approach gives greater weight to the larger studies (24).
The effect size of treatment in individual studies that compared typical and second-generation antipsychotics was determined by using Cohen’s d. An effect size is an indication of the amount of variability in the dependent variable (in this case, BPRS total score after treatment) that can be accounted for by the treatment. An effect size of d=0.20 is considered small; d=0.50 is a medium effect size; and d=0.80 is a large effect size (25). We used published estimates of means and standard deviations to determine an effect size for five studies that compared clozapine to typical antipsychotics, two studies that compared olanzapine to typical antipsychotics, and two studies that compared clozapine to risperidone (Table 1). Individual values of d were then combined across studies that compared clozapine to typical antipsychotics and weighted according to their variance to provide a global effect size (25).
Results
Clinical Outcome
Of the 10 comparisons of second-generation versus typical antipsychotics, six (1, 3–6, 14) found a significant difference that favored the second-generation antipsychotic on measures of treatment efficacy; four found no significant difference between treatments (2, 15–17). Five of the seven studies that compared clozapine to a typical antipsychotic medication in treatment-resistant patients found a significant difference favoring clozapine (3–6, 14).
The superiority of second-generation over typical antipsychotics was more definitive with regard to extrapyramidal symptom liability. Seven studies (1–3, 5, 14μ/cross-ref>16) reported less extrapyramidal symptom liability in patients receiving second-generation antipsychotics, one (4) did not report differences in extrapyramidal symptom liability, and two (6, 17) reported no difference. Of the two studies that compared clozapine to risperidone, one (13) found clozapine superior in terms of both treating positive symptoms and extrapyramidal symptom liability but reported no difference in total BPRS scores or negative symptoms. The other (12) found no difference in treatment efficacy or extrapyramidal symptom liability between the two drugs.
With regard to effects on tardive dyskinesia, two (1, 16) of five studies reported reduction in tardive dyskinesia in patients treated with second-generation versus typical antipsychotics, whereas the remaining three studies (5, 6, 17) showed no difference in tardive dyskinesia reduction.
Overall Psychopathology
An ANCOVA (with baseline score as a covariate) and weighted least squares analysis were performed to compare the efficacy of clozapine to typical antipsychotics in treatment-resistant patients in terms of BPRS total score. For the ANCOVA, seven studies (2–6, 14, 16) contributed two observations for a total of 14 observations in this analysis (seven observations from groups treated with clozapine and seven observations from groups treated with a typical antipsychotic). There was a main effect of treatment (F=8.51, df=1, 8, p<0.05), with greater reduction in psychopathology in the clozapine-treated groups compared to those treated with a typical antipsychotic. There was also a main effect of study (F=9.04, df=1, 12, p<0.03) and an interaction between baseline total BPRS score and treatment (F=28.45, df=1, 12, p<0.006), with patients treated with clozapine having greater BPRS score reductions when their initial scores were larger. The weighted least squares analysis revealed a nonsignificant effect of treatment (F=3.78, df=1, 14, p=0.07), with clozapine-treated patients having greater reductions in total BPRS scores than patients treated with typical antipsychotics.
An estimate of the magnitude of the treatment effect in individual studies was obtained by computing treatment effect sizes. The effect sizes in the five individual studies that compared clozapine to a typical antipsychotic were highly variable, ranging from 0.14 (very low) to 0.81 (high). An overall effect size for the effect of treatment with clozapine compared to a typical antipsychotic on BPRS total scores was obtained by computing the weighted mean across the five studies. The overall estimate of effect size for treatment with clozapine in these five studies was 0.48 (moderate).
In contrast to the BPRS total score, there were no significant treatment effects for clozapine over typical antipsychotics on scores for the BPRS positive symptom subscale or the SANS in the ANCOVA model. The weighted least squares model was not fit because of the small number of studies for which these variables were reported.
Categorical Response to Treatment
Response was categorically defined by a liberal but generally accepted definition (a 20%–30% decrease from baseline BPRS total score) as favorable or unfavorable response to treatment. The response rates for the studies that used this criterion are shown in Table 1. This categorical approach to data analysis was implemented by using the Cochran-Mantel-Haenszel method (24), which allows for combining of data across several studies.
Pooled across the five studies that categorically compared patients treated with clozapine to those treated with a typical antipsychotic (2–5, 16) (N=1,028), clozapine-treated patients were 2.45 times more likely to meet treatment response criteria (χ2=45.41, df=1, p=0.001). Pooled across the two studies that compared treatment with olanzapine to treatment with a typical antipsychotic (1, 15) (N=610), olanzapine-treated patients were 1.71 times more likely to meet categorical response criteria (χ2=7.96, df=1, p=0.005). The categorical comparison of 115 patients treated with clozapine to those treated with risperidone (12, 13) was not significant (χ2=0.08, df=1, p=0.79).
Extrapyramidal Symptoms
Since there is evidence that several second-generation antipsychotic medications have a better extrapyramidal symptom profile than typical antipsychotics, we performed an ANCOVA for studies comparing patients treated with olanzapine or clozapine to patients treated with typical antipsychotics in terms of score on the Simpson-Angus Rating Scale. Six studies (1, 5, 6, 13, 15, 16) contributed two observations for a total of 12 observations (four observations from groups treated with clozapine, two observations from groups treated with olanzapine, and six observations from groups treated with a typical antipsychotic). There was a significant treatment effect (F=54.95, df=1, 10, p<0.002), with subjects treated with clozapine or olanzapine exhibiting fewer extrapyramidal symptoms than those treated with a typical antipsychotic. There was also a main effect of study (F=37.08, df=1, 10, p<0.002). The weighted least squares model was not fit because of the small sample sizes for this variable.
Tardive Dyskinesia
Since there is evidence that olanzapine and clozapine may result in an amelioration of tardive dyskinesia compared to typical antipsychotics, we performed an ANCOVA that compared endpoint AIMS scores of patients treated with olanzapine or clozapine to patients treated with a typical antipsychotic, with baseline AIMS score as a covariate. Four studies (1, 5, 6, 16) contributed two observations for a total of eight observations in an analysis (three observations from groups treated with clozapine, one observation from a group treated with olanzapine, and four observations from groups treated with a typical antipsychotic). The effects of both study and treatment were not significant (study: F=2.79, df=1, 7, p=0.27; treatment: F=6.60, df=1, 7, p=0.13). The weighted least squares model was not fit because of the small sample sizes for this variable.
Adverse Events
Nonmotor adverse events were reported in six studies (4–6, 12, 14, 15). Hypotension was most common with chlorpromazine treatment (42%) but was also seen in patients treated with clozapine, olanzapine, and risperidone (11%, 10%, and 12%, respectively). Sedation was commonly reported by patients receiving haloperidol (33.3%), chlorpromazine (21.8%), clozapine (37.4%), olanzapine (35.7%), and risperidone (30.2%). Weight gain was reported in patients treated with chlorpromazine (1%), clozapine (7.1%), and risperidone (23.3%) but in none of the patients taking haloperidol. Data on weight gain were not reported in olanzapine treatment studies. Concentration problems were reported in patients treated with chlorpromazine (3.4%), clozapine (2.9%), and risperidone (25.6%) but in none of the patients treated with haloperidol. Other adverse events reported in patients receiving clozapine included neutropenia (2.0%), enuresis (1.0%), and seizures (0.4%). Neuroleptic malignant syndrome developed in 0.5% of the patients treated with chlorpromazine.
Study Completion Rates
The completion rates by treatment group by study are displayed in Table 1. Examining the raw completion rates, patients treated with risperidone had the highest completion rate (84.8%, N=78), followed by patients treated with clozapine (69.6%, N=400), olanzapine (65.7%, N=259), and typical antipsychotics (56.1%, N=398).
The data on completion rates were also analyzed categorically. In the seven studies (2–6, 14, 16) that compared completion rates in patients treated with clozapine to those treated with a typical antipsychotic (N=1,124), clozapine-treated patients were significantly more likely to complete the clinical trial (odds ratio=1.49; χ2=8.95, df=1, p=0.003). In the two studies (1, 15) that compared treatment with olanzapine versus a typical antipsychotic (N=610), the olanzapine-treated patients were more likely to complete the treatment trial (odds ratio=1.81; χ2=11.73, df=1, p=0.001). In the two studies (12, 13) that compared patients treated with clozapine to those treated with risperidone (N=115), there was no difference in completion rate (χ2=0.00, df=1, p=1.00).
Discussion
In the aggregate, the results indicate that the second-generation antipsychotics as a class, to the extent that they have been studied in patients with treatment-resistant schizophrenia, exhibit superiority in measures of treatment compliance and reduction of extrapyramidal symptoms compared to typical antipsychotics. However, the efficacy of second-generation antipsychotics, other than clozapine, in the reduction of symptoms in treatment-resistant schizophrenia is not established. This is principally because there are relatively little data with which to evaluate this question for second-generation antipsychotics apart from clozapine. Using an ANCOVA model with baseline score as a covariate, we found that the clozapine treatment group had a greater reduction in BPRS total score than the typical antipsychotic treatment group. The association of greater reductions from baseline in BPRS total score in the clozapine treatment group did not reach statistical significance in the weighted least squares analysis model. One possible reason for this discrepant result was that the ANCOVA model used the endpoint as the dependent variable with the initial time point as a covariate, whereas the weighted least squares model used the change score as the dependent variable. Another possible reason for the discrepancy is that the two models weighted the studies differently. As we noted, the ANCOVA model weighted each study equally, which, in effect, gave greater weight to small studies that showed large effects. This might give a small study with a large effect size greater influence than would the weighted least squares model. The weighted least squares analysis, by weighting studies proportionally to their sample size, in effect gave greater weight to larger studies that did not show differences between the treatment groups. Finally, the discrepancy may be the result of using estimated variance. Typically, a weighted least squares analysis weights by a function of the sample size and the variance; however, the actual variance data were not available for all of the studies included in this analysis so estimated variance was used.
In any case, the difference between the ANCOVA model and the weighted least squares model with regard to BPRS total score was minimal and should not affect the interpretation of the results. In both analyses, the direction of the effect was the same (BPRS total score for the clozapine treatment group was lower than for the typical antipsychotic treatment group). This provides evidence that clozapine is effective in reducing overall psychopathology in patients with treatment-resistant schizophrenia compared to typical antipsychotics. However, it is important to note that the effect size of the clozapine treatment effect varied considerably across individual studies, although the combined effect size was moderate.
The significant interaction between baseline psychopathology and treatment in the ANCOVA meta-analytic comparison of clozapine-treated patients to patients treated with a typical antipsychotic suggests that treatment-resistant patients who are more severely symptomatic at baseline are most likely to benefit from treatment with clozapine. Since baseline psychopathology was controlled for as a covariate in the ANCOVA, this appears to be a real effect and not an artifact or a result of regression toward the mean.
Individual categorical analyses performed to evaluate the efficacy of both clozapine and olanzapine suggested that both of these treatments were superior to typical antipsychotic medications in producing clinically significant improvement. However, the analysis comparing olanzapine to typical antipsychotics was based on only two studies (1, 15), which found contrary results. Conley et al. (15) found no advantage of olanzapine compared to chlorpromazine in very ill, chronically institutionalized schizophrenic patients, whereas Breier and Hamilton (1) found an advantage of olanzapine compared to haloperidol in a group of treatment-resistant schizophrenic and schizoaffective patients, 50% of whom were outpatients at the time of study entry. One reason for the contradictory findings of these two studies may be that they involved different patient groups, although both were ostensibly refractory. The Conley et al. study was conducted in the United States, and its patients had a baseline total BPRS score of 57, whereas the Breier and Hamilton study was done in Europe with patients who had a mean baseline BPRS score of 37.5. These two studies suggest that, unlike clozapine, olanzapine (at least at the doses used in these studies) may be effective in treatment-resistant subjects who are less severely ill but not as effective in the sickest treatment-resistant subjects.
The two studies that compared risperidone to clozapine found no difference in the efficacy of these two second-generation antipsychotic medications in the treatment-resistant population that they studied (12, 13). From these studies, and our evidence that clozapine is more effective in resistant patients than are typical antipsychotics (3–6, 9), we could infer that risperidone is more effective than typical antipsychotics by extrapolation of its noninferior efficacy to clozapine. However, characteristics of sample selection may have contributed to the inability to detect a differential effect of clozapine and risperidone in the treatment of the subjects in these studies. The subjects of Breier et al. (13) were outpatients with relatively low baseline psychopathology (mean baseline BPRS score was 38) and were considered partially responsive with residual symptoms. Bondolfi et al. (12) did not prospectively treat subjects with a typical neuroleptic to screen out treatment responders and included subjects who were treatment-intolerant as well as treatment-resistant, without reporting the proportion of each of these groups in the sample. The Wirshing et al. study (17) found no difference in the treatment efficacy of risperidone compared to haloperidol in patients with high levels of baseline psychopathology. These studies, although clearly not definitive, do not provide convincing evidence to support the hypothesis that the sickest treatment-resistant patients would do as well with risperidone as they did with clozapine.
The ANCOVA models did not reveal differential efficacy of clozapine compared to typical antipsychotics in the treatment of negative symptoms. This may be in part due to a lack of power in the analysis, since only four studies contributed observations to this analysis. When examining the effects of clozapine or olanzapine on negative symptoms in individual studies, a reduction of negative symptoms is not definitive. Kane et al. (5), Kumra et al. (6), and Breier et al. (13) found that second-generation antipsychotics reduced negative symptoms, whereas Buchanan et al. (14), Conley et al. (15), and Kane et al. (4) did not. Since the Kane et al. sample (5) had high baseline extrapyramidal symptoms, the observed improvements in negative symptoms in the clozapine-treated group in that study could be secondary to continued reduction of extrapyramidal symptoms throughout the duration of the trial. This interpretation is supported by studies that have found an association between improvement of negative symptoms and extrapyramidal symptoms (8). In contrast, Kumra et al. (6) found clozapine effective in treating negative symptoms in adolescents with childhood-onset schizophrenia who began clozapine treatment at an average age of 14.4 years (SD=2.95). Since these patients had a low level of extrapyramidal symptoms at baseline, the reduction in negative symptoms might be due to a reduction in primary negative symptoms. This suggests that early intervention with clozapine in young refractory patients may result in enhanced efficacy of clozapine in the reduction of negative symptoms. The differential effects of olanzapine on negative symptoms as reported by Breier and Hamilton (1) and Conley et al. (15) may be due to the fact that Conley et al. studied a sicker population of patients who had been chronically institutionalized in state or VA hospital systems and had a mean baseline BPRS score of 57, compared to a mean baseline BPRS score of 38 reported in the Breier and Hamilton study. However, additional studies are needed to clarify the basis for this difference.
Some of the most troublesome side effects associated with typical antipsychotic drugs are extrapyramidal symptoms, including acute dystonic reactions, drug-induced parkinsonism, and akathisia. Extrapyramidal symptoms occur in up to 75% of patients treated with typical antipsychotics and significantly contribute to medication noncompliance (26–28). Another troublesome adverse consequence of long-term treatment with typical antipsychotics is tardive dyskinesia (29, 30). The risk of developing tardive dyskinesia is believed to increase with total cumulative drug exposure. This risk, on average, is about 5% per treatment year for the first 5 to 7 years (29–31).
Despite the limited number of studies that contributed to these analyses, advantages of clozapine and olanzapine treatment compared to typical antipsychotic medications in reducing extrapyramidal symptoms were seen. Wirshing et al. (17) did not find a difference in reduction of drug-induced parkinsonism in the risperidone-treated group compared to the haloperidol-treated group, although patients treated with risperidone received less concomitant anticholinergic medication.
Even though the reductions in total AIMS scores in patients treated with olanzapine and clozapine ranged on average from 30% to 40% (Table 2), the analyses did not demonstrate a greater reduction in AIMS scores compared to patients treated with typical antipsychotics. This failure to demonstrate superior efficacy of second-generation antipsychotic medication in the amelioration of tardive dyskinesia may be due to the small sample size (only four studies contributed data), which may have resulted in inadequate power to detect a difference between groups. Therefore, further studies are needed to examine the effects of these medications on tardive dyskinesia in this population.
Despite the important advantages conferred by the second-generation antipsychotics with regard to motor side effects, concern about other side effects may affect ultimate treatment choice. Second-generation antipsychotics have been associated with weight gain and obesity, which increases the risk for diabetes, hypertension, and coronary artery disease (32). Among antipsychotics, clozapine and olanzapine appear to cause the most weight gain, with risperidone causing a lesser amount of weight gain and haloperidol the least (although haloperidol may not be representative of all typical antipsychotics in this respect) (33). Given this effect, it is not unreasonable to consider that when treating obese patients with underlying medical problems such as diabetes, hypertension, or hypercholesterolemia, there may be advantages conferred by treatment with the second-generation antipsychotics ziprasidone, aripripazole, iloperidone (each currently awaiting approval from the Food and Drug Administration) or typical antipsychotics such as haloperidol, since these are less likely to result in significant weight gain and exacerbate underlying medical problems. Despite the significant medical consequences of drug-induced obesity, neither of the two studies that used olanzapine as a treatment condition (1, 15) reported on weight changes, and only three of the studies that used clozapine as a treatment condition (3, 6, 13) reported on weight changes.
An additional troublesome side effect of antipsychotic medications is hyperprolactinemia, which is induced by the dopamine D2 receptor antagonist properties of typical antipsychotic drugs and risperidone. The resultant hyperprolactinemia can lead to impotence, galactorrhea, amenorrhea, and a higher risk of osteoporosis in psychiatric patients (34). These associated sexual and reproductive side effects may affect the ultimate choice of treatment for a given patient. However, none of the 12 studies reviewed reported on these side effects. Similarly, differences in effects of antipsychotic medications on memory and concentration may affect treatment choice and need to be further studied (35).
An important measure of outcome is compliance with treatment. A substantial proportion of patients with schizophrenia continue to relapse frequently, often as a result of nonadherence to treatment (36–38). Our analyses demonstrated a superiority of second-generation over typical antipsychotics with regard to completion rates, which implies that patients with treatment-resistant schizophrenia given these drugs are more likely to comply with treatment.
Finally, the variable and, in many cases, limited effect sizes found in the analyses of comparative drug studies indicate that although there may be some measure of superior efficacy (as well as considerable advantages in safety in terms of neurologic side effects) for the second-generation antipsychotics, they clearly have substantial limitations in their efficacy. The majority of patients improved minimally to moderately, and although they may have been less symptomatic, they still had substantial residual symptoms. Thus, there is a critical need for additional drug development and research on new treatment strategies such as the use of adjunctive treatments with antipsychotic drugs.
Conclusions
A meta-analysis confirmed that patients with treatment-resistant schizophrenia have more favorable outcomes when treated with clozapine rather than a typical antipsychotic with respect to BPRS total score, Simpson-Angus Rating Scale score, and compliance rate. There is at present insufficient evidence on which to definitively evaluate the other second-generation antipsychotics for this patient population.
Despite the superior clinical efficacy of clozapine in the treatment of resistant patients, the extent of this superior efficacy in terms of scope (symptom dimensions improved) and magnitude (effect size) is variable. Using what might be regarded as a nonstringent criterion of 20%–30% reduction in total psychopathology scores, we found that fewer than half of the patients responded in most studies, suggesting that many resistant patients were still left with substantial impairments and symptoms. In addition, the “responsive” patients may still have debilitating pathology and only slight improvement in their overall functioning. Therefore, there is a growing need to consider new and different treatment strategies for patients who continue to be refractory or are partial responders, whether they be adjunctive or monotherapeutic.
 |
 |
Received March 14, 2000; revision received Aug. 25, 2000; accepted Sept. 1, 2000. From the Department of Psychiatry, University of North Carolina School of Medicine. Address reprint requests to Dr. Chakos, Department of Psychiatry, University of North Carolina School of Medicine, CB 7160, Chapel Hill, NC 27599-7160; [email protected] (e-mail).
1. Breier A, Hamilton S: Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol Psychiatry 1999; 45:403–411Crossref, Medline, Google Scholar
2. Essock SM, Hargreaves WA, Hohm FA, Goethe CL, Hipshman L: Clozapine eligibility among state hospital patients. Schizophr Bull 1996; 22:15–25Crossref, Medline, Google Scholar
3. Hong CJ, Chen JY, Chiu HJ, Sim CB: A double-blind comparative study of clozapine versus chlorpromazine on Chinese patients with treatment-refractory schizophrenia. Int Clin Psychopharmacol 1997; 12:123–130Crossref, Medline, Google Scholar
4. Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW, Wirshing DA, Safferman A, Ganguli R, Borenstein M: Clozapine and haloperidol in moderately refractory schizophrenia: a six-month double-blind comparison. Arch Gen Psychiatry (in press)Google Scholar
5. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
6. Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Rapoport JL: Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 1996; 53:1090–1097Google Scholar
7. Lindenmayer JP, Grochowski S, Mabugot L: Clozapine effects on positive and negative symptoms: a six-month trial in treatment-refractory schizophrenics. J Clin Psychopharmacol 1994; 14:201–204Crossref, Medline, Google Scholar
8. Lieberman JA, Safferman AZ, Pollack S, Szymanski S, Johns C, Howard A, Kronig M, Bookstein P, Kane JM: Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry 1994; 151:1744–1752Google Scholar
9. Bradford DW, Chakos MH, Sheitman B, Lieberman JA: Atypical antipsychotic drugs in treatment-refractory schizophrenia. Psychiatr Annals 1998; 28:618–628Crossref, Google Scholar
10. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry 1997; 154(April suppl)Google Scholar
11. Schmid JE, Koch GG, LaVange LM: An overview of statistical issues and methods of meta-analysis. J Biopharm Stat 1991; 1:103–120Crossref, Medline, Google Scholar
12. Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P (Risperidone Study Group): Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry 1998; 155:499–504Link, Google Scholar
13. Breier AF, Malhotra AK, Su TP, Pinals DA, Elman I, Adler CM, Lafargue RT, Clifton A, Pickar D: Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry 1999; 156:294–298Abstract, Google Scholar
14. Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT Jr: Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry 1998; 155:751–760Link, Google Scholar
15. Conley RR, Tamminga CA, Bartko JJ, Richardson C, Peszke M, Lingle J, Hegerty J, Love R, Gounaris C, Zaremba S: Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155:914–920Link, Google Scholar
16. Rosenheck R, Cramer J, Xu W, Thomas J, Henderson W, Frisman L, Fye C, Charney D (Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia): A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med 1997; 337:809–815Crossref, Medline, Google Scholar
17. Wirshing DA, Marshall BD Jr, Green MF, Mintz J, Marder SR, Wirshing WC: Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999; 156:1374–1379Google Scholar
18. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
19. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
20. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
21. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
22. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
23. Kay SR: Positive and Negative Syndromes in Schizophrenia: Assessment and Research. New York, Brunner & Mazel, 1991Google Scholar
24. Fleiss JL: Statistical Methods for Rates and Proportions. New York, John Wiley & Sons, 1981Google Scholar
25. Rosenthal R: Meta-Analytic Procedures for Social Research. London, Sage Publications, 1986Google Scholar
26. Keepers GA, Clappison VJ, Casey DE: Initial anticholinergic prophylaxis for neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry 1983; 40:1113–1117Google Scholar
27. Casey DE: Motor and mental aspects of acute extrapyramidal syndromes. Acta Psychiatr Scand 1994; 89:14–20Crossref, Medline, Google Scholar
28. Casey DE: Neuroleptic-induced acute extrapyramidal syndromes and tardive dyskinesia. Psychiatr Clin North Am 1993; 16:589–610Crossref, Medline, Google Scholar
29. Chakos M, Alvir JM, Bilder R, Mayerhoff D, Woerner MG, Borenstein M, Geisler S, Kane JM, Lieberman JA: Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry 1996; 53:313–319Crossref, Medline, Google Scholar
30. Kane JM, Woerner M, Borenstein M, Wegner J, Lieberman J: Integrating incidence and prevalence of tardive dyskinesia. Psychopharmacol Bull 1986; 2:254–258Google Scholar
31. Morganstern H, Glazer WM: Identifying risk factors for tardive dyskinesia among chronic outpatients maintained on neuroleptic medications: results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiatry 1993; 50:723–733Crossref, Medline, Google Scholar
32. Fertig MK, Brooks VG, Shelton PS, English CW: Hyperglycemia associated with olanzapine. J Clin Psychiatry 1998; 59:687–688Crossref, Medline, Google Scholar
33. Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR: Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999; 60:358–363Crossref, Medline, Google Scholar
34. Kartaginer J, Ataya K, Mercado A, Abbasi A: Osteoporosis associated with neuroleptic treatment. J Reprod Med 1990; 35:198–202Medline, Google Scholar
35. Keefe RS, Silva SG, Perkins DO, Lieberman JA: The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 1999; 25:201–222Crossref, Medline, Google Scholar
36. Green JH: Frequent rehospitalization and noncompliance with treatment. Hosp Community Psychiatry 1988; 39:963–966Abstract, Google Scholar
37. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA: Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry 1995; 152:856–861Link, Google Scholar
38. Lang FH, Forbes JF: Service provision for people with schizophrenia, I: clinical and economic perspective. Br J Psychiatry 1997; 171:159–164Crossref, Medline, Google Scholar



