Cytokines and the Brain: Implications for Clinical Psychiatry
Abstract
OBJECTIVE: This article reviews recent developments in cytokine biology that are relevant to clinical psychiatry.METHOD: The authors reviewed English-language literature of the last 15 years that pertains to the biology of cytokines with emphasis on central nervous system effects in general and psychiatric disorders in particular.RESULTS: Growing evidence suggests that, in addition to providing communication between immune cells, specific cytokines play a role in signaling the brain to produce neurochemical, neuroendocrine, neuroimmune, and behavioral changes. This signaling may be part of a generalized, comprehensive mechanism to mobilize resources in the face of physical and/or psychological stress and to maintain homeostasis. The clinical implications of these findings are far-reaching and include a possible role for cytokines in the pathophysiology of specific psychiatric disorders such as major depression, schizophrenia, and Alzheimer’s disease. The effects of cytokines in the central nervous system may provide a possible mechanism for the “sickness behavior” of patients with severe infection or cancer, as well as for the neuropsychiatric adverse effects of treatment with interferons and interleukins.CONCLUSIONS: A better understanding of the role of cytokines in various brain activities will enhance knowledge of specific psychobiological mechanisms in health and disease and provide opportunities for novel treatment interventions.
Neuroscience and immunology are two of the fastest growing fields of knowledge in the medical sciences. One area where these two fields overlap is the biology of cytokines. Cytokines are generally known as chemical messengers between immune cells. As such, they play a crucial role in mediating inflammatory and immune responses. The discovery a decade or two ago that cytokines signal the brain and serve as mediators between immune and nerve cells is far-reaching in its implications. The brain is now seen as capable of influencing immune processes. Conversely, changes in brain activity can occur in association with an immunologic response. In this context, the immune system is seen as a sensory organ, monitoring not so much the external world, but rather the internal milieu of the organism. Information regarding infection or injury is conveyed to the brain, which in turn sets in motion important metabolic and behavioral pathways directed toward maintenance of homeostasis and restoration of health. Many of these functions are mediated by cytokines, which can often act both as immunomodulators and as neuromodulators. A large body of literature is now emerging that details interactions between brain cells and immune cells and the role of specific cytokines in these complex interactions (1–3).
On the clinical level, advances in cytokine research have had a profound effect on our understanding of the pathophysiology of various medical conditions and on the formulation of new treatments (4). These developments are particularly relevant to immune-related disorders such as infection, allergy, autoimmune diseases, and cancer. Furthermore, clinicians dealing with various clinical entities are incorporating specific cytokines in their patients’ treatment regimens. Clinical psychiatrists can benefit from familiarizing themselves with principles of cytokine physiology, both to better understand the role cytokines may play in the pathophysiology of specific psychiatric disorders and to deal more effectively with the neuropsychiatric adverse effects of treatment with cytokines.
Structure and Function of Cytokines
Major Properties of Cytokines
The cytokines are a diverse group of proteins that may be regarded as the hormones of the immune system. These small molecules can be secreted by various cells and act as signals between cells to regulate the immune response to injury and infection. They may be considered to be hormones because their properties are similar to those of the classic hormones of the endocrine system. Cytokines are frequently regulated in cascades, where induction of the early cytokines serves to increase the production of later cytokines (e.g., interleukin-1 [IL-1] stimulates the release of IL-2, IL-6, and tumor necrosis factor [TNF]). The specificity of the response to cytokines is provided by unique cytokine receptors. Cells that express a functional receptor for a cytokine will respond to the presence of that cytokine. These interactions of cytokines and cytokine receptors are a necessary component of the physiologic response to cytokines. Cytokine receptors can also be found in a soluble form. Usually, a soluble receptor for a specific cytokine can inhibit the biological activity of the cytokine by inhibiting the binding of the cytokine to its membrane-anchored receptor (For example, soluble TNF receptors decrease the biological activity of TNF by inhibiting the binding of TNF to its specific surface receptor.) In some instances, the binding of the cytokine to its soluble receptor can form a complex that enhances the biological activity of the cytokine. This rare situation is seen, for example, when IL-6 binds to soluble IL-6 receptors, forming a biologically active complex that adds to the activity of IL-6.
Classification of Cytokines
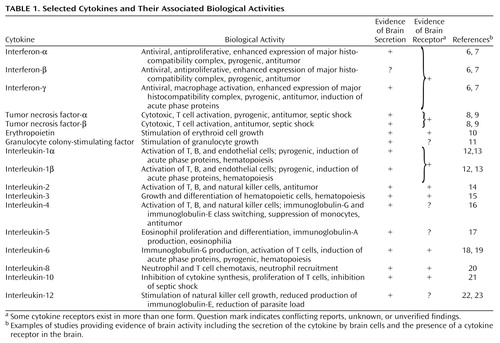
The nomenclature of the cytokines can be confusing, primarily because they are frequently named for their biological activity. The names of some of the early identified cytokines have become so ingrained in the literature that these names will probably not change despite attempts to standardize the nomenclature. Referring to the cytokines by their activity lacks specificity because cytokines are pleotropic and are involved in multiple biological activities. It is possible for two groups of investigators to be working on the same molecule, but to be focusing on different aspects of its functional capacity. Attempts at standardization have therefore included assigning interleukin numbers to cytokines as their genes are sequenced. This method has not been uniformly applied to all newly discovered cytokines, partly because many members of supergene families are often discovered. For example, IL-8 was initially described, and subsequently multiple additional members of this family have been cloned. This group of molecules associated with IL-8 are called chemokines (5). Table 1 provides a partial listing of cytokines that may be relevant to psychiatry and highlights evidence of secretion of cytokines by brain cells (astrocytes and/or microglia) and evidence of cytokine receptors in the brain. A good deal of the work on activity of cytokines in the brain is still in progress.
Several cytokines (e.g., TNF, interferon, and IL-1) are available in more than one form, as they are the product of more than one gene. They are given a suffix from the Greek alphabet (α, β, γ) to distinguish them from one another. Although these cytokines have limited structural homology, they usually bind to the same surface receptors and have similar if not identical biological properties. The complete list of cytokines is constantly being updated (24). At the time this article was written, the list extended to IL-18.
Proinflammatory cytokines
Cytokines such as IL-1, IL-6, and TNF may act in a proinflammatory manner, augmenting the immune response to help speed the elimination of pathogens and the resolution of the inflammatory challenge. There are multiple mechanisms for accomplishing this effect. Individual cells may be activated so that they function more efficiently. Within the host many aspects of physiology such as rate of metabolism and temperature regulation may be altered to achieve a more proinflammatory state. Some of the effects of cytokines are direct, some are indirect, and many are both direct and indirect. For example, recruitment of neutrophils to sites of inflammation is a multistep process that involves the direct action of IL-8 as a chemotactic agent and the indirect actions of TNF and IL-1 to up-regulate adhesion molecules on endothelial cells.
Antiinflammatory cytokines
Cytokines may also serve to dampen the immune response. Examples of antiinflammatory cytokines are IL-4, IL-10, and IL-13. Some of these cytokines serve to decrease cell function and synthesis of other cytokines. IL-10 was originally called cytokine synthesis inhibitory factor because one of its major biological activities was to block the production of cytokines by other T cells. The receptors for the cytokines also may serve as major inhibitors of the immune response. Some cytokines may serve both a proinflammatory and an antiinflammatory role, although this combination is rather unusual. In many instances, the inflammatory state or condition dictates how the cytokine will perform. For example, at sites of local inflammation, IL-8 serves to recruit neutrophils. Yet high circulating levels of IL-8 will act to decrease infiltration of neutrophils to inflammatory sites.
Cytokine production has also been divided into two broad categories depending on the functional profile of the secreting T helper cells: type 1 T helper responses (TH1) generally mediate cellular immune reactions and include production of the cytokines interferon, TNF, and IL-12; type 2 T helper responses (TH2) enhance antibody-mediated immune reactions and include production of the cytokines IL-4 and IL-5 (25). Shifts in the balance of type 1 and type 2 reactions mediated by stress have also been reported (22).
Hematopoietic cytokines
One of the principal functions of cytokines is their ability to alter the hematopoietic response. These cytokines may be called hematopoietic cytokines and include interleukins (e.g., IL-3 and IL-5) and the colony-stimulating factors. The colony-stimulating factors are usually named for the type of colonies that are formed when the cytokine is mixed with bone marrow cells.
Cytokines and Immunogenetics
The study of the genetic basis of disease is a rapidly growing science. Because cytokines play a central role in the pathophysiology of various medical illnesses (Figure 1), genetic polymorphism of specific cytokines and their association with specific disease entities have been widely investigated. Much of the work has centered on TNF-α, although other cytokines have also been studied (26). Polymorphism in TNF genes has been associated with several illnesses, including multiple sclerosis, asthma, myasthenia gravis, and septic shock (27). No association has yet been reported between cytokine polymorphism and psychiatric illness. The association with septic shock, however, presents interesting theoretical ramifications for psychiatry. Although septic shock is undeniably caused by identifiable bacterial pathogens (environmental factors), genetic polymorphism at the TNF-α locus, and in particular the presence of the TNF-2 allele, increases the host’s risk of developing septic shock and the chance of mortality associated with it (28). This model of gene-environment interaction may be of relevance to psychiatry. Genetic polymorphism at a yet unidentified locus or loci relevant to a specific psychiatric illness may have profound implications for the risk of developing the illness in the face of varying environmental stressors. Polymorphism within the promoter of the serotonin transporter gene, for instance, has been associated with a differential response to specific antidepressant medications (29).
Quantitative Assessment of Cytokines
Cytokine measurements may be divided into two broad categories: assays based on the immunological detection of peptides (immunoassays) and assays based on a biological response (bioassays). There are distinct advantages and disadvantages to each (30).
Immunoassays
All immunoassays rely on at least one antibody that specifically detects the cytokine that is being measured. The immunoassay may be either in the form of a radioimmunoassay or enzyme-linked immunosorbent assay (ELISA). In practice, most immunoassays that are currently being done use an ELISA format. Most immunoassays have exquisite specificity, which is due to the use of monoclonal antibodies. Because the immunoassay is based on an antibody that recognizes only a small portion of the total protein, it is possible that the assay will measure degraded fragments of the protein, not material that is biologically active. In addition, many immunoassays will detect cytokines that are bound to inhibitory substances. In the normal immune response, soluble receptors or receptor antagonists are often produced shortly after the production of the cytokines. These serve to block the biological activity of the cytokines and also to maintain them in circulation for a prolonged period.
Bioassays
Bioassays measure the functional activity of cytokines. They require that some measure of biological activity be recorded. In practice, this usually means using cell lines and evaluating either cell proliferation or cell lysis. Depending on the particular method, a bioassay may be more or less sensitive than an immunoassay. One difficulty with bioassays is their lack of specificity. As an example, the thymocyte co-proliferation assay was routinely used in the past to measure IL-1. However, these cells have since been shown to respond to TNF, IL-2, IL-6, and IL-10. Bioassays may also be blocked by potential inhibitors present in the samples. These include soluble receptors, naturally occurring anticytokine antibodies, and antagonist proteins. Many of these products are produced as part of the normal immune response, where they serve to limit the actions of the cytokine in vivo. In addition, cytokines may be proteolytically degraded at sites of active inflammation, and the fragments will not retain biological activity. Use of bioassays as compared to immunoassays allows understanding of the functional participation of the cytokine in the inflammatory response, because bioassay results indicate not only whether the cytokine is present, but whether it is biologically active.
Cytokines and Neuroimmunology
The brain has for many years been considered an immunologically privileged site, suggesting reduced or altered immunological responsivity. Evidence in this regard includes the brain’s lack of adequate lymphatic systems to capture antigens, protection from circulating blood by the blood-brain barrier, and failure to exhibit a “classic inflammatory response” characterized by early invasion of macrophages and leukocytes. Recent developments in neuroimmunology however have challenged some of these concepts (31). The brain can certainly exhibit many of the hallmarks of inflammation in response to infection or injury. They include edema, activation of resident phagocytes (microglia), local invasion of circulating immune cells, and production of cytokines. The role of these various cytokines in different brain activities is a topic of intense investigation and debate at the present time.
Cytokine Localization in the Brain
Before we discuss any role for cytokines in brain activity, we need to address the issue of the sources of brain cytokines, because cytokines as such cannot cross the blood-brain barrier, at least under physiologic conditions. How then do cytokines communicate with brain cells? Four mechanisms for brain signaling by cytokines have been postulated (32): 1) passive transport of cytokines into the brain at circumventricular sites lacking a blood-brain barrier; 2) binding of cytokines to cerebral vascular endothelium, thereby inducing the generation of secondary messengers such as prostaglandins and nitric oxide; 3) carrier-mediated transport of cytokines into the brain, across the blood-brain barrier; and 4) activation by cytokines of peripheral afferent nerve terminals at the site where cytokines are released. These mechanisms are not mutually exclusive. They depend in part on the location of the inflammatory stimulus and the disease state of the organism.
But cytokines do not always have to reach the brain, either directly or indirectly, from the periphery to be able to play a role in brain activity. As shown in Table 1, most cytokines can be synthesized and released within the central nervous system. Although most cytokines in the brain are secreted by astrocytes and/or microglia, some evidence suggests that under certain conditions, neurons can also produce cytokines (33). Furthermore, although cytokines are usually secreted in response to specific stimuli such as infection or injury, there is evidence that low-level expression of specific cytokines is constitutive in blood vessels within the brain (6). Cytokines in the brain are also regulated in cascades, with evidence of feedback loops, both positive and negative, at different levels. As for the localization of cytokine pathways within the brain, IL-1 has been found in several brain regions, including the hippocampus and specific hypothalamic structures such as the paraventricular nucleus and the arcuate nucleus. IL-1 immunoreactive nerve fibers have also been described in the human brain, particularly in the hypothalamus (12). Widespread TNF and other cytokine immunoreactivity has been detected in the murine brain (8). Cytokine receptors have also been detected in several areas of the brain (Table 1). The precise mapping of various cytokine pathways and their receptors in the brain, however, remains incomplete.
Cytokines and Associated Brain Activities
Once in the brain, cytokines have been associated with various brain activities. These include immunologic, neurochemical, neuroendocrine, and behavioral activities. Here again, the bulk of the experimental evidence centers on IL-1 (13). Figure 2 illustrates selected effects of IL-1 in the brain, as well as elsewhere in the body. For a more complete presentation of IL-1 effects, several reviews are available (13, 34).
Immunologic effects
Consistent with its activities in other organs, IL-1 in the brain stimulates the production of other cytokines by specialized cells, in this case astrocytes and microglia. Human astroglial cell lines stimulated by IL-1 have been shown to produce colony-stimulating factor, TNF-α, additional IL-1, and IL-6 (9, 11). There is also evidence that intracerebroventricular injections of IL-1b in rats are associated with a decrease in natural killer (NK) cell activity of circulating lymphocytes (35) and the release of IL-6 into the blood stream (36). IL-1 directly injected into the brain can also stimulate astrogliosis and produce neovascularization (37).
Neurochemical effects
A number of studies suggest that specific neurochemical changes occur in the brain of animals in response to an immunological challenge (38, 39). The same effects could also be obtained with systemic injection of cytokines. Dunn and Wang (40) have shown that intraperitoneal injections of IL-1b to mice were accompanied an hour later by increases in brain concentrations of 3-methoxy;4-hydroxyphenylethyleneglycol (MHPG) (mostly in the hypothalamus), 5-hydroxyindoleacetic acid (5-HIAA) (in different parts of the brain), and much smaller increases in 3,4-dihydroxyphenylacetic acid (DOPAC). Similar results have been reported for IL-2 and to some extent IL-6. TNF and interferon, however, do not seem to produce such effects.
Neuroendocrine effects
Although it has been known for many years that infection is often accompanied by an increase in hypothalamic-pituitary-adrenal (HPA) activity, the exact mechanism has only been elucidated in the last 15 years. Besedovsky and colleagues (41) first reported that the immune response to an injection of sheep red blood cells into rats was accompanied by increases in plasma levels of corticosterone. Several investigators later reported that administration of IL-1 was associated with increases in corticotropin-releasing hormone, ACTH, and corticosteroids (42, 43). There is also evidence that IL-6, TNF, and interferon are capable of stimulating the HPA axis both in rodents and in man (44). The results are equivocal for IL-2. Regardless of the mechanism involved, HPA activation and the resultant hypercortisolemia frequently act as a negative feedback mechanism to suppress an otherwise exaggerated immune/inflammatory response initiated by the chain of cytokines (Figure 1). This delicate balance seems to be part of a homeostatic mechanism, the failure of which could result in serious disorders such as infection, cancer, or autoimmune disease. Because the brain plays a central role in this feedback loop, factors such as emotions and/or psychosocial stressors can tilt the balance one way or the other. There is also evidence that cytokines can modulate the hypothalamic-pituitary-thyroid axis (45) and the hypothalamic-pituitary-gonadal axis (46).
Behavioral effects
Although part of the response of the organism to an infection or injury is local and occurs at the cellular and molecular levels, another part is more general, occurs at the level of the entire organism, and involves specific adaptive behaviors. These effects can also be seen as part of the overall mechanism to maintain homeostasis. We know now that several of the behaviors associated with infection, such as increased sleep, decreased appetite, and decreased sexual drive, which are often referred to as “sickness behavior,” may be at least partly attributed to the specific effects of cytokines. These behaviors can be “adaptive” during the course of an acute infection or trauma, allowing the organism to mobilize all necessary resources to the task of healing itself.
Although it is well recognized that infection is commonly associated with somnolence and fatigue, the mechanism of such behaviors has only been investigated within the last 10 years. It is now believed, for instance, that muramyl peptide, a common microbial product, and endotoxin stimulate the release of IL-1 and that IL-1 interacts with specific neurohormones and neurotransmitters in the brain to produce somnogenic activity (47). Additional evidence suggests that plasma levels of IL-1 peak at the onset of slow-wave sleep in healthy human volunteers (48) and levels of IL-1 in cerebrospinal fluid increase during sleep (49). Specific cytokines therefore seem to play a role in sleep regulation, particularly during an infection. Although most research in this area has focused on IL-1, there is growing evidence that TNF-α and interferon may also have somnogenic activities, while IL-2, IL-6 and TNF-b probably do not (50). Sleep regulation is a complex phenomenon and involves interactions between neuropeptides, biogenic amines, and other neurotransmitters. The exact role played by specific cytokines remains to be determined.
Another commonly observed behavior during infection and cancer is diminished appetite or anorexia. Plata-Salaman and colleagues (51) studied the effects of chronic intracerebroventricular microinfusion of various cytokines on feeding and drinking behaviors in rats. They found that IL-1b decreased nighttime feeding in these animals in a dose-dependent manner. This effect was abolished if IL-1b was heat-inactivated or was given along with IL-1 receptor antagonist. Water intake was not affected in this paradigm. TNF-α and IL-8 were less effective than IL-1b in producing these changes. IL-2, IL-4, and IL-10 had no such effects. In human studies, treatment with cytokines is often accompanied by nausea and anorexia as common adverse effects. Furthermore, there is evidence that patients with anorexia nervosa, despite a decreased capacity to produce IL-2, have in their sera one or more factors that stimulate the production of cytokines (52).
Stress and Cytokine Secretion
The physical stress of infection or trauma has long been known to stimulate cytokine production, and the effects of psychological stress on cytokine secretion have also been investigated. Weiss and colleagues (35), using an animal (rat) model of stress (intermittent tail shock), found that in vitro IL-2 and interferon production were significantly reduced in lymphocytes from stressed animals compared to nonstressed control animals. These effects were largely independent of the effects of glucocorticoids, as they could be reproduced in animals after their adrenals had been removed. The authors also noted that the effects of stress were not uniformly immunosuppressive and that enhancement of cytokine production was observed when certain specific conditions were met: Le May and coinvestigators (18) used exposure of rats to an open field as the psychological stressor in a series of experiments. They found that plasma IL-6 activity increased within 30 minutes of exposure to the stressor. Unlike the stress from tail shock, the open-field stress was purely “psychological” and involved no physical contact with the animal. In a different line of experiments, Persoons and colleagues (53) found that isolated alveolar macrophages from rats exposed to stress showed a marked rise in IL-1b and TNF-αa secretion, but no changes in the production of IL-6. In addressing discrepancies between results, it is important to note that 1) the effects of stress are not uniform and vary with such factors as nature of the stressor, its intensity and duration, and control over the stressor; 2) the effects vary depending on the organ/tissue or cells being investigated; and 3) different cytokines may be affected differently by the same stress paradigm.
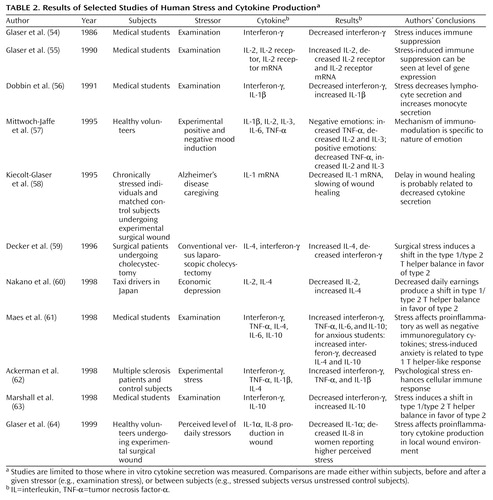
The effects of stress on cytokine regulation in humans have also been investigated. Many studies have measured the circulating levels of various cytokines in relation to one or more specific stressors. However, the measurement of plasma levels is not very reliable. Values are often undetectable or highly variable and therefore difficult to interpret. In vitro cytokine secretion by a subject’s stimulated peripheral blood lymphocytes (or whole blood) usually provides more useful information regarding the quantity and/or activity of a particular cytokine. We have therefore limited our review of the literature on human stress and cytokines to studies that have measured in vitro production of cytokines (Table 2). As shown in Table 2, a common paradigm has been the evaluation of examination stress in medical students and comparison of patterns of cytokine secretion in the same subjects before and after the examination (54–56, 61, 63). Other strategies have included comparisons of cytokine secretion between chronically stressed individuals (e.g., caregivers for patients with Alzheimer’s disease) and age-matched comparison subjects (58), as well as the use of various other natural (60, 64) and/or experimental (57, 59, 62) stressors. Research methods have also differed, ranging from the direct measurement of one or more cytokines (54, 56, 57) to measurement of the cytokine receptor and its mRNA (55) and including indirect assessments such as the speed of wound healing and the quantification of related cytokines (58, 64). Results have varied. Although no definite pattern has emerged, several investigators have interpreted their results as consistent with the hypothesis that stress induces a shift in the balance of type 1 and type 2 T helper responses in favor of type 2 (59, 60, 63). However, this pattern has not always been confirmed (61). The relationship between stress, hormones, and cytokine secretion is complex and not fully understood. More research is needed to clarify some of the unresolved issues.
Cytokines and Psychiatric Disorders
Because cytokines are closely associated with central neurotransmitters and because cytokine regulation is affected by stress, a number of studies have investigated a possible role for cytokines in major psychiatric disorders. These studies have been described in an emerging literature on cytokine regulation in major depression, schizophrenia, Alzheimer’s disease, and other psychiatric disorders.
Major Depression
Research on cytokine regulation in major depression has been surprisingly limited. Maes and colleagues reported an increase in the plasma concentration and in vitro production of IL-1 (65) and IL-6 (66) in patients with major depression. They also reported increases in plasma concentrations of soluble IL-2 receptors, soluble IL-6 receptors, and acute phase proteins (66). These authors concluded that there is an increase in proinflammatory cytokines in patients with major depression that seems to correlate with severity of the illness and measures of HPA hyperactivity. Unfortunately, these observations have not been consistently replicated. Weizman and associates (67) found a reduction in the secretion of IL-1b and IL-2 and in IL-3-like activity in depressed patients compared to control subjects. Most investigators, however, have seemed to confirm an increase in the plasma levels of acute phase proteins, notably haptoglobin (68). This finding, along with reports of mild leukocytosis, neutrophilia, and elevated C-reactive protein and complement components (69), suggests a mild inflammatory response in depression, possibly initiated by cytokines. Any possible role for cytokines in the pathophysiology of mood disorder however remains highly speculative (70).
Schizophrenia
The idea that some forms of schizophrenia may be associated with an autoimmune response is not new. This topic has been the subject of excellent reviews (71). One reason for skepticism in this area, however, is the inconsistent replication of laboratory findings, particularly when dealing with antibodies directed toward specific brain antigens. With advances in cytokine research, a number of studies have examined specific cytokine abnormalities in patients with schizophrenia. The results have been more encouraging. Several studies have found a decrease in mitogen-stimulated IL-2 production in patients with schizophrenia (72, 73). Furthermore, Ganguli and colleagues (73) have shown that diminished IL-2 production was associated with a younger age at onset and a preponderance of negative symptoms. Other frequently replicated immune abnormalities in schizophrenia include an increase in circulating soluble IL-2 receptors (74) and an increase in serum IL-6 concentrations (75). Results regarding levels and/or production of IL-1 and TNF have been inconclusive. A great deal of the discrepancy among results may be related to clinical factors such as subjects’ duration of illness, medication status, and clinical subtype of the disorder. Whether cytokines are involved in the pathophysiology of certain forms of schizophrenia, possibly in relation to a viral and/or neurodevelopmental insult, remains to be determined.
Alzheimer’s Disease and Other Psychiatric Disorders
It has frequently been suggested that inflammatory and immune mechanisms play an important role in the pathophysiology of Alzheimer’s disease (76). Acute phase proteins such as a-1-antichymotrypsin are frequently elevated in the serum and cerebrospinal fluid of patients with Alzheimer’s disease (77) and may become part of the amyloid deposit of senile plaques, the histopathological hallmark of this illness (78). Acute phase proteins are mediated by cytokines, particularly IL-1, IL-6, and TNF. Studies have shown that the levels of IL-1, IL-6, and TNF are elevated both in the serum and brain extracts in patients with Alzheimer’s disease (79, 80). Both IL-1 and IL-6 have been shown to increase the synthesis of amyloid precursor proteins by specialized cells (81). The pathophysiology of Alzheimer’s disease is complex and involves both genetic and environmental factors. Although cytokine dysregulation is evident during different phases of the disease, whether this process represents a reaction to or a major reason for tissue destruction remains controversial (76).
The literature includes reports of cytokine dysregulation in other psychiatric disorders, such as panic disorder (82), obsessive-compulsive disorder (83), and autism (84).
Cytokines and Consultation-Liaison Psychiatry
Use of Cytokines in Selected Medical Conditions
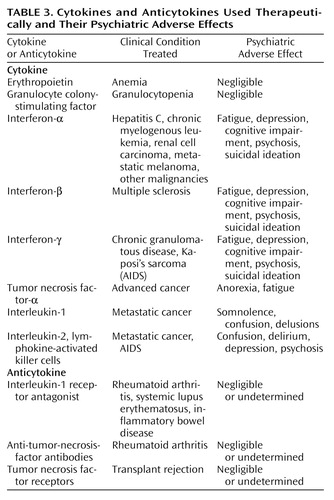
Cytokines have a number of potential therapeutic applications (Table 3). In treating many inflammatory states, physicians wish to alter the immune response to improve the outcome of their patients. Because cytokines are a prominent component of the immune response, they represent a natural target for modulation. Exogenous cytokines may be given in some situations (e.g., certain infections, cancer) where it is desirable to have increased levels of cytokines, or they may be blocked in other situations (e.g., autoimmune diseases and transplant rejection) where it is believed that the cytokine levels are too high.
Administration of exogenous cytokines
The most frequent clinical use of exogenous cytokines is the administration of hematopoietic growth factors to patients who are deficient in one or more of the components of the bone marrow, i.e., patients with anemia, leukopenia, or thrombocytopenia. A good example of such treatment is administration of granulocyte colony-stimulating factor to patients with neutropenia. Other frequent uses include treatment of chronic infection, e.g., hepatitis C, where interferon-a has been used (85), and several cancerous conditions such as malignant melanoma and metastatic breast cancer, where TNF-α, IL-1, and IL-2 have been used (86, 87).
Inhibition of cytokines
Several specific disease states, including autoimmune diseases and certain infections and their sequelae, are associated with an exaggerated release of cytokines. Cytokines may be inhibited by using several different methods, including administration of monoclonal antibodies against the cytokine, as well as administration of antireceptor antibodies, soluble receptors that inhibit the activity of their cytokines, and receptor antagonists such as the naturally occurring IL-1 receptor antagonist that binds to the IL-1 receptor but fails to transduce a signal (34).
Psychiatric Adverse Effects of Treatment With Cytokines
As treatment with cytokines and/or cytokine inhibitors is gaining ground, psychiatrists, particularly those in consultation-liaison services, may need to become more familiar with the principles of cytokine therapy. Increased attention to these principles is especially needed because the medical conditions that require cytokine treatment, such as cancer and autoimmune diseases, have high psychiatric comorbidity and because these treatments commonly have psychiatric adverse effects (Table 3).
Interferon
Interferon therapy is now an integral part of various clinical protocols. Because of their antiviral as well as antiproliferative and immunomodulatory properties, interferons have been used in the treatment of various medical conditions such as malignant melanoma, multiple sclerosis, and hepatitis C. The most common side effects appear early in treatment and include flu-like symptoms such as fever, tachycardia, headache, malaise, arthralgia, and myalgia (85). These side effects tend to decrease in severity as treatment continues. Neuropsychiatric side effects typically appear later in treatment (88–90). Fatigue is one of the most frequently encountered adverse effects; it is usually dose-related and tends to remit when treatment is discontinued. Other neuropsychiatric side effects include mood changes (depression, anxiety, irritability, manic symptoms, and even suicidal behavior), cognitive changes (decreased concentration, confusion, delirium), and, rarely, psychosis. The exact mechanism by which these side effects develop is poorly understood, but it may involve neurotransmitter changes produced by the cytokines (40). Attempts have been made to treat and even prevent some of these side effects by using appropriate psychopharmacological agents (91). The goal of such treatment is not only to reduce psychiatric symptoms, but also to allow completion of the course of medical treatment, as neuropsychiatric side effects are the most commonly reported causes for dose limitation or treatment discontinuation (85).
IL-2 and lymphokine-activated killer cells
IL-2 and lymphokine-activated killer cells have also been used to treat patients with metastatic malignancies. In a longitudinal survey of consecutive patients who received this treatment, 50% of the patients experienced severe cognitive and/or behavioral impairment (92). In 34% of the patients, the behavioral changes necessitated acute intervention. The neuropsychiatric side effects usually were dose-related and tended to appear at the end of the treatment phase. They seemed, however, to be completely reversible.
Other cytokines
Treatment with TNF-α is often accompanied by anorexia, nausea, headache, and fatigue (86). Side effects of treatment with colony-stimulating factor are infrequent and are usually mild (93).
Anticytokine therapy
Use of cytokine inhibitors is a very new treatment approach. Preliminary data have suggested that this treatment is safe and effective for specific medical conditions, such as rheumatoid arthritis (94) and inflammatory bowel disease (95). The psychiatric adverse effects, if any, seem to be mild or undetermined.
Effects of Psychiatric Treatment on Cytokine Production
Any somatic treatment in psychiatry is likely to be associated with changes in specific neurotransmitter regulation in the brain. Because changes in neurotransmitter function can lead to alterations in cytokine production in the central nervous system (96), it is not surprising that the effects of psychopharmacologic agents on cytokine production should be examined. What is surprising is the very limited number of studies addressing this issue. Furthermore, most of the studies that have been conducted have involved psychiatric patients who are receiving treatment, so it is often difficult to determine whether any changes in the levels of cytokines are a result of a pharmacological property of the medication or of changes in the clinical status of the patient receiving the treatment. In any event, a few studies have reported changes in the levels of specific cytokines in conjunction with one or more psychopharmacologic agents. In vitro studies have shown that chlorpromazine and other neuroleptics have an inhibitory effect on the production of IL-2, TNF, and interferon by human lymphocytes (97). Animal studies seem to confirm these observations (98). In humans, neuroleptic treatment has been associated with an increase in the serum levels of soluble IL-2 receptor and a decrease in the levels of soluble IL-6 receptor (99). Clozapine-induced agranulocytosis has also been associated with the inhibitory effects of cytokines (100). The effects of antidepressant medications have rarely been investigated. Animal studies have suggested an inhibitory influence by selective serotonin reuptake inhibitors on the production of acute phase proteins and cytokines (101). In depressed patients, treatment with clomipramine has been associated with an increase in IL-1b and in IL-3-like activity by stimulated lymphocytes (67). Plasma levels of IL-6, soluble IL-6 receptor, and soluble IL-2 receptor, however, were not affected by antidepressant medications (66). In a different line of studies, we have shown that ECT was associated with a significant increase in plasma levels of IL-6 (102). However, associations between changes in the levels of cytokines and the therapeutic response have not been firmly established.
Conclusions
The last decade has witnessed a rapid expansion of research in cytokine biology. In addition to their role as messengers among inflammatory and immune cells, these proteins seem to interact with different tissues and organs and assume new roles outside their traditionally known functions. One such role involves the central nervous system. Cytokines seem capable of acting as neuromodulators within the brain. As such, they affect important brain activities such as sleep, appetite, and neuroendocrine regulation. Discoveries about these processes have led to speculations about a possible link between cytokines and psychopathology. We should, however, emphasize that our knowledge of the role of cytokines in human brain activity is still in its infancy. Most of our information comes from animal studies. Human studies remain scarce and frequently suffer from methodological flaws that make comparisons between the results difficult to achieve. We therefore feel that more hypothesis-driven research protocols involving human subjects are needed to assess the role of specific cytokines in neuropsychiatric symptom production as well as their role in the pathophysiology of specific psychiatric disorders. Once a defect has been identified, novel treatment strategies involving cytokines and/or cytokine inhibitors should be tested clinically. This work may lead to a whole new approach in the way we understand and treat psychiatric illness.
 |
 |
 |
Received June 15, 1999, revision received Dec. 1, 1999, accepted Dec. 3, 1999. From the Departments of Psychiatry and Pathology, University of Michigan Health System, Ann Arbor. Supported in part by the National Alliance for Research on Schizophrenia and Depression. Dr. Kronfol is a Van Ameringen Investigator.
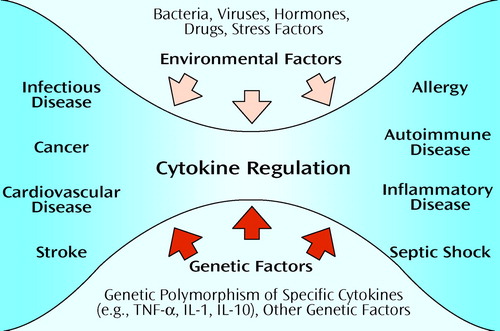
Figure 1. Cytokine Regulation in Health and Diseasea
aCytokine regulation is influenced by genetic factors (e.g., genetic polymorphism) and environmental factors (e.g., microbes, hormones, stress factors). Physiological interactions help keep the organism healthy. Pathological interactions can lead to a variety of medical illnesses, such as infection, allergy, and autoimmune disease (shaded area). TNF-α=tumor necrosis factor-α, IL=interleukin.
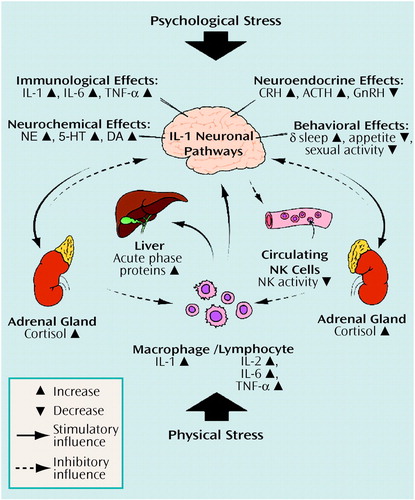
Figure 2. Interleukin-1 and the Stress Responsea
aBoth psychological stress (e.g., academic stress, depression) and physical stress (e.g., infection, trauma) can activate interleukin-1 (IL-1). IL-1 activation is associated with various phenomena, both peripherally (e.g., cascade activation of other cytokines, induction of acute phase proteins) and centrally (e.g., various immunologic, neurochemical, neuroendocrine, and behavioral effects). Feedback mechanisms occur at several levels and include negative feedback exerted by cortisol. Only selected effects are shown as an example. IL=interleukin, TNF-α=tumor necrosis factor-α, CRH=corticotropin-releasing hormone, GnRH=gonadotropin-releasing hormone, NE=norepinephrine, 5-HT=serotonin, DA=dopamine, NK=natural killer.
1. Rothwell NJ (ed): Cytokine in the Nervous System. Georgetown, Tex, PG Landes, 1996Google Scholar
2. Ransohoff RM, Benveniste EN (eds): Cytokines and the CNS. Boca Raton, Fla, CRC Press, 1996Google Scholar
3. Plotnikoff N, Murgo A, Faith R, Good R (eds): Cytokines: Stress and Immunity. Boca Raton, Fla, CRC Press, 1999Google Scholar
4. Kunkel SL, Remick DG (eds): Cytokines in Health and Disease. New York, Marcel Dekker, 1992Google Scholar
5. Baggiolin M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997; 15:675–705Crossref, Medline, Google Scholar
6. Licinio L, Kling M, Hauser P: Cytokines and brain function: relevance of interferon a-induced mood and cognitive changes. Semin Oncol 1998; 25:30–38Medline, Google Scholar
7. Lin JS, Amaral TD, Brosnan CF, Lee SC: Interferons as critical regulators of IL-1 receptor antagonist and IL-1 expression in human microglia. J Immunol 1998; 16:1989–1996Google Scholar
8. Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB: Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Compr Neurol 1993; 337:543–567Crossref, Medline, Google Scholar
9. Bethea JR, Chung IY, Sparacio SM, Gillespie GY, Beneveniste EN: Interleukin-1b induction of tumor necrosis factor-alpha gene expression in human astroglioma cells. J Neuroimmunol 1992; 36:179–191Crossref, Medline, Google Scholar
10. Juul SE, Anderson DK, Li Y, Christensen RD: Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res 1998; 43:40–49Crossref, Medline, Google Scholar
11. Tweardy D, Mott P, Glazer E: Monokine modulation of human astroglial cell production of granulocyte colony-stimulating factor and granulocyte-macrophage colony stimulating factor, I: effects of IL-1a and IL-1b. J Immunol 1990; 144:2233–2241Google Scholar
12. Breder CD, Dinarello CA, Saper CB: Interleukin-1 immunoreactive innervation of the human hypothalamus. Science 1988; 240:321–324Crossref, Medline, Google Scholar
13. Rothwell NJ, Luheshi G: Pharmacology of interleukin-1 actions in the brain. Adv Pharmacol 1994; 25:1–20Crossref, Medline, Google Scholar
14. Hanisch UK, Quirion R: Interleukin-2 as a neuroregulatory cytokine. Brain Res Brain Res Rev 1995; 21:246–284Crossref, Medline, Google Scholar
15. Konishi Y, Chui DH, Kunishita T, Yamemura T, Higashi Y, Tabira T: Demonstration of interleukin-3 receptor-associated antigen on the central nervous system. J Neurosci Res 1995; 41:572–582Crossref, Medline, Google Scholar
16. Racke MK, Burnett D, Pak SH, Albert PS, Camella B, Raine CS, McFarlin DE, Scott DE: Retinoid treatment of experimental allergic encephalomyelitis: IL-4 production correlates with improved disease course. J Immunol 1995; 154:450–451Medline, Google Scholar
17. Saweda N, Suzumura A, Itoh Y, Marunouchi T: Production of interleukin 5 by mouse astrocytes and microglia in culture. Neurosci Lett 1993; 155:175–178Crossref, Medline, Google Scholar
18. LeMay LB, Vander AJ, Kluger MJ: The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav 1990; 47:957–961Crossref, Medline, Google Scholar
19. Benveniste EN, Sparacia SM, Norris JG, Grenott HE, Muller GM: Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol 1990; 30:201–212Crossref, Medline, Google Scholar
20. Ehrlich LC, Hu S, Sheng WS, Sutton RC, Rocks-Wolf GC, Peterson PK, Chao CC: Cytokine regulation of human microglia cell IL-8 production. J Immunol 1998; 160:1944–1948Google Scholar
21. Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J: Interleukin-1 beta, interleukin-1 receptor antagonist, IL-10 and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proc Natl Acad Sci USA 1997; 94:227–232Crossref, Medline, Google Scholar
22. Elenkov I, Papanicolaon D, Wilder R, Chrousos GP: Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians 1996; 108:334–381Medline, Google Scholar
23. Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CG, Hobbs MV, Bloom FE, Campbell IL: Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J Immunol 1997; 159:1344–1351Google Scholar
24. Thompson A (ed): The Cytokine Handbook, 3rd ed. San Diego, Academic Press, 1998Google Scholar
25. Mosmann TR, Coffman RL: TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7:145–173Crossref, Medline, Google Scholar
26. Hurme M, Lahdenpohja N, Santtila S: Gene polymorphisms of interleukins 1 and 10 in infectious and autoimmune diseases. Ann Med 1998; 30:469–473Crossref, Medline, Google Scholar
27. Wilson AG, di Giovine FS, Duff GW: Genetics of tumour necrosis factor alpha in autoimmune, infectious, and neoplastic diseases. J Inflamm 1995; 45:1–12Medline, Google Scholar
28. Mira J-P, Cariou A, Grall F, Delclaux C, Losser M-R, Heshmati F, Cheal C, Monchi M, Teboul J-L, Riché F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut J-F: Association of TNF2, a TNF-α promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA 1999; 282:561–568Crossref, Medline, Google Scholar
29. Smeraldi E, Sanardi R, Benedetti F, DiBella D, Perez J, Catalano M: Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 1998; 3:508–511Crossref, Medline, Google Scholar
30. Remick DG: Quantitation of cytokines, in Cytokines in Health and Disease, 2nd ed. Edited by Remick DG, Friedland JS. New York, Marcel Dekker, 1997, pp 281–298Google Scholar
31. Cserr HF, Knopf PM: Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 1992; 13:507–512Crossref, Medline, Google Scholar
32. Watkins LR, Maier SF, Goehler LE: Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci 1995; 57:1011–1026Google Scholar
33. Freidin M, Bennett MVL, Kessler JA: Cultured sympathetic neurons synthesize and release the cytokine interleukin-1b. Proc Natl Acad Sci USA 1992; 89:10440–10443Google Scholar
34. Dinarello C: Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol 1988; 16:457–499Crossref, Google Scholar
35. Weiss JM, Sundar SK, Becker KJ, Cierpial MA: Behavioral and neural influences on cellular immune responses: effects of stress and interleukin-1. J Clin Psychiatry 1989; 50:43–53Medline, Google Scholar
36. Romero LI, Kakueska I, Lechan RM, Reichlin S: Interleukin-6 (IL-6) is secreted from the brain after intracerebroventricular injection of IL-1 beta in rats. Am J Physiol 1996; 270:R518–R524Google Scholar
37. Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB: Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci 1988; 8:2485–2490Google Scholar
38. Besedovsky H, del Rey A, Sorkin E, Da Prada M, Burri R, Honegger C: The immune response evokes changes in brain noradrenergic neurons. Science 1983; 221:564–566Crossref, Medline, Google Scholar
39. Dunn AJ, Powell ML, Meitin C, Small PA Jr: Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol Behav 1989; 45:591–594Crossref, Medline, Google Scholar
40. Dunn AJ, Wang J: Cytokine effects on CNS biogenic amines. Neuroimmunomodulation 1995; 2:319–328Crossref, Medline, Google Scholar
41. Besedovsky H, Sorkin E, Keller M, Müller J: Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med 1975; 150:466–470Crossref, Medline, Google Scholar
42. Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale WW: Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 1987; 238:522– 524Crossref, Medline, Google Scholar
43. Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HGZ: Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science 1987; 238:519–521Crossref, Medline, Google Scholar
44. Besedovsky H, del Rey A, Sorkin E, Da Prada M, Burri R, Honegger C: Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol 1991; 40:613–618Crossref, Medline, Google Scholar
45. Rivier C: Neuroendocrine effects of cytokines in the rat. Rev Neurosci 1993; 4:223–237Crossref, Medline, Google Scholar
46. Rivest S, Rivier C: Centrally injected interleukin-1b inhibits the hypothalamic LHRH secretion and circulating LH levels via prostaglandins in rats. J Neuroendocrinol 1993; 5:445–450Crossref, Medline, Google Scholar
47. Krueger JM, Majde JA: Microbial products and cytokines in sleep and fever regulation. CRC Crit Rev Immunol 1994; 14:355–379Crossref, Google Scholar
48. Moldofsky H, Lue FA, Eisen J, Keystone E, Gorczynski RM: The relationship of interleukin-1 and immune functions to sleep in humans. Psychosom Med 1986; 48:309–318Crossref, Medline, Google Scholar
49. Lue FA, Bail M, Jephthah-Ochola J, Carayanniotis K, Gorczynski R, Moldofsky H: Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. Int J Neurosci 1988; 42:179–183Crossref, Medline, Google Scholar
50. Krueger JM: Somnogenic activity of immune response modifiers. Trends Pharmacol Sci 1990; 11:122–126Crossref, Medline, Google Scholar
51. Plata-Salaman CR, Sonti G, Borkoski JP, Wilson CD: Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav 1996; 60:867–875Crossref, Medline, Google Scholar
52. Bessler H, Karp L, Notti I, Apter A, Tynus S, Djaldetti M, Weizman R: Cytokine production in anorexia nervosa. Clin Neuropharmacol 1993; 16:237–243Crossref, Medline, Google Scholar
53. Persoons JH, Schornagel K, Breve J, Berkenbosch F, Kraal G: Acute stress affects cytokines and nitric oxide production by alveolar macrophages differently. Am J Respir Crit Care Med 1995; 152:619–624Crossref, Medline, Google Scholar
54. Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK: Stress depresses interferon production by leukocytes concomitant with a decrease in NK cell activity. Behav Neurosci 1986; 100:675–678Crossref, Medline, Google Scholar
55. Glaser R, Kennedy S, Lafuse WP, Bonneau RH, Speicher C, Hillhouse J, Kiecolt-Glaser JK: Psychological stress-induced modulation of interleukin 2 receptor gene expression and interleukin 2 production in peripheral blood leukocytes. Arch Gen Psychiatry 1990; 47:707–712Crossref, Medline, Google Scholar
56. Dobbin JP, Harth M, McCain GA, Martin RA, Cousin K: Cytokine production and lymphocyte transformation during stress. Brain Behav Immun 1991; 5:339–348Crossref, Medline, Google Scholar
57. Mittwoch-Jaffe T, Shalit F, Srendi B, Yehuda S: Modification of cytokine secretion following mild emotional stimuli. Neuroreport 1995; 6:789–792Crossref, Medline, Google Scholar
58. Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R: Slowing of wound healing by psychological stress. Lancet 1995; 346:1194–1196Google Scholar
59. Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA: Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119:316–325Crossref, Medline, Google Scholar
60. Nakano Y, Nakamura S, Hirata M, Harada K, Ando K, Tabuchi T, Matunaga I, Oda H: Immune function and lifestyle of taxi drivers in Japan. Ind Health 1998; 36:32–39Crossref, Medline, Google Scholar
61. Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS: The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 1998; 10:313–318Crossref, Medline, Google Scholar
62. Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS: Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med 1998; 60:484–491Crossref, Medline, Google Scholar
63. Marshall GD, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ: Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun 1998; 12:297–307Crossref, Medline, Google Scholar
64. Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB: Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry 1999; 56:450–456Crossref, Medline, Google Scholar
65. Maes M, Bosmans E, Meltzer HY, Scharpé S, Suy E: Interleukin-1b: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry 1993; 150:1189– 1193Google Scholar
66. Maes M, Meltzer H, Bosmans E, Bergmans R, Vandoolaeghe E, Rajan R, Desnyder R: Increased plasma concentrations of interleukin-6, soluble interleukin-6 receptor, soluble interleukin-2 receptor and transferrin receptor in major depression. J Affect Disord 1995; 34:301–309Crossref, Medline, Google Scholar
67. Weizman R, Laor N, Podliszewski E, Notti I, Djaldetti M, Bessler H: Cytokine production in major depressed patients before and after clomipramine treatment. Biol Psychiatry 1994; 35:42–47Crossref, Medline, Google Scholar
68. Kronfol Z, Singh VJ, Zhang Q: Plasma cytokines, acute phase proteins and cortisol in major depression (abstract). Biol Psychiatry 1995; 37(suppl):609AGoogle Scholar
69. Kronfol Z, House JD: Lymphocyte mitogenesis, immunoglobulin and complement levels in depressed patients and normal controls. Acta Psychiatr Scand 1989; 80:142–167Crossref, Medline, Google Scholar
70. Maes M, Smith RS: Fatty acids, cytokines, and major depression. Biol Psychiatry 1998; 43:313–314Crossref, Medline, Google Scholar
71. Ganguli R, Rabin BS, Kelly RH, Lyte M, Ragu U: Clinical and laboratory evidence of autoimmunity in acute schizophrenia. Ann NY Acad Sci 1987; 496:676–685Crossref, Medline, Google Scholar
72. Villemain F, Chatenoud L, Galinowski A, Homo-Delarche F, Ginestet D, Loo H, Zarifian E, Bach J-F: Aberrant T-cell-mediated immunity in untreated schizophrenic patients: deficient interleukin-2 production. Am J Psychiatry 1989; 146:609–616Link, Google Scholar
73. Ganguli R, Brar JS, Chengappa KR, Deleo M, Yang ZW: Mitogen-stimulated interleukin 2 production in never-medicated first episode schizophrenics—the influence of age of onset and negative symptoms. Arch Gen Psychiatry 1995; 52:668–672Crossref, Medline, Google Scholar
74. Rapaport MH, Torrey EF, McAllister CG, Nelson DL, Pickar D, Paul SM: Increased serum soluble interleukin-2 receptors in schizophrenic monozygotic twins. Eur Arch Psychiatr Clin Neurosci 1993; 243:7–10Crossref, Medline, Google Scholar
75. Ganguli R, Young Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, Rabin BS: Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatr Res 1994; 51:1–10Crossref, Medline, Google Scholar
76. Aisen PS, Davis KL: Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry 1994; 151:1105–1113Google Scholar
77. Matsubara E, Hirrai S, Amari M, Shoji M, Yamaguchi H, Okomoto K, Ishiguro K, Harigaya Y, Wakabayashi K: Alpha-1-antichymotripsin as a possible biochemical marker for Alzheimer-type dementia. Ann Neurol 1990; 28:561–567Crossref, Medline, Google Scholar
78. Abraham CR, Selkor DJ, Potter H: Immunohistochemical identification of the serum protein inhibitor alpha-1 antichymotripsin in the brain amyloid deposits of Alzheimer’s disease. Cell 1988; 52:487–501Crossref, Medline, Google Scholar
79. Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL III, Araoz C: Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA 1989; 86:7611–7615Google Scholar
80. Bauer J, Gauter U, Strauss S, Stadtmuller G, Frommberger U, Bauer H, Volk B, Berger M: The participation of interleukin-6 in the pathogenesis of Alzheimer’s disease. Res Immunol 1992; 143:650–657Crossref, Medline, Google Scholar
81. Altstiel L, Sperber K: Cytokines in Alzheimer’s disease. Prog Neuropychopharmacol Biol Psychiatry 1991; 15:481–495Crossref, Medline, Google Scholar
82. Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P: Plasma interleukin-1b concentrations in panic disorder. Psychiatry Res 1994; 54:135–142Crossref, Medline, Google Scholar
83. Brambilla F, Perna G, Bellodi L, Arancio C, Bertani A, Perini G, Carraro C, Gava F: Plasma interleukin-1b and tumor necrosis factor concentrations in obsessive-compulsive disorders. Biol Psychiatry 1997; 42:976–981Crossref, Medline, Google Scholar
84. Warren RP: An immunologic theory for the development of some cases of autism. CNS Spectrums 1998; 3:71–79Google Scholar
85. Dusheiko G: Side effects of alpha interferon in chronic hepatitis C. Hepatology 1997; 26:1125–1215Google Scholar
86. Schiller JH, Storer BE, Witt PL, Alberti D, Tombes MB, Arzoomanian R, Proctor RA, McCarthy D, Brown RR, Voss SD, Remick SC, Grem JL, Borden EC, Trump DL: Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Res 1991; 51:1651–1658Google Scholar
87. Rosenberg SA: The development of new immunotherapies for the treatment of cancer using interleukin-2: a review. Ann Surg 1988; 208:121–135Crossref, Medline, Google Scholar
88. Renault PF, Hoofnagle JH, Park H, Mullen ED, Peters M, Jones B, Rustgi V, Jonas EA: Psychiatric complications of long-term interferon alpha therapy. Arch Intern Med 1987; 147:1577–1580Google Scholar
89. Stritz D, Valentine AD, Meyers CA: Manic episodes in two patients treated with interferon alpha. J Neuropsychiatr Clin Neurosci 1997; 9:273–276Crossref, Medline, Google Scholar
90. Janssen HL, Brouwer JT, van der Mast RC, Schalm SW: Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol 1994; 21:241–243Crossref, Medline, Google Scholar
91. Levenson J, Fallon HJ: Fluoxetine treatment of depression caused by interferon-alpha. Am J Gastroenterol 1993; 88:760–761Medline, Google Scholar
92. Denicoff KD, Rubinow DR, Papa MZ, Simpson C, Seipp CA, Lotze MT, Chang AE, Rosenstein D, Rosenberg SA: The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med 1987; 107:293–300Crossref, Medline, Google Scholar
93. Klingemann HG: Clinical applications of recombinant human colony stimulating factors. Can Med Assoc J 1989; 140:137–142Google Scholar
94. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ: A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999; 340:253–259Crossref, Medline, Google Scholar
95. Rogler G, Andus T: Cytokines in inflammatory bowel disease. World J Surg 1998; 22:382–389Crossref, Medline, Google Scholar
96. Norris JG, Benveniste EN: Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. J Neuroimmunol 1993; 45:137–146Crossref, Medline, Google Scholar
97. Schleuning MJ, Duggan A, Reem GH: Inhibition by chlorpromazine of lymphokine-specific m-RNA expression in human thymocytes. Eur J Immunol 1989; 19:1491–1496Google Scholar
98. Bertini S, Garattini R, Delgado P, Ghezzi P: Pharmacological activities of chlorpromazine involved in the inhibition of tumor necrosis factor production in vivo in mice. Immunology 1993; 79:217–219Medline, Google Scholar
99. Maes M, Meltzer HY, Bosmans E: Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand 1994; 89:346–351Crossref, Medline, Google Scholar
100. Sperner-Unterweger B, Gaggl S, Fleischhacker WW, Barnas C, Herold M, Geissler D: Effects of clozapine on hematopoiesis and the cytokine system. Biol Psychiatry 1993; 34:536–543Crossref, Medline, Google Scholar
101. Song C, Leonard E: An acute phase protein response in the olfactory bulbectomised rat: effect of sertraline treatment. Med Sci Res 1994; 22:313–314Google Scholar
102. Kronfol Z, LeMay L, Nair M, Kluger M: Electroconvulsive therapy increases plasma levels of interleukin-6. Ann NY Acad Sci 1990; 594:463–465Crossref, Google Scholar



