Neuroanatomical Hypothesis of Panic Disorder, Revised
Abstract
OBJECTIVE: In a 1989 article, the authors provided a hypothesis for the neuroanatomical basis of panic disorder that attempted to explain why both medication and cognitive behavioral psychotherapy are effective treatments. Here they revise that hypothesis to consider developments in the preclinical understanding of the neurobiology of fear and avoidance. METHOD: The authors review recent literature on the phenomenology, neurobiology, and treatment of panic disorder and impressive developments in documenting the neuroanatomy of conditioned fear in animals. RESULTS: There appears to be a remarkable similarity between the physiological and behavioral consequences of response to a conditioned fear stimulus and a panic attack. In animals, these responses are mediated by a “fear network” in the brain that is centered in the amygdala and involves its interaction with the hippocampus and medial prefrontal cortex. Projections from the amygdala to hypothalamic and brainstem sites explain many of the observed signs of conditioned fear responses. It is speculated that a similar network is involved in panic disorder. A convergence of evidence suggests that both heritable factors and stressful life events, particularly in early childhood, are responsible for the onset of panic disorder. CONCLUSIONS: Medications, particularly those that influence the serotonin system, are hypothesized to desensitize the fear network from the level of the amygdala through its projects to the hypothalamus and the brainstem. Effective psychosocial treatments may also reduce contextual fear and cognitive misattributions at the level of the prefrontal cortex and hippocampus. Neuroimaging studies should help clarify whether these hypotheses are correct.
In 1989, we (1) articulated a neuroanatomical hypothesis of panic disorder that was essentially an attempt to understand how two seemingly diverse treatments—medication and cognitive behavioral therapy—could both be effective interventions. This theory posited that a panic attack itself stems from loci in the brainstem that involve serotonergic and noradrenergic transmission and respiratory control, that anticipatory anxiety arises after the kindling of limbic area structures, and, finally, that phobic avoidance is a function of precortical activation. The hypothesis then asserted that medication exerts its therapeutic effect by normalizing brainstem activity in patients with panic disorder, whereas cognitive behavioral therapy works at the cortical level.
In succeeding years, studies have continued to accumulate demonstrating that medications, particularly those that affect serotonergic neurotransmission (2), and cognitive behavioral therapy (3) are indeed robustly effective for patients with panic disorder. Further, the notion that respiratory (4) and cardiovascular reactivity (5) is abnormal in panic disorder, pointing to brainstem involvement, has also been strengthened. However, our original hypothesis is now seen as clearly deficient because it is almost completely divorced from exciting preclinical and basic research that has elegantly mapped out the neuroanatomical basis for fear. We now wish to revisit our neuroanatomical hypothesis of panic disorder and address these critical novel elements. The result will propose that panic disorder may involve the same pathways that subserve conditioned fear in animals, including the central nucleus of the amygdala and its afferent and efferent projections.
Anatomy of Conditioned Fear
One of the greatest challenges in modern psychiatry is to make use of advanced preclinical information from basic and behavioral neuroscience. Strides have been made in developing animal models of emotional and behavioral states, a task seen as critical in developing a coherent understanding of any human disease state. However, it has been difficult to understand how any animal, lacking the capacity to express in words its emotional state, could meaningfully reflect human psychopathology. It is problematic to conceive of an animal model for depression or psychosis, for example, because these illnesses seemingly depend on the ability of the human patient to inform us verbally about symptoms that are necessary for diagnosis. Although even in these instances cogent animal models are now available, two human psychiatric syndromes seem best suited for analogy with animal states: anxiety and substance abuse. In the latter case, it is possible to addict animals to a variety of substances and then study the neural pathways that subserve the continued acquisition of these substances. This has led to remarkable insights into brain pathways necessary to sustain the abuse of substances like cocaine (6), some of which can be confirmed in humans with neuroimaging techniques (7).
In the case of anxiety, it is well understood that fear, escape, or avoidance behavior and panic-like responses are ubiquitous throughout animal phylogeny. It takes relatively little intuition to recognize that a rodent that avoids entering a cage in which an adverse stimulus has been presented in the past appears similar to a phobic patient refusing to drive across a bridge on which panic attacks previously occurred. Similarly, an animal manifesting increases in heart rate, blood pressure, respiration, and glucocorticoid release after the presentation of a tone that had previously been paired with a mild electric shock demonstrates many of the autonomic features characteristic of a panic attack.
The analogy of panic attacks to animal fear and avoidance responses is, to be sure, imperfect. Most animal models of anxiety states involve conditioning, and it is not at all clear that panic disorder or any other anxiety disorder except posttraumatic stress disorder (PTSD) involves prior exposure to any aversive stimulus. Further, because animals cannot tell us about the experience of anxiety and fear, we miss a rich source of information that is readily obtained from humans with anxiety disorders. In addition, animal models of fear may not truly reflect anxiety status. Klein (8) has written persuasively that there may be major biological differences in humans between fear and the manifestations of anxiety disorders.
Nevertheless, as we will detail, there are many aspects of conditioned fear in animals that make the analogy with panic attacks irresistible. Most important, it would be a mistake, in our view, to miss the opportunity to study pathways in humans that have been carefully elucidated in animals during anxious responses. We take, then, as a starting point that a consideration of the neuroanatomy of conditioned fear in rodents and other animals may give us important insights that can then be studied in patients with panic disorder.
The conditioned-fear paradigm used in neurobiological studies is well known to the most elementary students of behavior. In a typical study, a rat is presented with a stimulus—usually a tone or flash of light—at the same time it receives a mild electric shock. The former is called the conditioned stimulus, and the latter, the unconditioned stimulus. After several pairings, the rat learns to respond to the conditioned stimulus with the same autonomic and behavioral array even when no unconditioned stimulus is presented. The paradigm originates from the work of Pavlov (9) and has occupied the time of countless psychology students for generations.
What is new is an understanding of the brain pathways and neurotransmitters that are required for the acquisition of conditioned fear. The critical pathways are shown in figure 1. The sensory input for the conditioned stimulus runs through the anterior thalamus to the lateral nucleus of the amygdala and is then transferred to the central nucleus of the amygdala (10). The central nucleus of the amygdala stands as the central point for dissemination of information that then coordinates autonomic and behavioral responses (11, 12). In preclinical work, amygdalar projections have been established that may carry out these responses. Efferents of the central nucleus of the amygdala have many targets: the parabrachial nucleus, producing an increase in respiratory rate (13); the lateral nucleus of the hypothalamus, activating the sympathetic nervous system and causing autonomic arousal and sympathetic discharge (14); the locus ceruleus, resulting in an increase in norepinephrine release and contributing to increases in blood pressure, heart rate, and the behavioral fear response (15); and the paraventricular nucleus of the hypothalamus, causing an increase in the release of adrenocorticoids (16). A projection from the central nucleus of the amygdala to the periaqueductal gray region is responsible for additional behavioral responses, including defensive behaviors and postural freezing, that may be the animal equivalent of phobic avoidance (17). In fact, it can readily be seen from figure 1, which is based mainly on the work of Joseph LeDoux et al. (11) and Michael Davis (12), that the autonomic, neuroendocrine, and behavioral responses that occur during panic attacks are remarkably similar to the symptoms occurring in animals as a result of activity in these brain regions during fearful response to conditioned stimuli. This striking overlap between the consequences of stimulation by the central nucleus of the amygdala of brainstem sites and the biological events that occur in humans during panic attacks is compelling. However, this is not the complete story. It is clear that there are important reciprocal connections between the amygdala and the sensory thalamus, prefrontal cortex, insula, and primary somatosensory cortex (18). So although the amygdala receives direct sensory input from brainstem structures and the sensory thalamus, enabling a rapid response to potentially threatening stimuli, it also receives afferents from cortical regions involved in the processing and evaluation of sensory information. Potentially, a neurocognitive deficit in these cortical processing pathways could result in the misinterpretation of sensory information (bodily cues) known to be a hallmark of panic disorder, leading to an inappropriate activation of the “fear network” via misguided excitatory input to the amygdala. Although much remains to be elucidated regarding the amygdala’s role in panic, it seems reasonable to speculate that there may be a deficit in the relay and coordination of “upstream” (cortical) and “downstream” (brainstem) sensory information, which results in heightened amygdalar activity with resultant behavioral, autonomic, and neuroendocrine activation.
One must pause at this point, however, to consider how often patients with panic disorder actually demonstrate autonomic and neuroendocrine activation during attacks. Studies here are incomplete and somewhat inconsistent. For example, some, but not all, ambulatory monitoring studies have shown an increase in heart rate (19) and respiration (20) during panic attacks recorded outside of the laboratory. Although patients with panic disorder respond to CO2 inhalation with more anxiety, panic, and increase in respiratory rate than do normal volunteers or patients with other psychiatric illnesses (21), studies of the most sensitive measure of physiological response to CO2 inhalation—the ratio of change in minute ventilation to change in end tidal CO2 concentration—have yielded conflicting results (22). Although some investigators have found evidence for CO2 hypersensitivity, others have found that patients with panic fall within the normal range on this measure. Cortisol elevation in patients with panic disorder is reliably observed only during the anticipation of panic attacks (23), not during the attacks themselves (24). Taken together, the evidence suggests that some, but not all, panic attacks are accompanied by autonomic and neuroendocrine activation.
This last point serves to highlight what we think is an important error in our original neuroanatomic hypothesis of panic disorder. If, as argued there, panic attacks are the direct result of abnormal autonomic control at the level of the brainstem, we would expect to find autonomic and neuroendocrine activation a common property of all panic attacks. The fact that this is not the case suggests that brainstem activation is probably an epiphenomenon of activity in another area of the brain. The finding in preclinical research that activity in the central nucleus of the amygdala initiates stimulation of all of the relevant brainstem centers and that disrupting specific projections from the central nucleus of the amygdala to brainstem neurons selectively interferes with autonomic responses is consonant with this idea. Thus, for example, if the projection from the central nucleus of the amygdala to the central gray region is lesioned, all autonomic responses are seen, but the animal does not posturally freeze or attempt to flee (17).
Another finding that continues to contradict the idea that there is a specific abnormality in autonomic brainstem control in panic disorder is that a variety of agents with dissimilar biological properties all produce panic attacks in patients with panic disorder but not in healthy comparison subjects or patients with other psychiatric illnesses. The list of such agents is long, seems to grow annually, and includes sodium lactate (25), CO2 (26), yohimbine (27), fenfluramine (28), m-CPP (29), noradrenaline (30), adrenaline (31), hypertonic sodium chloride (32), and analogues of cholecystokinin (33). It remains difficult to understand what abnormal brainstem nucleus could be specifically triggered by such a diverse group of agents.
An alternative explanation that takes into account the inconsistency of autonomic responses and the heterogeneity of agents capable of producing panic attacks in susceptible patients is that panic originates in an abnormally sensitive fear network, which includes the prefrontal cortex, insula, thalamus, amygdala, and amygdalar projections to the brainstem and hypothalamus. When administering an agent that causes panic attacks, sometimes called a panicogen, we are not interacting with a specific brainstem autonomic area but, rather, activating the entire fear network. Patients with panic disorder experience unsettling somatic sensations on a regular basis. The administration of a panicogenic substance represents nonspecific activation; because each of the panicogens produces acutely uncomfortable physical sensations, we suppose that they act to provoke a sensitized brain network that has been conditioned to respond to noxious stimuli. Over time, various projections from the central nucleus of the amygdala to brainstem sites such as the locus ceruleus, periaqueductal gray region, and hypothalamus may become stronger or weaker. There may be interindividual differences in the strength of these afferent projections as well. Hence, the actual pattern of autonomic and neuroendocrine responses during panic may vary within any given patient with panic over time and even widely across patients with panic. These observations are clearly in line with what both researchers and clinicians observe.
Role of Medication
It is useful to consider how medications might act at the level of the central nucleus of the amygdala and its projections to reduce the severity and frequency of panic attacks. Although many classes of medication have been shown to be more effective than placebo in treating panic disorder, we select only one class for this discussion—the selective serotonin reuptake inhibitors (SSRIs). We do this because SSRIs are rapidly becoming the first-line medication treatment for panic disorder (34), their acute mechanism of action in the central nervous system is fairly well understood (35), and they may be more effective than other classes of medication for panic disorder (36).
SSRIs have in common the property of inhibiting the protein responsible for transporting serotonin (5-HT) back into the presynaptic neuron. This effectively increases the amount of 5-HT available in the synapse to bind to both pre- and postsynaptic receptors. There are at least 13 subtypes of 5-HT receptors established in humans (37), although the function and anatomical distribution of all of them have not been fully characterized. Nevertheless, functional studies demonstrate that over time, treatment with SSRIs leads to an increase in overall serotonergic neurotransmission in the central nervous system (38).
When attempting to understand the mechanism of action of SSRIs in panic disorder, a paradox arises as soon as animal models are considered. Acutely increasing 5-HT levels either globally or regionally in the brain of an experimental animal has been shown by many—although not all studies—to increase fear and avoidance (39). Although long-term studies are not available, and there are contradictory data, even some longer-term administrations of 5-HT precursors or agonists appear to maintain this increase in fear. By contrast, although some patients initially complain of increased anxiety and agitation when given treatment with SSRIs, these adverse events are generally mild and subside over subsequent weeks. By 4 weeks, most patients with panic report feeling less anxious and are having fewer panic attacks (and of diminished intensity) than before medication was initiated.
The solution to this paradox may come from a more refined understanding of the pathways involved in serotonergic neurotransmission. Serotonergic neurons originate in the brainstem raphe region and project widely throughout the entire central nervous system (40). Three of these projections are of particular relevance to an understanding of the SSRI antipanic effect.
First, the projection of 5-HT neurons to the locus ceruleus is generally inhibitory (41), such that the greater the activity of the serotonergic neurons in the raphe, the smaller the activity of the noradrenergic neurons in the locus ceruleus. Indeed, Coplan et al. (42) showed that after 12 weeks of fluoxetine treatment, patients with panic who responded to treatment showed a decrease in plasma levels of 3-methoxy-4-hydroxyphenylglycol, the main metabolite of noradrenaline. This suggests that SSRIs, by increasing serotonergic activity in the brain, have a secondary effect of decreasing noradrenergic activity. This would lead to a decrease in many of the cardiovascular symptoms associated with panic attacks, including tachycardia and increased diastolic blood pressure.
Second, the projection of the raphe neurons to the periaqueductal gray region appears to modify defense/escape behaviors. Viana and colleagues (43) have shown that stimulation of the dorsal raphe nucleus dramatically increases 5-HT release acutely in the dorsal periaqueductal gray region, resulting in diminished periaqueductal gray region activity. This finding supports the original suggestion of Deakin and Graeff (44) that serotonergic projections from the dorsal raphe nuclei play a role in modifying defense/escape responses by means of their inhibitory influence on the periaqueductal gray region.
Third, evidence now suggests that long-term treatment with an SSRI may reduce hypothalamic release of corticotropin-releasing factor (CRF) (45). CRF, which initiates the cascade of events that leads to adrenal cortical production of cortisol, is also a neurotransmitter in the central nervous system and has been shown on numerous occasions to increase fear in preclinical models (46). When administered directly into the brain, CRF also increases the firing rate of the locus ceruleus (47). CRF antagonists decrease both physiological (48) and behavioral consequences of CRF stimulation (49). Indeed, CRF antagonists are now being examined for their potential as anxiolytics in both animals and humans.
Hence, by looking at the specific pathways that originate from serotonergic neurons in the raphe, one can find at least three mechanisms by which increased serotonergic activity produced by long-term SSRI administration might produce an antipanic effect. By diminishing arousal, defense/escape behavior, and levels of the anxiogenic substance CRF, SSRIs are likely to block many of the downstream manifestations of panic.
Equally intriguing as the three examples given above is the possibility that SSRIs, by increasing serotonergic activity, have an effect on the central nucleus of the amygdala itself. Studies here are in the early stages, but it is already known that serotonergic neurons originating in the dorsal and medial raphe nuclei project directly to the amygdala via the medial forebrain bundle (40). At the level of the amygdala, Stutzmann and LeDoux (50) have demonstrated that 5-HT modulates sensory input at the lateral nucleus of the amygdala, inhibiting excitatory inputs from glutamatergic thalamic and cortical pathways. Because the amygdala is known to receive dense serotonergic input from the raphe nuclei, this may be a prime site for the anxiolytic action of the SSRIs, whereby an increase in 5-HT inhibits excitatory cortical and thalamic inputs from activating the amygdala.
We propose, then, that it is most likely that medications known to be effective in treating panic disorder diminish the activity of brainstem centers that receive input from the central nucleus of the amygdala and control autonomic and neuroendocrine responses during attacks. In addition to their psychic effects, drugs such as SSRIs may eliminate most of the troubling physical effects that occur during panic by affecting heart rate, blood pressure, breathing rate, and glucocorticoid release. This would then lead to a secondary decrease in anticipatory anxiety as a patient recognizes that the seemingly life-threatening physical manifestations of panic have been blocked. It is not uncommon, particularly in the early stages of medication treatment of a patient with panic disorder, to hear “I sometimes feel as if the attack is coming on, especially when I am in a situation in which attacks have occurred in the past. I get afraid, but then nothing happens. No palpitations, no dizziness, no difficulty breathing. My thoughts don’t seem to be able to cause a panic attack anymore.” We interpret this to suggest that the projections from the central nucleus of the amygdala to the brainstem and hypothalamus have been inhibited by medication treatment.
Neuroimaging and Panic Disorder
If the amygdala, thalamus, periaqueductal gray region, parabrachial nucleus, locus ceruleus, and hypothalamus are so important in coordinating the manifestations of panic, it would be a logical next step to attempt to image these brain regions during attacks and determine if they are particularly active compared to other brain regions. This has proven to be a daunting task. First, although the amygdala occupies a significant portion of the anterior part of the temporal lobe, it is a small structure in the human brain and is therefore difficult to distinguish with all but the most sensitive positron emission tomography (PET) cameras. Separating the amygdala from adjoining brain structures and the cortex is not a trivial task. Further, it is not yet possible with PET to distinguish among smaller brainstem nuclei. Functional magnetic resonance imaging (fMRI) potentially has the resolution to make this possible, but the technology is still in development to clearly image deeper subcortical and brainstem structures.
Sensitive PET cameras can now resolve the amygdala and other structures in the fear network like the thalamus as distinct neuroanatomical loci, but at least two other problems exist in learning whether they play a special role in panic. The first is in trying to capture the attack itself. Because panic attacks occur at seemingly random intervals, it is obviously impossible to place a patient in a scanner and wait for a spontaneous panic attack to occur. This means that some panicogenic agent must be administered that will reliably produce an attack. Several are available, as discussed above, but the attacks that occur when they are administered are, like naturally occurring panic attacks, brief. Radiotracers like [18F]fluorodeoxyglucose (FDG) that enable quantification of regional brain metabolism cannot capture events that occur over periods of time as short as a few minutes. Hence, PET scanning using FDG may not be able to tell us definitively if a change in metabolism has occurred acutely during the actual panic attack itself. Radiotracers such as [15O]water do provide temporal resolution compatible with capturing a panic attack, but they only allow us to measure regional cerebral blood flow (rCBF), not metabolism. The majority of studies have shown that there is a high correlation between brain metabolism and blood flow in healthy subjects (51, 52), such that increased neuronal activity in a given region is accompanied by increased blood flow to that region. However, other studies have suggested that this relationship may not hold consistently during physiologic activation (53). The possibility always exists that the uncoupling of CBF and metabolism may occur under circumstances of heightened stress, such as a panic attack. However, this uncoupling would be expected to be global, and, therefore, specific regional differences in CBF would be expected to still reflect changes in local neuronal metabolic activity.
A second problem for brain imaging studies is that patients with panic hyperventilate when they are anxious, and the resultant hypocapnia-induced vasoconstriction may obscure results, especially by counteracting expected increases in blood flow, reflecting neuronal activation and increased metabolism in certain structures. Most of the neuroimaging studies in populations with panic disorder have reported regional reductions in CBF or metabolism. Because the degree of hypocapnia (end tidal CO2) has not generally been controlled in these studies, it is difficult to interpret the results knowing that patients with panic disorder have a greater tendency to hyperventilate in stressful situations than normal comparison subjects (22). In support of this idea is a study by Stewart and colleagues (54), in which rCBF was measured during rest and immediately after a lactate infusion with xenon-133 single photon emission computed tomography ([133Xe]-SPECT). Normal comparison subjects and nonpanicking patients with panic disorder showed an increase in CBF after lactate infusion, whereas patients with panic disorder who panicked after lactate infusion demonstrated a smaller increase or an actual decrease in CBF. This finding may be accounted for by the vasoconstrictive effect of hyperventilation in overcoming the expected lactate-induced increase in CBF in the panicking patients. We suspect this is likely because we have previously shown that patients who panic during lactate infusion do hyperventilate more than those who do not panic (55).
In addition, there is growing evidence that patients with panic disorder are more sensitive to the vasoconstrictive effects of hyperventilation-induced hypocapnia than are comparison subjects. Ball and Shekhar (56) have reported a differentially greater hyperventilation-induced decrease in basilar arterial blood flow in patients with panic attacks than in comparison subjects by means of transcranial Doppler ultrasonography. Dager et al. (57) measured brain lactate levels by using magnetic resonance spectroscopy during voluntary hyperventilation in a group of patients with panic disorder and a group of normal comparison subjects. Although they controlled for differing degrees of hyperventilation by monitoring Pco2 with capnometry, hyperventilating subjects with panic disorder increased their brain lactate levels disproportionately, suggesting that they may have an exaggerated hypersensitivity to the vasoconstrictive effect of hypocapnia.
Our group has recently generated pilot data consistent with this notion (figure 2). A small number of patients with panic disorder, one of whom had been treated with cognitive behavioral therapy and was entirely asymptomatic at the time of the study, one of whom had been successfully treated with fluoxetine, and healthy comparison subjects, were asked to hyperventilate during [133Xe]-SPECT scanning. When corrected for level of hypocapnia, as measured by the end tidal CO2 level, the patients showed significantly greater decreases in CBF than the healthy comparison subjects. The only exception was the remitted patient who was taking fluoxetine at the time and who appeared by inspection to have the same level of vasoconstriction as the healthy comparison subjects.
Conclusions from so preliminary a study are obviously perilous, but the results are consistent with those of many other neuroimaging observations with patients with panic disorder. The greater vasoconstriction found in the patients with panic disorder cannot be secondary to more hyperventilation or greater hypocapnia because these were carefully controlled, and observed levels were equivalent to those found in the healthy comparison subjects. Some additive mechanism must be involved. Two possibilities arise. First, as discussed earlier, it is well known from the work of Goddard et al. (58) and others that the noradrenergic system is more active and more sensitive in patients with panic than in healthy comparison subjects. Matthews (59) has argued convincingly that this, plus the fact that noradrenergic fibers innervate cerebral blood vessels and cause vasoconstriction (60), explains the heightened vasoconstriction in patients with panic during hyperventilation. Patients with panic may respond with more fear during hyperventilation than do healthy comparison subjects, thus activating the locus ceruleus and causing noradrenergic-mediated vasoconstriction that adds to the effects of hyperventilation.
A second possibility arises from the preclinical studies of Marovitch and colleagues (61), showing that stimulation of the parabrachial nucleus, which is adjacent to the locus ceruleus in the brainstem, also causes cerebral vasoconstriction. The widespread reduction in CBF seen with stimulation of the parabrachial nucleus may be mediated by yet another subcortical site, since projections of the parabrachial nucleus do not reach the entire cortex. Marovitch and colleagues have suggested that the rostral raphe nuclei, which are capable of modulating cortical vasoconstriction through serotonergic projections, may be such a site. In this role, the raphe nuclei might relay information from the parabrachial nucleus to the cerebral cortex, resulting in a more global vasoconstrictive effect. In either event, we postulate once again that activation of the amygdala during a fearful episode—in this case, hyperventilating in the enclosed space of a PET or SPECT scanner—may produce by its projections an increase in activity in either the locus ceruleus, the parabrachial nucleus, or both. This would increase the amount of vasoconstriction in patients with panic relative to healthy comparison subjects, leading to the exaggerated decreases in CBF observed in several neuroimaging studies.
The observation that one patient with panic treated with an SSRI did not show exaggerated vasoconstriction during hyperventilation is consonant with our theory. If, indeed, SSRIs produce inhibition of the locus ceruleus or parabrachial nucleus, either directly or through an effect on the amygdala, one would expect to see a normalization of the vasoconstrictive effect of hyperventilation. This speculation obviously calls for further studies because it could be a marker for the success of SSRI therapy in the treatment of panic disorder.
Although neuroimaging studies to date in patients with panic disorder have not implicated fear network structures established in preclinical models (perhaps because of methodological and technical limitations), neuroimaging studies in human fear conditioning have demonstrated the importance of these structures. Early PET studies attempting to look at changes in the brains of healthy subjects during aversive classical (fear) conditioning (62) demonstrated significant increases in rCBF in several subcortical structures implicated in the fear network, including the thalamus, hypothalamus, and periaqueductal gray region, in addition to somatosensory and associative cortices and the cingulate. However, most PET studies have not found activation of the amygdala during fear conditioning paradigms using subtractive PET methods (62–65). Although these studies did not find an outright increase in amygdalar rCBF in fear conditioning, a positive correlation between nonspecific electrodermal fluctuations (reflecting degree of conditioning) and rCBF in the right amygdala was reported (64).
Recently, fMRI has been used to overcome some of the difficulties evident in PET approaches, such as temporal sequencing and nonassociative effects, and one group has successfully demonstrated activation of the amygdala and periamygdaloid cortical areas during conditioned fear acquisition and extinction (66). In addition, several investigators have shown specific activation of the amygdala in response to presentations of affectively negative or fearful visual stimuli using fMRI techniques (67). fMRI permits far better anatomical and temporal resolution than PET or SPECT; however, its ability to delineate deeper subcortical and brainstem structures has been limited in the past by field inhomogeneity. With the advent of cardiac gating and other advances in fMRI technology, this problem is being overcome. fMRI is therefore a logical technology to extend to the study of patients with panic disorder, since its superior anatomical and temporal resolution should allow the investigation of fear network structures during a real-time panic attack.
What is specifically needed for the study of panic disorder is a method that can provoke anxiety or panic without causing hyperventilation-induced hypocapnia. One possibility is to ask subjects to inhale small amounts of CO2 during brain imaging studies. Concentrations as low as 5% in room air have been shown to increase anxiety levels in most subjects, with patients with panic disorder showing significantly more anxiety and higher rates of frank panic (21). Inhalation of CO2 does produce increases in both tidal volume and respiratory rate (68), but because CO2 is continuously supplied, there is no opportunity for hypocapnia to develop. The difficulty with this technique, however, is that CO2 inhalation produces a global increase in CBF, making it unclear whether regional activation of a network responsible for panic would once again be obscured by blood flow changes, albeit in the opposite direction as those caused by hyperventilation. Corfield and colleagues (69) recently showed that there are regional differences in response to CO2 inhalation, with the amygdala showing particularly strong effects. Such preliminary findings suggest that CO2 inhalation may provide a method of overcoming the problems associated with hyperventilation-induced hypocapnia that attend most neuroimaging studies in panic disorder reported so far.
Genetics of Fear and Panic Disorder
If we accept the hypothesis that patients with panic disorder suffer from an abnormally sensitive fear network that includes the central nucleus of the amygdala, hippocampus, periaqueductal gray region, and other brainstem areas, the next issue to address is why this is the case. One possibility is that there is an inherited tendency for fearfulness, perhaps manifested as a neurocognitive deficit resulting in an abnormal response to or modulation of the fear network. Once again, an appeal to preclinical work is helpful.
A number of studies have now indicated that quantitative trait loci on specific chromosomes are associated with heightened emotionality and with fear-conditioning in rodents. For example, Flint et al. (70) showed that three loci on mouse chromosomes 1, 12, and 15 were associated with decreased activity and increased defecation in a novel environment. They concluded that these loci were responsible for heightened “emotionality” and speculated that “there are cogent reasons for expecting that the genetic basis of emotionality is similar in other species and that it may underlie the psychological trait of susceptibility [emphasis added] to anxiety in humans. The pattern of behavioral effects of anxiolytic drugs in rodents together with the results of electrophysiological and lesion experiments suggest conservation between species of a common neural substrate, probably determined by homologous genes” (p. 1434). Both Wehner et al. (71) and Caldarone et al. (72) found quantitative trait loci associated with contextual fear conditioning in rodents on several chromosomes, with both groups implicating chromosome 1. In an editorial accompanying the Wehner et al. and Calderone et al. studies, Flint (73) commented that “the locus on chromosome 1 that was identified by both groups has in fact already been shown to influence fearfulness in two independent studies…the fact that four studies working on such different measures of the same trait have succeeded in identifying the same chromosomal region is very encouraging” (p. 251).
Is there evidence that panic disorder is genetic? There is no question that it is highly familial. A number of studies have now shown that the chance of having panic disorder is substantially elevated over the base rate in the population if one has a first-degree relative with panic disorder (74). But this is hardly evidence of a genetic cause; one can easily imagine that growing up with anxious relatives could condition an individual to develop heightened levels of fear, anxiety, or even panic attacks. The next best data come from studies comparing the concordance rate for panic disorder between monozygotic (identical) and between dizygotic (fraternal) twins. For most medical illnesses, finding a higher rate of concordance between monozygotic than between dizygotic twins for a disease implies a genetic basis. For psychiatric illness we must make the additional assumption that parents maintain as similar an environment for dizygotic twins as they do for monozygotic twins. At least three studies (75–77) have examined the concordance rates for panic disorder among twins, and all find a higher concordance for monozygotic than for dizygotic twins, with one (75) specifically showing a higher concordance rate for panic attacks than for the syndromal disorder itself. The conclusion at first glance seems clear: panic disorder must have a genetic component.
A closer examination of the twin studies, however, suggests a retreat from such a broad statement. In none of the studies was the concordance rate for panic between monozygotic twins even close to 50% (range=14%–31%). This means that although genetically identical twins are more likely to both have panic disorder than are twins who are no more genetically identical than ordinary brothers and sisters, there are many cases in which one identical twin has panic disorder and the other does not. The conclusion seems clear. If genes are involved in causing panic disorder, they cannot be the whole story.
Taking the preclinical studies and the twin studies together, the most logical assumption is that what is inherited is a susceptibility to panic, not panic disorder itself. It is well known that children exhibit varying levels of anxiety and fear. Some children are extremely anxious and have been called “behaviorally inhibited to the unfamiliar” by Kagan and colleagues (78). The presence of an anxiety disorder in childhood or adolescence confers a substantially increased risk for having a recurrent anxiety disorder in young adulthood (79). Nevertheless, many anxious children do not develop anxiety disorders as adults, and many people with first-degree relatives, even identical twins, with panic disorder never have a panic attack themselves.
The elegant behavioral genetic work of Flint and others suggests that it is possible to inherit a vulnerability to fearfulness and anxiety. Further, this may be traced to relatively few genes and may involve areas of the brain such as the amygdala and its projection sites or the cortical areas involved in modulating amygdalar activity. We conclude, then, that patients with panic disorder very likely inherit a susceptibility to panic that has its basis in an unusually sensitive fear network, with the central nucleus of the amygdala playing a significant role.
Environmental Basis for Panic Disorder
The most likely candidate for the part of panic disorder etiology not likely to be explained by genetics is an environmental insult. In our original neuroanatomical hypothesis, we made a similar claim, but since then the evidence that this is plausible has increased both from animal and human studies.
Several studies have now suggested that disruptions of early attachment to parents may be associated with the later development of panic disorder. For example, using data from the Epidemiological Catchment Area study, Tweed et al. (80) reported that adults whose mothers died before they were age 10 were almost seven times more likely than those without a history of early maternal death to be diagnosed with agoraphobia with panic attacks. Those adults whose parents separated or divorced before age 10 also had a greater likelihood of being diagnosed with agoraphobia with panic attacks—almost four times the rate of those without a history of early parental separation. Stein et al. (81) found that patients with panic disorder reported more instances of childhood sexual and physical abuse than did healthy comparison subjects. The idea that disrupted emotional attachments with significant caregivers during childhood may be a risk factor for panic disorder is consonant with the clinical observation that patients with panic disorder are unusually sensitive to perceived, threatened, or actual separations. Indeed, patients with panic are less likely to experience a panic attack when surrounded by trusted companions. One study showed that having a companion present reduced the likelihood of a panic attack during CO2 inhalation (82). This is striking because our attempts to reduce the rate of panic during CO2 inhalation by cognitive manipulation have not proven successful (83).
The preclinical literature now strongly indicates that early disruptions of the attachment between infants and mothers (in animals fathers rarely play a substantial role in rearing offspring) produce long-lived behavioral and biological changes. For example, Michael Meaney, Paul Plotsky, and others (84–87; Plotsky et al., unpublished 1996 presentation) have shown that in rodents, alterations in mother-offspring interactions produce changes in the infant’s subsequent hormonal and physiological responses to stress that endure throughout the lifetime. These altered responses appear to be mediated at least in part by changes in central CRF function. Furthermore, these changes vary across different genetic strains of rodent so that “in relatively hardy animals the early-life manipulations may have less obvious effects” (85).
Andrews, Rosenblum, Coplan, and colleagues (88–90; Coplan et al., unpublished 1997 presentation) have pursued an interesting behavioral paradigm in nonhuman primates. Infant bonnet macaques are exposed to either a low foraging demand condition, in which mothers are given immediate direct access to food, or a variable foraging demand condition, in which, at randomly selected intervals, the mother is forced to search for food, thus interfering with her attention to the infant. Both infant and mother receive adequate nutrition in both conditions. It is of interest that infants exposed to the variable foraging demand condition do not seem particularly distressed while the mother searches for food, but the mothers appear stressed, and some begin to neglect the emotional needs of the infant over time. The infants raised under the variable foraging demand condition appear normal in most respects, but they exhibit more fearfulness and less social gregariousness throughout their lives when compared to animals raised under low foraging demand conditions (88). As adults, the animals raised under the variable foraging demand condition also exhibit increased CRF levels in CSF (89), exaggerated behavioral response to noradrenergic stimulation, blunted behavioral response to serotonergic agonists (90), and blunted growth hormone response to a clonidine treatment condition (Coplan et al., unpublished 1997 presentation). Thus, even years after a relatively subtle disruption of maternal-infant interaction, animals whose mothers underwent an uncertain variable foraging demand condition are fearful and shy and show evidence of enduring biological disruption.
There is evidence that experiencing traumatic events during childhood and adulthood is associated with the development of panic disorder (91–94). Nevertheless, our model asserts that patients with panic disorder, by virtue of a genetically imposed abnormality in the intrinsic brain fear network, are more susceptible to the effects of trauma, particularly those involving separation and disrupted attachment, than are individuals without panic disorder. That recent traumatic stress may also play a role in stimulating the onset of panic is clearly compatible with this model. This abnormality could take a number of forms, including tonic autonomic hyperactivity or a neurocognitive defect that would prevent the appropriate interpretation of fear network signals and/or the appropriate cortical feedback to limit anxiety and panic responses. Hence, we propose an interaction between life stress and genetic susceptibility as the root cause of panic disorder in adults.
The invocation of adverse life events may help us understand why anxious children may grow up to be depressed, have one or more of a number of different anxiety disorders, or exhibit no psychopathology at all. It is possible that a similar genetic vulnerability is common to several of these conditions and whether, or which, one of them is actually expressed in adult life depends on the kind of environmental influences to which a child is exposed. The same fear network that we have posited to be abnormal in panic disorder may also function abnormally in social phobia, PTSD, generalized anxiety disorder, or depression. The relationships among the different parts of the network may differ, however, among these disorders. Such a theory would explain the commonly observed high levels of comorbidity among anxiety disorders and between anxiety and depression. It would also explain why the same medications are effective for all of these conditions—presumably because all involve some hyperactivity of the amygdala and its projections—but cognitive behavioral therapies differ, perhaps because of different degrees of abnormality in the hippocampus and prefrontal cortex.
This hypothesis imposes learned reactions and unconscious emotional states onto genetic vulnerability. It predicts in at least two ways that psychotherapy of several types should be useful in treating panic disorder. First, we will argue that phobic avoidance represents a type of contextual learning analogous to that seen in fear-conditioned animals. This learned phenomenon has its neural basis in the memory systems of the hippocampus. Second, we will argue that sensitivity to separation, fear of impending doom and death, and overreaction to somatic cues are mediated by higher cortical centers. In both cases, behavioral, cognitive, and possibly psychodynamic therapies play a role in modifying these systems.
Contextual Learning in Panic Disorder
As we noted in our introduction, one of the puzzles in understanding panic disorder is the frequent observation that even when patients report few or no panic attacks, they still exhibit avoidant behavior. This has led to the widespread recommendation that clinical trials examining antipanic treatments should measure the effect of the putative treatment on more domains than just the reduction in frequency or severity of panic attacks (95). A patient who has stopped having attacks but will not leave the house is probably not appropriately considered a responder to treatment.
It is well known that animals who have undergone a fear-conditioning experience also become conditioned to the context in which the experience occurred (96). For example, a rat conditioned to have the same behavioral and autonomic reaction to a tone as was elicited by mild electrical shock will also demonstrate these reactions when simply placed in the cage in which the conditioning experiment took place, even without presentation of the tone. This contextual conditioning is known to require intact hippocampal neurons (97). If after conditioning to the tone has occurred the amygdala is lesioned, the animal will no longer exhibit a fearful response to the tone but will respond fearfully when placed back in the cage. If on the other hand, the hippocampus is lesioned, the animal maintains the response to the tone but no longer responds to placement in the cage.
The analogy with patients with panic disorder appears obvious. Phobic avoidance arises, in part, from an association of panic attacks with the context in which they occurred. Patients who have had, for example, a panic attack in a car while driving over a bridge in rush hour traffic remain fearful of cars, traffic, and bridges. This can generalize to any situation in which egress is not immediately possible and help not automatically available. It is of interest that clinicians observe that some patients treated with medication no longer panic but still maintain some level of phobic avoidance. This may improve over time as deconditioning occurs, but in many patients it becomes a life-long problem despite adequate pharmacological treatment. Such patients often require and do well when offered behavioral therapy aimed at desensitizing them to feared contexts.
Perhaps the reaction of the patient with panic to minor physical discomfort also represents a kind of contextual fear conditioning. Many patients with panic disorder are unbearably sensitive to relatively trivial somatic sensations such as mild dizziness, increase in heart rate, or slight tingling in a limb. Although it is difficult to make analogies with animal models of fear conditioning, it is plausible that somatic sensation is a kind of context that becomes capable of triggering panic over time.
Cognitive behavioral therapy for panic disorder focuses, in part, on eliminating contextual fear by desensitizing the patient to both physical and somatic cues for panic (98). This may represent an effect on memory mediated by the hippocampus. Imaging studies should be very helpful in determining if this is indeed the case.
Role of Higher Cortical Centers in Panic Disorder
Beyond contextual learning, patients with panic disorder frequently exhibit widespread catastrophic thinking (99) and an increased risk for the development of major depression (100). They can become tied to “phobic partners,” sometimes maintaining attachments that are not in their best interest because of an exaggerated fear that separation will cause life-threatening consequences. The toll on the patient and significant others can be substantial, and marital discord among patients with panic disorder is frequently observed (101).
There are many neuroanatomical sites that likely subserve the cognitive and relationship difficulties that ultimately may be the worst part of panic disorder. We have suggested that cortical sites, particularly those that process higher-order sensory information such as the medial prefrontal cortex, are important in modulating anxiety responses. As LeDoux has suggested (102), psychotherapy may work, in part, by strengthening the ability of these cortical projections to assert reason over automatic behavioral and physical responses. Medications, in our opinion, are only partially helpful in reversing the incessantly gloomy predictions of patients with panic disorder or in helping them rearrange relationships that are based on safety and dependency. Depending on the severity of these problems, which often correlates with how long a patient has had panic disorder, psychotherapy becomes invaluable.
We propose then that psychotherapies that are effective for panic disorder, including cognitive behavioral therapy and possibly psychodynamic treatment, operate upstream from the amygdala and exert powerful inhibitory effects on a variety of learned responses. At least in the case of cognitive behavioral therapy, for which most evidence now exists, these treatments are capable of reducing the frequency of panic attacks themselves and also of decreasing phobic avoidance and catastrophic thinking (103). Dynamic therapy may be most helpful for dealing with the effects of early disruptions in parental/child attachments that appear important for some patients in the development of panic disorder, but this requires empirical validation.
Conclusions
We are left with a model for panic disorder that asserts, exactly as did our original neuroanatomical hypothesis, that medication and psychotherapy can both be effective treatments for panic disorder and that they operate at different levels of the brain. Beyond this, the revised hypothesis presented here is substantially altered from the original. Using information from preclinical science, we now hypothesize that patients with panic disorder inherit an especially sensitive central nervous system fear mechanism that has at its center the central nucleus of the amygdala and includes the hippocampus, thalamus, and hypothalamus, as well as the periaqueductal gray region, locus ceruleus, and other brainstem sites. Medications such as SSRIs may reduce panic attacks by decreasing the activity of the amygdala and interfering with its ability to stimulate projection sites in the hypothalamus and brainstem. Once the physiologic/somatic symptoms of anxiety are lessened, there is a secondary reduction in anticipatory anxiety and phobic avoidance. Cognitive behavioral and other effective psychotherapies most likely operate upstream from the amygdala, reducing phobic avoidance by deconditioning contextual fear learned at the level of the hippocampus and decreasing cognitive misattributions and abnormal emotional reactions by strengthening the ability of the medial prefrontal cortex to inhibit the amygdala.
This model suggests many experimental tests. We have already outlined the promise of neuroimaging studies in detailing the exact neuroanatomical substrates for panic and phobic avoidance and also the sites of action of effective therapies. Genetic research should be targeted at uncovering susceptibility genes, rather than attempting to find genes for panic disorder itself. The continued development of animal models will help elucidate the exact neural mechanisms that translate early rearing stress into permanent disorders of behavior and neurobiology.
Although many aspects of our revised neuroanatomical hypothesis are likely to prove incorrect, it is our profound hope that it will stimulate a renewed interest in basic and clinical research of anxiety disorders such as panic. In our opinion, anxiety disorders are among the most likely psychiatric disturbances to yield to modern scientific inquiry. The results should be better treatment for our patients.
Received Feb. 25, 1999; revision received Aug. 3, 1999; accepted Aug. 4, 1999. From the Department of Psychiatry, College of Physicians and Surgeons, Columbia University; and the Department of Clinical Psychobiology, New York State Psychiatric Institute, New York. Address reprint requests to Dr. Gorman, Department of Psychiatry, College of Physicians and Surgeons, Columbia University, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail). Supported in part by an NIMH grant to Drs. Kent and Sullivan (MH-15144), a Senior Scientist Award to Dr. Gorman (MH-00416), and a Research Scientist Development Award to Dr. Coplan (MH-01039). The authors thank Barbara Barnett, Christopher Tulysewski, David Ruggiero, and Jose Martinez for their help in the preparation of this manuscript.
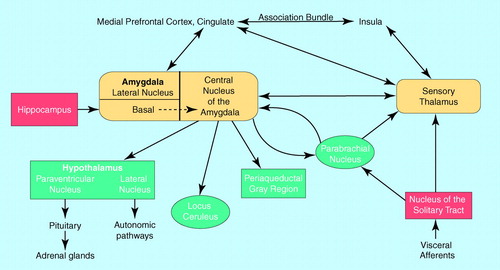
FIGURE 1. Neuroanatomical Pathways of Viscerosensory Information in the Braina
aViscerosensory information is conveyed to the amygdala by two major pathways: downstream, from the nucleus of the solitary tract via the parabrachial nucleus or the sensory thalamus; and upstream, from the primary viscerosensory cortices and via corticothalamic relays allowing for higher-level neurocognitive processing and modulation of sensory information. Contextual information is stored in memory in the hippocampus and conveyed directly to the amygdala. Major efferent pathways of the amygdala relevant to anxiety include the following: the locus ceruleus (increases norepinephrine release, which contributes to physiologic and behavioral arousal), the periaqueductal gray region (results in defensive behaviors and postural freezing), the hypothalamic paraventricular nucleus (activates the hypothalamic-pituitary-adrenal axis, releasing adrenocorticoids), the hypothalamic lateral nucleus (activates the sympathetic nervous system), and the parabrachial nucleus (influences respiratory rate and timing).
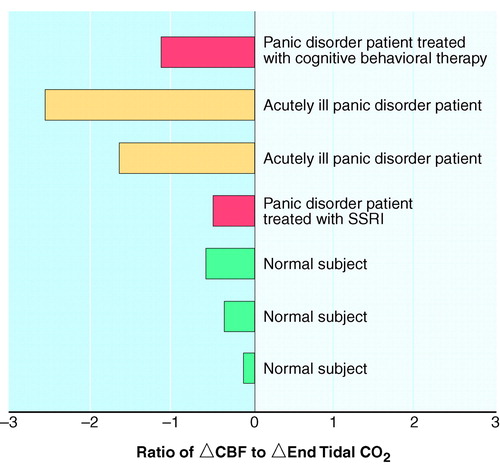
FIGURE 2. Change in Global CBF, Corrected for Degree of Hypocapnia, During Voluntary Hyperventilation in Three Patients With Panic Disorder and Three Normal Comparison Subjectsa
aSignificant difference between groups, with the patient taking an SSRI excluded (t=3.26, df=4, p=0.03, N=6).
1. Gorman JM, Liebowitz MR, Fyer AJ, Stein J: A neuroanatomical hypothesis for panic disorder. Am J Psychiatry 1989; 146:148–161Link, Google Scholar
2. Kent JM, Coplan JD, Gorman JM: Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry 1998; 44:812–824Crossref, Medline, Google Scholar
3. Welkowitz LA, Papp LA, Cloitre M, Liebowitz MR, Martin LY, Gorman JM: Cognitive-behavior therapy for panic disorder delivered by psychopharmacologically oriented clinicians. J Nerv Ment Dis 1991; 179:473–477Crossref, Medline, Google Scholar
4. Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, de Jesus MJ, Ross D, Goetz R, Gorman JM: Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatry 1997; 154:1557–1565Google Scholar
5. Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P: Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Res 1993; 46:89–103Crossref, Medline, Google Scholar
6. Callahan PM, de la Garza R II, Cunningham KA: Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 1997; 57:601–607Crossref, Medline, Google Scholar
7. Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE: Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19:591–611Crossref, Medline, Google Scholar
8. Klein DF: False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
9. Pavlov IP: Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex (1927). Edited by Anrep GV. New York, Boyer, 1960Google Scholar
10. LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM: The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 1990; 10:1062–1069Google Scholar
11. LeDoux JE, Iwata J, Cicchetti P, Reis DJ: Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8:2517–2519Google Scholar
12. Davis M: The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992; 15:353–375Crossref, Medline, Google Scholar
13. Takeuchi Y, McLean JH, Hopkins DA: Reciprocal connections between the amygdala and parabrachial nuclei: ultrastructural demonstration by degeneration and axonal transport of horseradish peroxidase in the cat. Brain Res 1982; 239:583–588Crossref, Medline, Google Scholar
14. Price JL, Amaral DG: An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1981; 1:1242–1259Google Scholar
15. Cedarbaum JM, Aghajanian GK: Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol 1978; 178:1–16Crossref, Medline, Google Scholar
16. Dunn JD, Whitener J: Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 1986; 42:211–217Crossref, Medline, Google Scholar
17. De Oca BM, DeCola JP, Maren S, Fanselow MS: Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci 1998; 18:3426–3432Google Scholar
18. de Olmos J: Amygdaloid nuclear gray complex, in The Human Nervous System. Edited by Paxinos G. San Diego, Academic Press, 1990, pp 583–710Google Scholar
19. Freedman RR, Ianni P, Ettedgui E, Puthezhath N: Ambulatory monitoring of panic disorder. Arch Gen Psychiatry 1985; 42:244–248Crossref, Medline, Google Scholar
20. Martinez JM, Papp LA, Coplan JD, Anderson DE, Mueller CM, Klein DF, Gorman JM: Ambulatory monitoring of respiration in anxiety. Anxiety 1996; 2:296–302Medline, Google Scholar
21. Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, Ross D, Klein DF: Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry 1994; 151:547–553Link, Google Scholar
22. Papp LA, Klein DF, Gorman JM: Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry 1993; 150:1149–1157Google Scholar
23. Coplan JD, Goetz R, Klein DF, Papp LA, Fyer AJ, Liebowitz MR, Davies SO, Gorman JM: Plasma cortisol concentrations preceding lactate-induced panic: psychological, biochemical, and physiological correlates. Arch Gen Psychiatry 1998; 55:130–136Crossref, Medline, Google Scholar
24. Woods SW, Charney DS, McPherson CA, Gradman AH, Heninger GR: Situational panic attacks: behavioral, physiologic, and biochemical characterization. Arch Gen Psychiatry 1987; 44:365–375Crossref, Medline, Google Scholar
25. Liebowitz MR, Gorman JM, Fyer AJ, Levitt M, Dillon D, Levy G, Appleby IL, Anderson S, Palij M, Davies SO, Klein DF: Lactate provocation of panic attacks, II: biochemical and physiological findings. Arch Gen Psychiatry 1985; 42:709–719Crossref, Medline, Google Scholar
26. Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF: Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 1988; 45:31–39Crossref, Medline, Google Scholar
27. Charney DS, Heninger GR, Breier A: Noradrenergic function in panic anxiety: effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch Gen Psychiatry 1984; 41:751–763Crossref, Medline, Google Scholar
28. Targum SD, Marshall LE: Fenfluramine provocation of anxiety in patients with panic disorder. Psychiatry Res 1989; 28:295–306Crossref, Medline, Google Scholar
29. Klein E, Zohar J, Geraci MF, Murphy DL, Uhde TW: Anxiogenic effects of m-CPP in patients with panic disorder: comparison to caffeine’s anxiogenic effects. Biol Psychiatry 1991; 30:973–984Crossref, Medline, Google Scholar
30. Pyke RE, Greenberg HS: Norepinephrine challenges in panic patients. J Clin Psychopharmacol 1986; 6:279–285Crossref, Medline, Google Scholar
31. Veltman DJ, van Zijderveld GA, van Dyck R: Epinephrine infusions in panic disorder: a double-blind placebo-controlled study. J Affect Disord 1996; 39:133–140Crossref, Medline, Google Scholar
32. Jensen CF, Peskind ER, Veith RC, Hughes J, Cowley DS, Roy-Byrne P, Raskind MA: Hypertonic saline infusion induces panic in patients with panic disorder. Biol Psychiatry 1991; 30:628–630Crossref, Medline, Google Scholar
33. Bradwejn J, Koszycki D: The cholecystokinin hypothesis of anxiety and panic disorder. Ann NY Acad Sci 1994; 713:273–282Crossref, Medline, Google Scholar
34. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Panic Disorder. Am J Psychiatry 1998; 155(May suppl)Google Scholar
35. Kreiss DS, Lucki I: Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther 1995; 274:866–875Medline, Google Scholar
36. Boyer W: Serotonin uptake inhibitors are superior to imipramine and alprazolam in alleviating panic attacks: a meta-analysis. Int Clin Psychopharmacol 1995; 10:45–49Crossref, Medline, Google Scholar
37. Hoyer D, Martin G:5-HT receptor classification and nomenclature: toward a harmonization with the human genome. Neuropharmacology 1997; 36:419–428Google Scholar
38. Blier P, DeMontigny C, Chaput Y: Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol 1987; 7:24S–35SCrossref, Medline, Google Scholar
39. Handley SL:5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther 1995; 66:103–148Google Scholar
40. Tork I, Hornung J-P: Raphe nuclei and the serotonergic system, in The Human Nervous System. Edited by Paxinos G. San Diego, Academic Press, 1990, pp 1001–1022Google Scholar
41. Aston-Jones G, Akaoka H, Charlety P, Chouvet G: Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci 1991; 11:760–769Crossref, Medline, Google Scholar
42. Coplan JD, Papp LA, Pine DS, Martinez JM, Cooper TB, Rosenblum L, Klein DF, Gorman JM: Clinical improvement with fluoxetine therapy and noradrenergic function in patients with panic disorder. Arch Gen Psychiatry 1997; 54:643–648Crossref, Medline, Google Scholar
43. Viana MB, Graeff FG, Loschmann PA: Kainate microinjection into the dorsal raphe nucleus induces 5-HT release in the amygdala and periaqueductal gray. Pharmacol Biochem Behav 1997; 58:167–172Crossref, Medline, Google Scholar
44. Deakin JFW, Graeff F:5-HT and mechanisms of defence. J Psychopharmacol 1991; 5:305–315Google Scholar
45. Brady LS, Gold PW, Herkenham M, Lynn AB, Whitfield HJ Jr: The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res 1992; 572:117–125Crossref, Medline, Google Scholar
46. Elkabir DR, Wyatt ME, Vellucci SV, Herbert J: The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept 1990; 28:199–214Crossref, Medline, Google Scholar
47. Butler PD, Weiss JM, Stout JC, Nemeroff CB: Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci 1990; 10:176–183Crossref, Medline, Google Scholar
48. Baram TZ, Mitchell WG, Haden E: Inhibition of pituitary-adrenal secretion by a corticotropin releasing hormone antagonist in humans. Mol Psychiatry 1996; 1:320–324Medline, Google Scholar
49. Griebel G, Perrault G, Sanger DJ: Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents: comparison with diazepam and buspirone. Psychopharmacology (Berl) 1988; 138:55–66Google Scholar
50. Stutzmann GE, LeDoux JE: GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci 1999; 19:RC8Google Scholar
51. Sokoloff L: Relationships among local functional activity, energy metabolism, and blood flow in the central nervous system. Fed Proc 1981; 40:2311–2316Google Scholar
52. Raichle ME, Grubb RL Jr, Gado MH, Eichling JO, Ter-Pogossian MM: Correlation between regional cerebral blood flow and oxidative metabolism. Arch Neurol 1976; 33:523–526Crossref, Medline, Google Scholar
53. Fox PT, Raichle ME: Cerebral blood flow and oxidative metabolism are focally uncoupled by physiological activation: a positron-emission tomographic study, in Cerebrovascular Diseases. Edited by Raichle ME, Powers WJ. New York, Raven Press, 1987, pp 129–138Google Scholar
54. Stewart RS, Devous MD Sr, Rush AJ, Lane L, Bonte FJ: Cerebral blood flow changes during sodium-lactate-induced panic attacks. Am J Psychiatry 1988; 145:442–449Link, Google Scholar
55. Gorman JM, Goetz R, Uy J, Ross D, Martinez J, Fyer AJ, Liebowitz MR, Klein DF: Hyperventilation occurs during lactate-induced panic. J Anxiety Disorders 1988; 2:193–202Crossref, Google Scholar
56. Ball S, Shekhar A: Basilar artery response to hyperventilation in panic disorder. Am J Psychiatry 1997; 154:1603–1604Google Scholar
57. Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA: Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. Am J Psychiatry 1995; 152:666–672Link, Google Scholar
58. Goddard AW, Gorman JM, Charney DS: Neurobiology of panic disorder, in Panic Disorder and Its Treatment. Edited by Rosenbaum JF, Pollack MH. New York, Marcel Dekker, 1998, pp 57–92Google Scholar
59. Matthews RJ: Sympathetic control of cerebral circulation: relevance to psychiatry. Biol Psychiatry 1995; 37:283–285Crossref, Medline, Google Scholar
60. Kalaria RN, Stockmeier CA, Harik SI: Brain microvessels are innervated by locus ceruleus noradrenergic neurons. Neurosci Lett 1989; 97:203–208Crossref, Medline, Google Scholar
61. Marovitch S, Iadecola C, Ruggiero DA, Reis DJ: Widespread reductions in cerebral blood flow and metabolism elicited by electrical stimulation of the parabrachial nucleus in the rat. Brain Res 1985; 314:283–296Crossref, Google Scholar
62. Fredrikson M, Wik G, Fischer H, Andersson J: Affective and attentive neural networks in humans: a PET study of Pavlovian conditioning. Neuroreport 1995; 7:97–101Crossref, Medline, Google Scholar
63. Morris J, Frith C, Perret D, Rowland D, Young A, Calder A, Dolan R: A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383:812–815Crossref, Medline, Google Scholar
64. Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M: The amygdala and individual differences in human fear conditioning. Neuroreport 1997; 8:3957–3960Google Scholar
65. Hugdahl K, Beraki A, Thompson W, Kosslyn S, Macy R, Baker D, Alpert N, LeDoux JE: Brain mechanisms in human classical conditioning: a PET blood flow study. Neuroreport 1995; 6:1723–1728Google Scholar
66. LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA: Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 1998; 20:937–945Crossref, Medline, Google Scholar
67. Grodd W, Schneider F, Klose U, Nagele T: [Functional magnetic resonance tomography of psychological functions exemplified by experimentally induced emotions]. Radiologe 1995; 35:283–289 (German)Medline, Google Scholar
68. Papp LA, Martinez J, Coplan JD, Gorman JM: Rebreathing tests in panic disorder. Biol Psychiatry 1995; 38:240–245Crossref, Medline, Google Scholar
69. Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JDG, Adams L, Frackowiak RSJ, Guz A: Activation of limbic structures during C02-stimulated breathing in awake man, in Modeling and Control of Ventilation. Edited by Semple SJG, Adams L, Whipp BJ. New York, Plenum, 1995, pp 331–334Google Scholar
70. Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, Collins AC: A simple genetic basis for a complex psychological trait in laboratory mice. Science 1995; 269:1432–1435Google Scholar
71. Wehner JM, Radcliffer RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW, Wiles M: Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet 1997; 17:331–334Crossref, Medline, Google Scholar
72. Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L: Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet 1997; 17:335–337Crossref, Medline, Google Scholar
73. Flint J: Freeze! Nat Genet 1997; 17:250–251Crossref, Medline, Google Scholar
74. Weissman MM, Wickramaratne P, Adams PB, Lish JD, Horwath E, Charney D, Woods SW, Leeman E, Frosch E: The relationship between panic disorder and major depression: a new family study. Arch Gen Psychiatry 1993; 50:767–780Crossref, Medline, Google Scholar
75. Togersen S: Genetic factors in anxiety disorders. Arch Gen Psychiatry 1983; 40:1085–1089Google Scholar
76. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: Panic disorder in women: a population-based twin study. Psychol Med 1993; 23:397–406Crossref, Medline, Google Scholar
77. Skre I, Onstad S, Togersen S, Lygren S, Kringlen E: A twin study of DSM-III-R anxiety disorders. Acta Psychiatr Scand 1993; 88:85–92Crossref, Medline, Google Scholar
78. Kagan J, Reznick JS, Snidman N: The physiology and psychology of behavioral inhibition. Child Dev 1987; 58:1459–1473Google Scholar
79. Pine DS, Cohen P, Gurley D, Brook J, Ma Y: The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64Crossref, Medline, Google Scholar
80. Tweed JL, Schoenbach VJ, George LK, Blazer DG: The effects of childhood parental death and divorce on six-month history of anxiety disorders. Br J Psychiatry 1989; 154:823–828Crossref, Medline, Google Scholar
81. Stein MB, Walker JR, Anderson G, Hazen AL, Ross CA, Eldridge G, Forde DR: Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry 1996; 153:275–277Link, Google Scholar
82. Carter MM, Hollon SD, Carson R, Shelton RC: Effects of a safe person on induced distress following a biological challenge in panic disorder with agoraphobia. J Abnorm Psychol 1995; 104:156–163Crossref, Medline, Google Scholar
83. Papp LA, Welkowitz LA, Martinez JM, Klein DF, Browne S, Gorman JM: Instructional set does not alter outcome of respiratory challenges in panic disorder. Biol Psychiatry 1995; 38:826–830Crossref, Medline, Google Scholar
84. Francis DD, Meaney MJ: Maternal care and the development of stress responses. Curr Opin Neurobiol 1999; 9:128–134Crossref, Medline, Google Scholar
85. Anisman H, Zaharia MD, Meaney MJ, Merali Z: Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci 1998; 16:149–164Google Scholar
86. Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney JM: Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 1998; 95:5335–5340Google Scholar
87. Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney JM: Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997; 277:1659–1662Google Scholar
88. Andrews MW, Rosenblum LA: The development of affiliative and agonistic social patterns in differentially reared monkeys. Child Dev 1994; 65:1398–1404Google Scholar
89. Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Gorman JM, Nemeroff CB: Increased cerebrospinal fluid CRF concentration in adult non-human primates previously exposed to adverse experiences as infants. Proc Natl Acad Sci USA 1996; 93:1619–1623Google Scholar
90. Rosenblum LA, Coplan JD, Friedman S, Bassoff TB, Gorman JM: Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biol Psychiatry 1994; 35:221–227Crossref, Medline, Google Scholar
91. Tweed JL, Schoenbach VJ, George LK, Blazer DG: The effects of childhood parental death and divorce on six-month history of anxiety disorders. Br J Psychiatry 1989; 154:823–828Crossref, Medline, Google Scholar
92. Faravelli C, Webb T, Ambonetti A, Fonnesu F, Sessarego A: Prevalence of traumatic early life events in 31 agoraphobic patients with panic attacks. Am J Psychiatry 1985; 142:1493–1494Google Scholar
93. Faravelli C, Pallanti S: Recent life events and panic disorder. Am J Psychiatry 1989; 146:622–626Link, Google Scholar
94. Horesh N, Amir M, Kedem P, Goldberger Y, Kotler M: Life events in childhood, adolescence and adulthood and the relationship to panic disorder. Acta Psychiatr Scand 1997; 96:373–378Crossref, Medline, Google Scholar
95. Shear MK, Masur JD: Standardized assessment for panic disorder research: a conference report. Arch Gen Psychiatry 1994; 51:346–354Crossref, Medline, Google Scholar
96. Phillips RG, LeDoux JE: Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106:274–285Crossref, Medline, Google Scholar
97. Kim JJ, Fanselow MS: Modality-specific retrograde amnesia of fear. Science 1992; 256:675–677Crossref, Medline, Google Scholar
98. Barlow DH: Cognitive-behavioral therapy for panic disorder: current status. J Clin Psychiatry 1997; 58(suppl 2):32–36Google Scholar
99. Hibbert GA: Ideational components of anxiety: their origin and content. Br J Psychiatry 1984; 144:618–624Crossref, Medline, Google Scholar
100. Klerman GL: Depression and panic anxiety: the effect of depressive comorbidity on response to drug treatment of patients with panic disorder and agoraphobia. J Psychiatr Res 1990; 24(suppl 2):27–41Google Scholar
101. Markowitz JS, Weissman MM, Ouellette R, Lish JD, Klerman GL: Quality of life in panic disorder. Arch Gen Psychiatry 1989; 46:984–992Crossref, Medline, Google Scholar
102. LeDoux JE: The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, Simon & Schuster, 1996Google Scholar
103. Otto MW, Whittal ML: Cognitive-behavior therapy and the longitudinal course of panic disorder. Psychiatr Clin North Am 1995; 18:803–820Crossref, Medline, Google Scholar



