Influence of Serotonin-Transporter-Linked Promoter Region Polymorphism on Platelet Activation in Geriatric Depression
Abstract
OBJECTIVE: Depression has been associated with increased platelet activation. Variations in the serotonin-transporter-linked promoter region (5-HTTLPR) polymorphism may influence the degree of activation. The authors examined the association among depression, platelet activation, and 5-HTTLPR genotype. METHOD: Elderly subjects with (N=61) and without (N=12) major depression were assessed for cognitive impairment, cardiovascular disease, and two indices of platelet activation. The depressed subjects were genotyped for the 5-HTTLPR polymorphism. RESULTS: The depressed subjects were older, were more cognitively impaired, and had higher platelet factor 4 and β-thromboglobulin levels; cardiovascular disease was minimal in both groups. In the depressed group, subjects with the 5-HTTLPR l/l genotype had significantly higher platelet factor 4 and β-thromboglobulin levels. CONCLUSIONS: Platelet activation is increased in elderly depressed patients, especially those with the 5-HTTLPR l/l genotype. This finding suggests how genetic differences may influence cardiovascular mortality in depressed patients with ischemic heart disease.
Depression is associated with higher than normal rates of mortality in patients with ischemic heart disease (1). This association may be due in part to the increased platelet activation seen in depressed subjects both with and without ischemic heart disease or risk factors for ischemic heart disease (2–6). The mechanism by which platelet activation is increased in depression is unknown. One possibility involves the serotonin system, as both platelets and brain neurons share the same serotonin transporter gene and protein and the same serotonin-transporter-linked promoter region (5-HTTLPR). The expression of the serotonin transporter gene is affected by the 5-HTTLPR polymorphism; the dominant s allele of the 5-HTTLPR polymorphism is associated with the production of fewer serotonin transporters, whereas the recessive l allele is associated with the production of a greater number of serotonin transporters (7). Variations in the 5-HTTLPR polymorphism have been associated with mood disorders (8). However, it is not known whether the 5-HTTLPR polymorphism influences platelet activation.
We investigated the relationship among depression, platelet activation, and 5-HTTLPR genotype in a group of elderly subjects without overt ischemic heart disease. We hypothesized that subjects with the l/l genotype would demonstrate greater platelet activation as their platelets should store more serotonin in their dense granules and therefore should release more serotonin and activate more platelets in response to subclinical vascular damage.
Method
From 96 participants in a study of pharmacotherapy of late-life depression (9), 61 elderly depressed subjects were recruited. The subjects included in this study were age 60 years or older, met the DSM-IV criteria for a major depressive episode without psychotic features, and had a baseline score on the Hamilton Depression Rating Scale (10) of 15 or higher, a Mini-Mental Status Examination (MMSE) (11) score of 15 or higher, and no current history of substance abuse. Two indices of platelet activation—platelet factor 4 and β-thromboglobulin—were measured as previously described (5). Cardiovascular disease burden was estimated by summing the heart and vascular disease subscales of the Cumulative Illness Rating Scale (12). Genotyping for the 5-HTTLPR polymorphism was performed as previously described (13) with 56 of the depressed subjects. Twelve nondepressed elderly subjects were recruited to form the comparison group and were assessed in the same manner as for the depressed subjects but were not genotyped. Written informed consent was obtained from all subjects.
We used t tests and Fisher’s exact tests to contrast the depressed subjects with the comparison group and an analysis of covariance (ANCOVA) to compare the platelet factor 4 concentrations while controlling for MMSE score and age. We calculated a Cohen’s r to measure effect size. In the analysis based on genotype, we used t tests and Fisher’s exact tests with log transformations of platelet factor 4 and β-thromboglobulin levels (to help normalize the distribution), an ANCOVA while controlling for Hamilton depression scale score, and a Cohen’s r to measure effect size.
Results
The depressed subjects were significantly older (mean age=72.4 years, SD=7.8) than the comparison subjects (mean age=67.3, SD=5.4) (t=–2.15, df=71, p=0.04) and more cognitively impaired, according to MMSE scores (mean=26.8, SD=3.3, versus mean=29.1, SD=1.2) (t=4.18, df=50.5, p=0.0001), but they did not differ from the comparison group in percentage of male subjects (26.2% [N=16] versus 33.3% [N=4]) (p=0.73, Fisher’s exact test) or percentage who smoked (15.3% [N=9] versus 0%) (p=0.34, Fisher’s exact test; data missing for two depressed subjects). The mean scores on the Cumulative Illness Rating Scale for heart and vascular disease were low in both groups, indicating minimal cardiovascular disease burden (mean=1.7, SD=1.5, versus mean=1.3, SD=1.3) (t=–1.07, df=71, p=0.29). Platelet factor 4 and β-thromboglobulin levels were significantly higher in the depressed subjects than in the comparison group; the mean platelet factor 4 levels were 34.7 IU/ml (SD=28.4) and 12.7 IU/ml (SD=22.0), respectively (t=–4.30, df=71, p<0.0001), and the mean β-thromboglobulin levels were 119.4 IU/ml (SD=103.1) and 50.1 IU/ml (SD=80.6) (t=–2.95, df=49, p=0.005). The differences remained significant even after we controlled for age (F=15.71, df=1, 70, p=0.0002; Cohen’s r=0.43) and MMSE score (F=15.38, df=1, 70, p=0.0002; Cohen’s r=0.42).
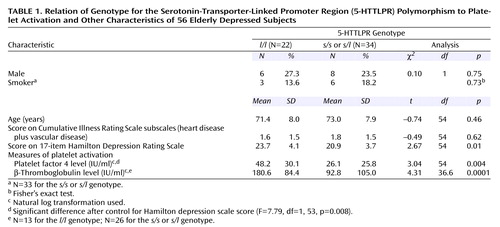
In the 56 depressed subjects classified by 5-HTTLPR polymorphism (Table 1), platelet factor 4 and β-thromboglobulin levels were highly correlated (r=0.75, p<0.0001, N=39). Depressed subjects with the l/l genotype had significantly higher Hamilton depression scale scores but were otherwise clinically similar to the other depressed subjects. The depressed subjects with the l/l genotype had significantly higher platelet factor 4 and β-thromboglobulin levels. The difference in platelet factor 4 remained significant after we controlled for the difference in Hamilton depression scale score; effect size was indicated by Cohen’s r=0.36.
Discussion
In our group of elderly subjects with low cardiovascular disease burden, major depression was associated with higher levels of platelet factor 4 and β-thromboglobulin. Depressed subjects who were homozygous for the l/l 5-HTTLPR allele had more severe depression and demonstrated the greatest elevation in platelet factor 4 and β-thromboglobulin.
These findings replicate those of studies demonstrating greater platelet activation in depressed subjects than in nondepressed comparison subjects (2–6), and we believe it is the first to demonstrate greater than normal platelet activation in depressed elderly subjects with low cardiovascular disease burden. To our knowledge, this is also the first study of the association between 5-HTTLPR polymorphism and platelet activation in depression. Our finding supports our hypothesis that platelets in persons with the l/l genotype are more efficient in the uptake (14) and storage of serotonin in their dense granules. Thus, one could imagine that when a platelet in an l/l carrier becomes activated by “bumping” into a subclinical atherosclerotic plaque (14), the activated platelet releases more serotonin and therefore activates a larger number of circulating platelets than in someone without the l/l genotype. This greater activation could lead to greater thrombus formation, which may be more likely to result in an adverse cardiovascular event, such as a myocardial infarction. Our finding is particularly interesting in light of the higher rate of mortality associated with depression in patients with ischemic heart disease (1); the observed increase in mortality might be accounted for by greater platelet activation in depressed individuals with the l/l 5-HTTLPR genotype. If this finding is replicated, genotyping could be used to help identify the patients with ischemic heart disease at greatest risk of depression-associated cardiovascular mortality and thus allow for the testing of focused research intervention(s). It should be noted, however, that our hypothesis regarding increased uptake and storing of serotonin conflicts with some evidence that the 5-HTTLPR genotype is not associated with platelet serotonin concentration (15).
The strengths of this study include the rigorous psychiatric and medical assessment of the subjects and the use of a group of subjects with minimal cardiovascular disease. The main limitation is the relatively small number of subjects and the use of a sample of convenience.
While this novel finding does not have immediate clinical implications, it does illustrate the potential influence of genetic differences on illness course and treatment outcome. Future studies are needed to replicate this association between the l/l 5-HTTLPR genotype and increased platelet activation in geriatric depression; to clarify the relationship among 5-HTTLPR polymorphism, depression, ischemic heart disease, and platelet activation in community-dwelling subjects; and to investigate the association of the l/l genotype with cardiovascular events in depressed patients with ischemic heart disease.
 |
Presented at the 14th annual meeting of the American Association of Geriatric Psychiatry, San Francisco, Feb. 23–26, 2001, and at the 41st New Clinical Drug Evaluation Unit Conference, Phoenix, May 28–31, 2001. Received Dec. 27, 2000; revision received June 7, 2001; accepted Aug. 11, 2001. From the Departments of Psychiatry, Surgery, and Neurology, University of Pittsburgh School of Medicine; and the Departments of Human Genetics and Biostatistics, Graduate School of Public Health, University of Pittsburgh. Address reprint requests to Dr. Whyte, Rm. 886-B, 3811 O’Hara St., Pittsburgh PA 15213; [email protected] (e-mail). Supported by grants MH-01509, MH-01613, MH-52247, MH-30915, MH-43832, MH-37869, and MH-19986 from NIMH and by grant AG-05133 from the National Institute on Aging.
1. Frasure-Smith N, Lespérance F, Talajic M: Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999-1005Crossref, Medline, Google Scholar
2. Musselman DL, Marzec UM, Manatunga A, Penna S, Reemsnyder A, Knight BT, Baron A, Hanson SR, Nemeroff CB: Platelet reactivity in depressed patients treated with paroxetine. Arch Gen Psychiatry 2000; 57:875-882Crossref, Medline, Google Scholar
3. Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, Marzec U, Harker LA, Nemeroff CB: Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313-1317Link, Google Scholar
4. Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS: Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry 1997; 42:290-295Crossref, Medline, Google Scholar
5. Pollock BG, Laghrissi-Thode F, Wagner WR: Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000; 20:137-140Crossref, Medline, Google Scholar
6. Markovitz JH, Shuster JL, Chitwood WS, May RS, Tolbert LC: Platelet activation in depression and effects of sertraline treatment: an open-label study. Am J Psychiatry 2000; 157:1006-1008Link, Google Scholar
7. Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP: Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66:2621-2624Crossref, Medline, Google Scholar
8. Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V: A serotonin transporter gene promoter polymorphism (5HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000; 57:729-738Crossref, Medline, Google Scholar
9. Mulsant BH, Pollock BG, Nebes RD, Miller MD, Little JT, Stack J, Houck PR, Bensasi S, Mazumdar S, Reynolds CF III: A double-blind randomized comparison of nortriptyline and paroxetine in the treatment of late-life depression:6-week outcome. J Clin Psychiatry 1999; 60(suppl 20):16-20Google Scholar
10. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
11. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
12. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF III: Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237-248Crossref, Medline, Google Scholar
13. Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, Davis S, Kirshner MA, Houck PR, Stack JA, Reynolds CF, Kupfer DJ: Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology 2000; 23:587-590Crossref, Medline, Google Scholar
14. del Zoppo GJ: The role of platelets in ischemic stroke. Neurology 1998; 51(3 suppl 3):S9-S14Google Scholar
15. Greenberg BD, Tolliver TJ, Haung S, Li Q, Bengel D, Murphy DL: Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet 1999; 88:83-87Crossref, Medline, Google Scholar



