Mental and Behavioral Disturbances in Dementia: Findings From the Cache County Study on Memory in Aging
Abstract
OBJECTIVE: The authors report findings from a study of 5,092 community residents who constituted 90% of the elderly resident population of Cache County, Utah. METHOD: The 5,092 participants, who were 65 years old or older, were screened for dementia. Based on the results of this screen, 1,002 participants (329 with dementia and 673 without dementia) underwent comprehensive neuropsychiatric examinations and were rated on the Neuropsychiatric Inventory, a widely used method for ascertainment and classification of dementia-associated mental and behavioral disturbances. RESULTS: Of the 329 participants with dementia, 214 (65%) had Alzheimer’s disease, 62 (19%) had vascular dementia, and 53 (16%) had another DSM-IV dementia diagnosis; 201 (61%) had exhibited one or more mental or behavioral disturbances in the past month. Apathy (27%), depression (24%), and agitation/aggression (24%) were the most common in participants with dementia. These disturbances were almost four times more common in participants with dementia than in those without. Only modest differences were observed in the prevalence of mental or behavioral disturbances in different types of dementia or at different stages of illness: participants with Alzheimer’s disease were more likely to have delusions and less likely to have depression. Agitation/aggression and aberrant motor behavior were more common in participants with advanced dementia. CONCLUSIONS: On the basis of their findings in this large community population of elderly people, the authors conclude that a wide range of dementia-associated mental and behavioral disturbances afflict the majority of individuals with dementia. Because of their frequency and their adverse effects on patients and their caregivers, these disturbances should be ascertained and treated in all cases of dementia.
Dementia is a common and growing public health problem. The most common causes of dementia are Alzheimer’s disease and vascular dementia. Both of these are progressive illnesses that lead to functional complications, debilitation, and eventually death (1). A serious and common complication of dementia is the occurrence of mental and behavioral disturbances (2). Many such disturbances have been described. Their classification has typically been phenomenological, relying on a description of mental state and problematic behaviors. The resulting categories range from “psychotic features” (delusions and hallucinations), to depressive symptoms, to a series of other behaviors such as agitation/aggression, wandering, and disinhibition. Such disturbances are associated with earlier nursing home referral, worse prognosis, greater costs, and increased caregiver burden (1, 2).
Several studies have described the frequency of mental and behavioral disturbances in dementia. Prevalence estimates have varied widely in studies using different methods, which have suggested that as few as 25% or as many as 80% of patients with dementia suffer from such disturbances (2–4). There is controversy about the prevalence of different disturbances in patients with different types of dementia. Some studies (5, 6) suggest that depression is specifically more common in vascular dementia than in Alzheimer’s disease. Others (7, 8) disagree. There is also controversy about the occurrence of these disturbances in patients with varying severity of dementia (9, 10), although psychotic symptoms seem to be more common in advanced dementia (11). Almost all relevant studies have come from clinical populations such as patients in dementia, geriatric, or psychogeriatric clinics or Alzheimer’s disease research centers. These sources are subject to referral bias. Estimates from general population samples, although more informative, are rare.
To our knowledge, the only published population-based study of psychiatric phenomena in dementia (12–15) relied on ascertainment of patients with Alzheimer’s disease from a defined area in the United Kingdom. This important study estimated the prevalence of individual mental and behavioral disturbances in 178 individuals with Alzheimer’s disease and reported the following cumulative prevalences of individual mental and behavioral disturbances: delusions, 16%; hallucinations, 17%; major depression, 24%; mania, 3.5%; agitation/aggression, 20%; wandering, 19%; and apathy, 41%. Because that study focused on Alzheimer’s disease, it did not make comparisons between patients with Alzheimer’s disease and nondemented patients. It did not investigate other types of dementia or study the prevalence of various disturbances at different stages of dementia severity. Also, the study was conducted in the United Kingdom, leaving open the possibility that there might be differences in dementia-associated mental and behavioral disturbances between the United Kingdom and the United States and other countries.
Cummings et al. (16) proposed a classification system and measurement method for dementia-associated mental and behavioral disturbances that used the Neuropsychiatric Inventory. The Neuropsychiatric Inventory distinguishes 10 different mental or behavioral disturbances in dementia. Each operationally defined disturbance is ascertained by a trained examiner through an interview with the caregiver. The Neuropsychiatric Inventory is reliable and valid and has received wide acceptance, as is evidenced by its wide use in a variety of studies of dementia. However, to our knowledge, no population-based study has used this instrument to investigate mental and behavioral disturbances in dementia.
In this article we report findings from a study of 5,092 participants who constituted 90% of the elderly resident population of Cache County, Utah. After screening and careful case ascertainment, we identified 329 participants with dementia. Thus, we identified a population-based panel of individuals with dementia who were carefully characterized clinically. We used the Neuropsychiatric Inventory (16) to study all participants with dementia as well as a comparison group of 673 individuals without dementia. We then estimated the population prevalence of individual Neuropsychiatric Inventory mental and behavioral disturbances in individuals with dementia and in the participants without dementia. We compared the prevalence of dementia-associated mental and behavioral disturbances in the two most common types of dementia—Alzheimer’s disease and vascular dementia—and, finally, estimated the prevalence of individual disturbances at different stages of dementia severity.
Method
Sampling and Screening
The methods of the Cache County Study of Memory in Aging are reported in detail elsewhere (17). The sampling method of this study is outlined in Figure 1. Briefly, we approached all permanent residents of Cache County, Utah, who were 65 years old or older in January 1995 (N=5,677). We enrolled 5,092 of these individuals (90%), who were then given the Modified Mini-Mental State (18). Individuals who could not participate directly were characterized by the results of an interview with the Informant Questionnaire on Cognitive Decline in the Elderly (19) conducted with a knowledgeable informant. Individuals whose screening scores suggested possible cognitive impairment were studied further with the Dementia Questionnaire (20). The Dementia Questionnaire was also used to study a stratified probability sample of the entire population (the subsample in Figure 1), irrespective of results on the Modified Mini-Mental State or the Informant Questionnaire on Cognitive Decline in the Elderly. The subsample, and all other participants with a Dementia Questionnaire rating of 4 (suspected dementia) or 5 (probable dementia), underwent a comprehensive clinical assessment in the presence of a collateral informant at their place of residence (including nursing homes).
The study was approved by the institutional review boards of Duke University Medical Center, the Johns Hopkins School of Public Health, and Utah State University. All study participants or their next of kin signed an informed consent document for each stage of assessment.
Participant Assessment, Diagnosis, and Staging of Dementia
A research nurse and a psychometric technician conducted the comprehensive evaluations, along with a board-certified geriatric psychiatrist (D.C.S. or J.C.S.B.). The geriatric psychiatrist directly evaluated 236 participants. Evaluations included history, mental status examination, standardized neurological examination, brief physical examination, and a 1-hour neuropsychological battery. In addition, we reviewed medical records when relevant and requested laboratory tests for participants with dementia, including CBC, chemistries, B12, folic acid, thyroid function tests, and urinalysis, when these tests had not been conducted in the last 6 months. Brain magnetic resonance images or, rarely and only when indicated, computerized tomography scans were also obtained for the majority of participants with dementia. Results of laboratory studies and brain imaging were used in final diagnostic formulations.
We used data from these evaluations to classify study participants into groups of participants who were evidently suffering from dementia or not and into distinct clinical diagnostic categories. These assignments were made at initial diagnostic conferences that included the staff who conducted the evaluation and a board-certified geriatric psychiatrist (D.C.S. or J.C.S.B.). Cases of participants with an initial diagnosis of dementia were then reviewed for final diagnosis at a conference that included the two geriatric psychiatrists, a board-certified neurologist, a senior neuropsychologist, and a cognitive neuroscientist.
Of 1,033 participants examined, 335 (32%) were diagnosed with dementia. Of these, 329 were rated on the Neuropsychiatric Inventory, along with 673 participants without dementia—the rest refused or could not be rated. The 329 participants with dementia who were rated on the Neuropsychiatric Inventory were further classified at the adjudication conference as to their type of dementia. On the basis of the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, 214 were diagnosed with probable or possible Alzheimer’s disease. According to Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria, 62 were diagnosed with probable or possible vascular dementia. An additional 53 received a DSM-IV diagnosis of dementia but did not meet the criteria for Alzheimer’s disease or vascular dementia; 32 of these participants were diagnosed with dementia of unknown etiology, four with frontotemporal dementia, four with hypoperfusion dementia, four with Lewy body dementia, three with alcohol-related dementia, and two each with posttraumatic dementia, normal-pressure hydrocephalus, or Pick’s disease.
Study participants with dementia were also classified by stage or severity of dementia by using the Clinical Dementia Rating (21), a broadly used method for rating stages of dementia. Of the 329 participants with dementia who were rated on the Neuropsychiatric Inventory, 134 were at Clinical Dementia Rating stages 0.5 or 1, 90 were at stage 2, and 105 were at stages 3–5.
Assessment of Mental and Behavioral Disturbances
The 1,002 participants with and without dementia were given the Neuropsychiatric Inventory by a trained psychometrician or nurse. The Neuropsychiatric Inventory is a fully structured informant interview that specifies verbatim the questions asked. Study participants were rated as experiencing or not experiencing each of 10 domains in the past month: delusions, hallucinations, agitation, depression, anxiety, elation, apathy, disinhibition, irritability, and aberrant motor behavior (e.g., wandering or pacing). Within each domain, the Neuropsychiatric Inventory includes a screening question. The rater is instructed to move to the next domain if the screening item is answered in the negative. Otherwise, the rater asks a series of individual questions describing behavior reflective of disturbance in that domain. Once the disturbances relevant to each domain (e.g., delusions) are defined for the informant, then the informant is asked about the frequency of these on a 4-point scale from 1 (occasionally) to 4 (very frequently, more than once a day). The informant is also asked to rate the severity of the behavior on a 3-point severity scale (mild, moderate, or severe).
Within each Neuropsychiatric Inventory domain, scores of 0 to 4 are possible for the frequency of the disturbance and 0 to 3 for its severity. The Neuropsychiatric Inventory then yields a domain rating of frequency times severity (range=0–12). Finally, there is a total Neuropsychiatric Inventory score that sums the individual domain scores.
Analyses
Our first goal was to estimate the prevalence and severity of mental and behavioral disturbances in participants with dementia and compare these with results from nondemented participants. We compared the proportion of participants with and without dementia who had a score of 1 or higher in any individual Neuropsychiatric Inventory domain. (A score of 1 or higher indicates the presence of any disturbance in the domain.) We also compared Neuropsychiatric Inventory mean scores for participants with and without dementia as an indicator of global severity in each domain.
To test the hypothesis that participants with dementia would experience more Neuropsychiatric Inventory disturbances, we constructed a series of logistic regression models, one for each Neuropsychiatric Inventory domain and one for the Neuropsychiatric Inventory as a whole. In these models, the presence or absence of a nonzero score within a given domain (or on the Neuropsychiatric Inventory as a whole) was the dependent variable and the presence or absence of dementia was the independent variable.
To test the hypothesis that participants with dementia had higher Neuropsychiatric Inventory domain or total scores, we constructed a series of linear regression models, one for each domain and one for the total Neuropsychiatric Inventory score. Individual Neuropsychiatric Inventory domain scores or the Neuropsychiatric Inventory total score were the dependent variables, and the presence or absence of dementia was again the independent variable.
Our other goal was to compare the prevalence of mental and behavioral disturbances in participants with different types of dementia, especially Alzheimer’s disease and vascular dementia (the group with other dementias was relatively small [N=53] and heterogeneous). As before, we used logistic regression to compare the prevalence of individual disturbances and linear regression to compare Neuropsychiatric Inventory domain and total scores.
Our final goal was to assess the prevalence and severity of dementia-associated mental and behavioral disturbances across the different stages of dementia. For this purpose we calculated the prevalence of different disturbances and the mean Neuropsychiatric Inventory domain (and total) scores for participants with dementia having different Clinical Dementia Rating ratings (those with ratings of 0.5 or 1, those with ratings of 2, and those with ratings of 3–5). We used logistic regression to compare the prevalence of individual disturbances across the groups with different Clinical Dementia Rating stages, and we used analysis of variance (ANOVA) to compare Neuropsychiatric Inventory domain and total scores across the three Clinical Dementia Rating stages.
There was a small amount of missing Neuropsychiatric Inventory data (less than 5%). We note in the tables the number of participants for whom data were available for each domain of the Neuropsychiatric Inventory.
Results
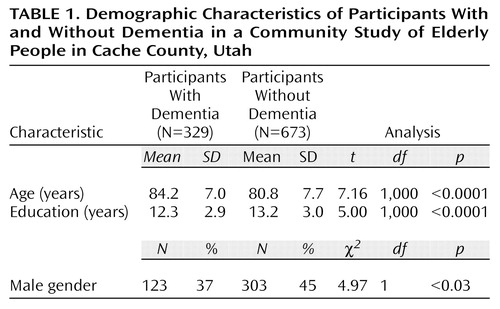
Table 1 shows the demographic characteristics of participants with and without dementia. The participants with dementia were older and less educated, and more of them were women.
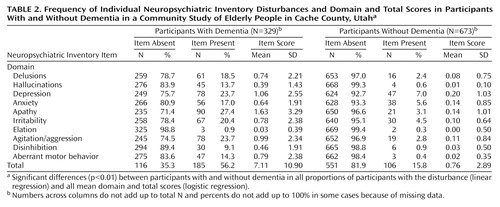
Table 2 compares participants with and without dementia. Among those with dementia, 201 (61%) suffered from at least one Neuropsychiatric Inventory disturbance, and 105 (32%) scored at least 6 total points on the Neuropsychiatric Inventory, indicating a moderate to severe disturbance. The mean Neuropsychiatric Inventory score for participants with dementia was about 7, almost 10 times higher than the comparable score for participants without dementia. The most frequent disturbance reported for participants with dementia was apathy (27%). Depression and agitation/aggression were nearly as common, each reported in 24% of participants with dementia. The least frequent symptom was elation, reported in only 1%. Neuropsychiatric Inventory domain scores also reflected these frequencies, with apathy, depression, and agitation/aggression having the highest mean scores. In every Neuropsychiatric Inventory domain, both the proportion of participants with the disturbance and the mean Neuropsychiatric Inventory score were significantly higher in the participants with dementia than in the participants without dementia (for all logistic and linear regression models, p<0.01).
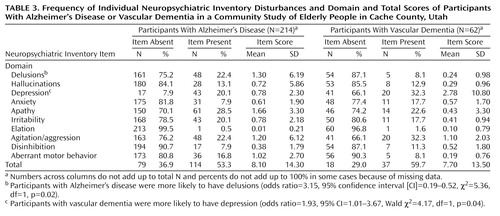
Table 3 compares the prevalence of Neuropsychiatric Inventory disturbances in participants with Alzheimer’s disease and those with vascular dementia. In general, the prevalence of Neuropsychiatric Inventory disturbances was similar in the two groups (p>0.10 for linear and logistic regression models of either total Neuropsychiatric Inventory scores or domain scores for hallucinations, agitation/aggression, anxiety, apathy, irritability, elation, disinhibition, and aberrant motor behavior).
There were two differences between Alzheimer’s disease and vascular dementia in the logistic regression models: participants with Alzheimer’s disease were more likely to have delusions, and participants with vascular dementia were more likely to have depression (Table 3). In linear regression models, participants with vascular dementia had higher mean scores in the depression domain than participants with Alzheimer’s disease (beta=0.145, t=2.42, df=1, p=0.02). There were no other significant differences in the comparison of total and domain Neuropsychiatric Inventory mean scores between individuals with vascular dementia and those with Alzheimer’s disease (in all domains, p>0.07 in linear regression models).
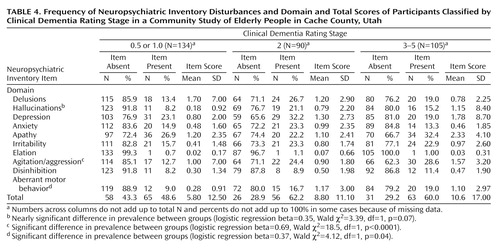
Table 4 shows the prevalence of individual Neuropsychiatric Inventory disturbances by dementia stage, as classified by the Clinical Dementia Rating. Differences in the prevalence of individual Neuropsychiatric Inventory disturbances across levels of severity of dementia were assessed in logistic regression models. Differences in the prevalence of agitation/aggression (13% in mild dementia, 24% in moderate dementia, and 29% in severe dementia) were statistically significant (Table 4). Differences in the prevalence of aberrant motor behavior (9% in mild, 17% in moderate, and 19% in severe dementia) were also statistically significant (Table 4). The differences in prevalence of hallucinations across Clinical Dementia Rating stages (8% in mild, 21% in moderate, and 15% in severe dementia) fell just short of statistical significance (Table 4). There were no other substantial differences in the prevalence of other Neuropsychiatric Inventory disturbances across Clinical Dementia Rating stages.
In the ANOVA of mean scores within the Neuropsychiatric Inventory domains, only depression showed statistically significant differences across Clinical Dementia Rating stages (F=5.86, df=1, 272, p=0.02). Scores in the depression domain increased with severity of dementia. There were no other significant differences in mean Neuropsychiatric Inventory domain or total scores (p>0.10 for all other linear and logistic regression models).
Discussion
We report findings on the prevalence and severity of 10 dementia-associated mental and behavioral disturbances in a population-based panel of elderly participants with dementia and in a probability sample of elderly participants without dementia. Because these rates are not subject to any substantial referral bias, they should represent direct estimates of the prevalence of these disturbances in the subject population. The described method of assessing mental and behavioral symptoms is widely considered to be at or near the state of the art (22). To our knowledge, these are the first U.S. estimates of the population prevalence of these disturbances in individuals with dementia and the first such estimates worldwide based on the Neuropsychiatric Inventory.
The point prevalence of any Neuropsychiatric Inventory disturbance was 61%; for any serious disturbance it was 32%. Apathy was the most common disturbance reported, and depression or agitation/aggression were present in one out of every four participants. The prevalence of all disturbances was much higher in participants with dementia than in those without dementia. Estimates for participants with Alzheimer’s disease are similar to those reported by Burns et al. (12–15). Thus, the two published population-based studies of these disturbances in participants with dementia agree on the frequency of individual disturbances. The comparison of participants with Alzheimer’s disease and vascular dementia yielded few consistent differences, a finding that has been observed elsewhere (5–8). Given that the estimates here are cross-sectional and considered only over the previous month, they are probably underestimates of the cumulative prevalence of these disturbances over the course of a dementing illness, which may approach 70%–80% (2, 4).
Our results lend modest support to previous reports of higher rates of depression in vascular dementia (5, 6). They also suggest that delusions may be more common in Alzheimer’s disease. These differences in the frequency of individual disturbances may reflect differences in the brain regions involved or the pathophysiology of Alzheimer’s disease and vascular dementia. Thus, vascular dementia, which is more likely to affect subcortical areas widely believed to be involved in mood disorders (23), is more closely associated with depression. In contrast, Alzheimer’s disease, which damages temporal lobe areas often associated with psychosis (24, 25), is more closely associated with delusions.
Differences in the prevalence of Neuropsychiatric Inventory disturbances across stages of dementia severity are of similar interest. In general, the differences observed were few and inconsistent. Several disturbances (delusions, anxiety, apathy, irritability, elation, and disinhibition) were reported with similar severity at all stages of dementia. By contrast, aggression/agitation and aberrant motor behavior were more common at later stages. The latter finding may reflect progressive disturbance in the control of behavior accompanying the advancing brain damage of dementing diseases. Our findings of increased occurrence of depression and hallucinations in moderately severe dementia were relatively weak but are consistent with previous reports (6, 9, 10).
The brain areas involved in Alzheimer’s disease and other dementias are heterogeneous. This gives rise to distinct clinical pictures, with substantial interpatient variability, including mental and behavioral disturbances. Many of these disturbances probably arise from the underlying disease affecting critical brain areas. For example, studies have shown that depression in Alzheimer’s disease is associated with damage to the locus ceruleus (26–28) and that aggression is associated with damage to the serotonergic centers of the brain (29) or with relative preservation of dopaminergic areas (30). Further careful investigation of brain/behavior relationships in dementia should reveal more such relationships, many involving damage to the limbic system, frontal lobes, or critical neurotransmitter systems.
A few study limitations require discussion. First, although this was an epidemiologic study, it sampled a population that may not be representative of others. The Cache County population is older and less ethnically diverse than the rest of the United States. It has higher rates of religious affiliation and lower rates of alcoholism and substance use than elsewhere (17). The comparability of these findings with those of Burns et al. (12–15) in the United Kingdom are somewhat reassuring in this regard. Second, the principal method of assessment of mental and behavioral disturbances relied on informants and not on direct examinations of participants. Again, however, we note that this method generated estimates similar to those of Burns et al. (12–15), who used psychiatric assessment. Also, the limitations of the Neuropsychiatric Inventory method should not apply to comparisons of Neuropsychiatric Inventory findings within our study population.
We conclude that mental and behavioral disturbances are a central component of dementia, regardless of cause and regardless of stage. Although the dementia syndrome is defined by its core cognitive features, it seems clear that almost all individuals with dementia will exhibit mental or behavioral disturbances at some point in their illness. These disturbances add substantially to the morbidity and disability of their illness and to its burden on their caregivers (4); therefore, it is unrealistic to tackle the problem of dementia without careful attention to these disturbances. Experience from general psychiatric practice seems to suggest an approach to the treatment of most mental and behavioral disturbances, but there are few intervention studies and clinical trials of methods for their treatment (26–34). Most of these studies have focused on symptom reduction, have been short-term, or have studied single pharmacological interventions. Thus, there is a critical need for research that explores more complex interventions (e.g., combinations of pharmacological and other strategies) and focuses on a broader range of outcomes (e.g., disability, nursing home admission, and caregiver burden). Clinicians in all settings, particularly those in primary care, where most dementia care is practiced, should pay increased attention to the identification and treatment of these disturbances in their patients.
 |
 |
 |
 |
Received April 20, 1999; revision received Sept. 27, 1999; accepted Nov. 5, 1999. From the Neuropsychiatry Service, Department of Psychiatry and Behavioral Sciences, School of Medicine, and the Department of Mental Hygiene, School of Public Health, Johns Hopkins University; the Department of Psychology and College of Family Life, Utah State University, Logan; and the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, N.C. Address reprint requests to Dr. Lyketsos, Osler 320, Johns Hopkins Hospital, Baltimore, MD 21287; [email protected] (e-mail). Supported by National Institute on Alcohol and Alcohol Abuse grant AG-11380 for the Cache County Study of Memory in Aging and by NIMH grant MH-56511 for the Depression in Alzheimer’s Disease Study (DIADS). The authors thank Jeannie-Marie E. Sheppard, B.A., for assistance with data analysis.
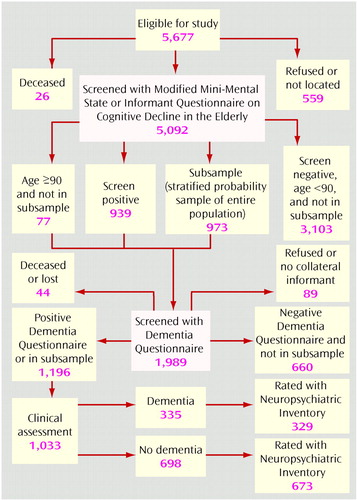
Figure 1. Sampling Method in a Community Study of Dementia Among Elderly People in Cache County, Utah
1. Rabins PV, Lyketsos CG, Steele CD: Practical Dementia Care. New York, Oxford University Press, 1999Google Scholar
2. Lyketsos CG, Steele C, Steinberg M: Behavioral disturbances in dementia, in Reichel’s Care of the Elderly: Clinical Aspects of Aging, 5th ed. Edited by Gallo JJ, Busby-Whitehead J, Rabins PV, Silliman RA, Murphy JB, Reichel W. Baltimore, Williams & Wilkins, 1999, pp 214–228Google Scholar
3. Mega MS, Cummings JL, Fiorello T, Gornbein J: The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996; 46:130–135Crossref, Medline, Google Scholar
4. Finkel S (ed): Behavioral Disturbance in Dementia. Int Psychogeriatrics 1996; 8(suppl 3)Google Scholar
5. Groves WC, Brandt J, Steinberg M, Warren A, Rosenblatt A, Baker AS, Lyketsos CG: Vascular dementia and Alzheimer disease: is there are difference? a comparison of symptoms by disease severity. J Neuropsychiatry Clin Neurosci (in press)Google Scholar
6. Payne JL, Lyketsos CG, Baker L, Warren A, Steele C, Brandt J, Steinberg M, Kopunek S, Baker A: The relationship of cognitive and functional impairment to depressive features in Alzheimer’s disease and other dementias. J Neuropsychiatry Clin Neurosci 1998; 10:440–447Crossref, Medline, Google Scholar
7. Molsa PK, Martilla RJ, Rinne UK: Long-term survival and predictors of mortality in Alzheimer’s disease and multi-infarct dementia. Acta Neurol Scand 1995; 91:159–164Crossref, Medline, Google Scholar
8. Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL: A comparison of psychiatric symptoms in vascular dementia and Alzheimer’s disease. Am J Psychiatry 1993; 150:1806–1812Google Scholar
9. Drevets WC, Rubin EH: Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry 1989; 25:39–48Crossref, Medline, Google Scholar
10. Rosen J, Zubenko GS: Emergence of psychosis and depression in the longitudinal evaluation of Alzheimer’s disease. Biol Psychiatry 1991; 29:224–232Crossref, Medline, Google Scholar
11. Rao V, Lyketsos CG: Delusions in Alzheimer’s disease: a review. J Neuropsychiatry Clin Neurosci 1998; 10:373–382Crossref, Medline, Google Scholar
12. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, I: disorders of thought content. Br J Psychiatry 1990; 157:72–76Crossref, Medline, Google Scholar
13. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, II: disorders of perception. Br J Psychiatry 1990; 157:76–81Crossref, Medline, Google Scholar
14. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, III: disorders of mood. Br J Psychiatry 1990; 157:81–86Crossref, Medline, Google Scholar
15. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, IV: disorders of behavior. Br J Psychiatry 1990; 157:86–94Crossref, Medline, Google Scholar
16. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Google Scholar
17. Breitner JCS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton M, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A: APOE-epsilon 4 count predicts age when prevalence of Alzheimer’s disease increases—then declines: the Cache County Study. Neurology 1999; 52:321–331Crossref, Medline, Google Scholar
18. Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48:314–318Medline, Google Scholar
19. Jorm AF: A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24:145–153Crossref, Medline, Google Scholar
20. Silverman JM, Breitner JCS, Mohs RC, Davis KL: Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am J Psychiatry 1986; 143:1279–1282Google Scholar
21. Morris J: Clinical Dementia Rating Scale. St Louis, Washington University, 1994Google Scholar
22. Lyketsos CG, Steinberg M: Cognitive disorders, non-cognitive measures, in Handbook of Psychiatric Measures. Washington, DC, American Psychiatric Association (in press)Google Scholar
23. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M: Vascular depression hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Medline, Google Scholar
24. Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, Kopp U: Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol 1991; 48:619–624Crossref, Medline, Google Scholar
25. Starkstein SE, Vazquez S, Petracca G: A SPECT study of delusions in Alzheimer’s disease. Neurology 1994; 44:2055–2059Google Scholar
26. Nyth AL, Gottfries CG: The clinical efficacy of citalopram in treatment of emotional disturbances of dementia subjects. Br J Psychiatry 1990; 157:894–901Crossref, Medline, Google Scholar
27. Reifler BV, Teri L, Raskind M, Veith R, Barnes R, White E, McLean P: Double-blind trial of imipramine in Alzheimer’s disease patients with and without depression. Am J Psychiatry 1989; 146:45–49Link, Google Scholar
28. Petracca G, Teson A, Chemerinski E, Leiguarda R, Starkstein SE: A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1996; 8:270–275Crossref, Medline, Google Scholar
29. Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M (Risperidone Study Group): Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry 1999; 60:107–115Crossref, Medline, Google Scholar
30. Street J, Clark WS, Gannon KS, Miran S, Sanger T, Tollefson GD: Olanzapine in the treatment of psychosis and behavioral disturbances associated with Alzheimer’s disease, in 1999 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1999, pp 225–226Google Scholar
31. Tariot PN, Erb R, Podgorski CA, Cox C, Patel S, Jakimovich L, Irvine C: Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998; 155:54–61Link, Google Scholar
32. Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B: Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology 1998; 50:1222–1230Google Scholar
33. Lyketsos CG, Veiel LL, Sheppard JME, Baker A, Steele CS: A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry 1999; 14:520–525Crossref, Medline, Google Scholar
34. Teri L: Behavioral treatment of depression in patients with dementia. Alzheimer Dis Assoc Disord 1994; 8(suppl 3):66–74Google Scholar



