Volumetric White Matter Abnormalities in First-Episode Schizophrenia: A Longitudinal, Tensor-Based Morphometry Study
Abstract
Objective: While schizophrenia has long been considered a disorder of brain connectivity, few studies have investigated white matter abnormalities in patients with first-episode schizophrenia, and even fewer studies have investigated whether there is progressive white matter pathology in the disease. Method: The authors obtained a T1-weighted structural magnetic resonance imaging (MRI) scan on 41 patients with first-episode schizophrenia. These first-episode schizophrenia patients were analyzed relative to 47 age- and sex-matched healthy comparison subjects who also underwent an MRI scan. Of the baseline participants, 25 first-episode schizophrenia patients and 26 comparison subjects returned 2 to 3 years later for a follow-up scan. To identify regional volumetric white matter differences between the two groups at baseline, voxel-based morphometry in statistical parametric mapping-2 (SPM2) was used, while tensor-based morphometry was used to identify the longitudinal changes over the follow-up interval. Results: The first-episode schizophrenia patients exhibited volumetric deficits in the white matter of the frontal and temporal lobes at baseline, as well as volumetric increases in the white matter of the frontoparietal junction bilaterally. Furthermore, these first-episode schizophrenia patients lost considerably more white matter over the follow-up interval relative to comparison subjects in the middle and inferior temporal cortex bilaterally. Conclusions: These results indicate that patients with schizophrenia exhibit white matter abnormalities at the time of their first presentation of psychotic symptoms to mental health services and that these abnormalities degenerate further over the initial years of illness. Given the role that white matter plays in neural communication, the authors suggest that these white matter abnormalities may be a cause of the dysfunctional neural connectivity that has been proposed to underlie the symptoms of schizophrenia.
A dysfunction in neural connectivity has long been proposed as the fundamental abnormality underlying schizophrenia (1 – 3) . Given that white matter constitutes the anatomical infrastructure for neural connectivity, it is reasonable to hypothesize the existence of white matter abnormalities in patients with schizophrenia (4) . The present study aimed to investigate for white matter abnormalities in patients with schizophrenia, both at the time of their first presentation to mental health services with psychotic symptoms and longitudinally over the first 2 to 3 years of illness.
Volumetric white matter reductions, particularly in the frontal lobe, have been the most consistently reported white matter abnormality in patients with chronic schizophrenia (5) . This volumetric reduction could potentially be indicative of abnormal axonal myelination, or it could represent axonal elimination resulting from neuronal death. In contrast to chronically ill patients, however, few studies have investigated white matter abnormalities in patients experiencing their first episode of schizophrenia. This is an issue that must be addressed, since investigating the structural underpinnings of schizophrenia early in its course gives significant insight into the nature of the disease, its origins, its clinical course, and the optimal path for therapeutic intervention. Furthermore, investigating patients with first-episode schizophrenia minimizes the confounds associated with long-term exposure to neuroleptic medication, which has been suggested to affect brain structure in and of itself (6) . Previous studies that have investigated for evidence of white matter irregularities in first-episode schizophrenia patients have produced equivocal results. For example, while several studies have reported white matter abnormalities in first-episode schizophrenia, including impaired myelination of the corpus callosum (7) , irregular shape of the corpus callosum (8) , reduced fractional anisotropy in frontotemporal white matter (9) , and a decreased magnetization transfer ratio in the fasciculus uncinatus (10) , others have failed to observe any white matter abnormalities (11 – 13) . Inconsistent results have also been reported in the very few studies that have investigated longitudinal white matter changes in patients with recent-onset schizophrenia. For example, while Ho et al. (14) reported progressive atrophy in frontal lobe white matter over 3 years in patients with recent-onset schizophrenia, Rapoport et al. (15) did not observe differential rates of white matter change over 4 years between patients with childhood-onset schizophrenia and matched healthy comparison subjects.
Aside from the clearly delineated corpus callosum, it is notoriously difficult to manually define white matter regions of interest consistently between subjects because of the dearth of referential anatomical landmarks. Hence, the majority of previous studies have investigated changes in white matter at the level of the whole brain or the brain lobe. The lack of sensitivity inherent in such a large-scale analysis could well have contributed to the inconsistency of previous findings. This is one area in which automated statistical imaging techniques (e.g., voxel-based morphometry) are advantageous. By looking for evidence of structural difference at every voxel in the brain, the statistical imaging techniques are able (given appropriate statistical correction for multiple comparisons) to identify small, discrete areas of regional abnormality without requiring the manual tracing of regions of interest which, as well as being difficult to define consistently, are also necessarily constrained to the regions defined by prior hypotheses.
In this study, we used voxel-based morphometry to investigate for evidence of white matter abnormality in patients with first-episode schizophrenia relative to matched healthy comparison subjects (baseline condition). We then used tensor-based morphometry to identify evidence of progressive white matter atrophy in the first-episode schizophrenia patients over the first 2 to 3 years of illness, over and above any corresponding longitudinal changes experienced by the healthy comparison subjects (follow-up condition). Based on the longitudinal gray matter reductions that we have previously reported in these patients (16) , we hypothesized that the first-episode schizophrenia patients would exhibit frontal, temporal, and parietal white matter reductions at baseline relative to the healthy comparison subjects and that these regional abnormalities would degenerate over the follow-up interval.
Method
Subjects
Forty-one patients experiencing their first episode of schizophrenia were recruited for the baseline condition as part of the Western Sydney, Australia, First Episode Psychosis project, a multimodal project investigating the clinical, neuroanatomical, neuropsychological and psychophysiological profiles of young people in western Sydney, Australia, who were experiencing their first episode of psychosis (17) . A stringent criterion for first-episode status was employed whereby all patients were recruited within 3 months of their first presentation to mental health services with psychotic symptoms (defined as hallucinations, delusions and/or formal thought disorder), although some patients had previously presented with symptoms of anxiety and depression that were not judged to be psychotic at the time. Diagnosis of schizophrenia was made using DSM-IV criteria (18) , by a consensus conference of at least three qualified senior psychiatrists, at least two of whom were independent of the study. Subjects with schizophrenia were interviewed and rated by psychiatrists who had reached an acceptable level of interrater reliability (r>0.8) on the Positive and Negative Syndrome Scale (19) .
Forty-seven healthy comparison subjects were recruited from parallel geographical regions in collaboration with the Brain Resource International Database (http://www.brainresource.com). Comparison subjects were screened for the presence of an axis I disorder using the SPHERE (20) , and subjects were also excluded if they reported a first-degree family member with an axis I diagnosis. Comparison subjects were within the normal range on depression, anxiety, and stress, which were assessed using an abbreviated version of the Depression Anxiety and Stress Scale (21) .
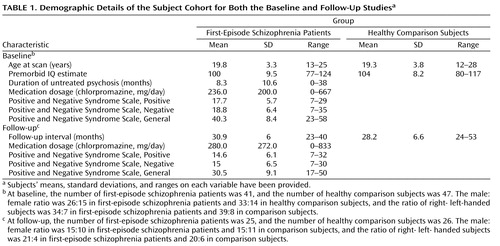
Exclusion criteria for both groups were a past history of substance dependence; exposure to electroconvulsive therapy (ECT); mental retardation (estimated premorbid IQ <75, based on the Wide Range Achievement Test, Revision 3 [22] and the Spot-the-Real-Word Test [23] ); neurological disorder, including epilepsy; a history of head injury causing loss of consciousness for at least 1 hour; and gross neurological abnormality as assessed by a senior radiologist who was independent of the study. Of the 41 first-episode schizophrenia patients and 47 matched healthy comparison subjects scanned in the baseline condition, 25 first-episode schizophrenia patients and 26 comparison subjects returned between 2 and 3 years later (on average) for a follow-up scan. The demographic data for the patients and comparison subjects at baseline and follow-up is presented in Table 1 . There were no significant differences, either at baseline or follow-up, between the first-episode schizophrenia patients and comparison subjects with respect to age at scan, gender, handedness, estimated premorbid IQ, or the time interval between scans (follow-up interval). Furthermore, there were no significant differences between the first-episode schizophrenia group at baseline and the first-episode schizophrenia group at follow-up or the comparison group at baseline and the comparison group at follow-up on male/female ratio, right/left handedness ratio, premorbid IQ, and medication dosage. This suggested that the follow-up group was representative of the baseline group for both the first-episode schizophrenia patients and comparison subjects. Subjects who reported having had a major neurological or cardiac event over the follow-up interval were excluded from the study. Over the course of the study, patients received treatment from a variety of services, and no fixed treatment protocol was used. Treatment combined second-generation antipsychotics (amisulpride, clozapine, olanzapine, risperidone, and quetiapine) with community case management, which varied from once or twice weekly contact with psychosocial rehabilitation to occasional contact from subjects refusing services. Most patients were exposed to at least two antipsychotics over the course of the follow-up interval. After a detailed description of the study, each subject gave written informed consent to participate in accordance with Australian National Health and Medical Research Council guidelines. This study was approved by the Western Sydney, Australia, Area Health Service Human Research Ethics Committee.
Magnetic Resonance Imaging (MRI) Acquisition
The full details of the magnetic resonance (MR) scanning protocols have been described previously (16) . Both first-episode schizophrenia patients and comparison subjects underwent a single T1-weighted volumetric MPRAGE structural MRI scan at baseline and again at follow-up on a Siemens 1.5-Tesla Vision Plus system at Westmead Hospital, Sydney, Australia (time to repeat=9.7 msec, echo time=4 msec, TI=200 msec, flip angle=12°, field of view=256 mm, voxel size=1 mm 3 ). There were no significant upgrades or modifications of the scanner between the baseline and follow-up stages of the study. To control for scanner drift, phantom data were collected weekly over the follow-up interval, and the scanner calibrated accordingly.
Image Preprocessing: Baseline Study
The baseline images were processed using voxel-based morphometry in statistical parametric mapping-2 (SPM2) (Wellcome Department of Cognitive Neurology, London), running on Matlab 6.5 (MathWorks, Natick, U.S.). The full details of the processing protocol used for voxel-based morphometry have been presented elsewhere (16 , 24 , 25) . Briefly, subjects’ brain images were first normalized spatially to the Montreal Neurological Institute T1-weighted template via a 12-parameter affine transformation and a linear combination (7×8×7) of smooth spatial basis functions to account for nonlinear shape differences. The normalized images were then segmented into gray matter, white matter, and CSF probability maps, cleaned of extracerebral voxels, modulated with the Jacobian determinants from the spatial normalization, and smoothed with a 12 mm Gaussian kernel.
Image Preprocessing: Longitudinal Study
The methodology we used for tensor-based morphometry, which was based on the procedure described by Kipps et al. (26) , has been described in full detail elsewhere (16) . What follows is a brief description of the procedures that were applied to the scans of each subject in SPM2:
1. Each subject’s baseline image was approximately registered to their follow-up image via a rigid-body transformation (three rotations and three translations).
2. Each subject’s baseline image was warped as closely as possible to their coregistered follow-up image via a high-dimensional deformation (27) . A deformation field describing this warp was generated for each subject, and the expansion or contraction at each point was calculated by taking the determinant of the gradient of the deformation (Jacobian determinant). An image consisting of the Jacobian determinants at each point (a “Jacobian map”) was generated in alignment with the follow-up image.
3. Each subject’s follow-up image was normalized to a customized whole brain template (in Montreal Neurological Institute space) by using the procedure described for the preprocessing of the baseline images. These normalized images were then segmented into gray matter, white matter, and CSF. The parameters for this normalization were then applied to each subject’s “Jacobian map” (from step 2).
4. Each subject’s normalized white matter segment (from step 3) was multiplied voxel-by-voxel with their normalized “Jacobian map” (from step 3) to form a product image. These images were then modulated with the Jacobian determinants from the spatial normalization described in step 3. These modulated images were then smoothed with a 12 mm Gaussian kernel and entered into the statistical analysis.
Statistical Analysis: Baseline Study
Statistical analyses (which can be regarded as analyses of covariance) were undertaken in SPM2 to identify the brain regions where the first-episode schizophrenia patients exhibited white matter volume reductions relative to the healthy comparison subjects. Subjects’ age at scan, gender, handedness, and global white matter volume (calculated by summing the voxel values in each subject’s preprocessed white matter image) were included as nuisance covariates in the analysis. Output was in the form of statistical parametric maps (SPMs), based on a voxel-level height threshold of p<0.05 (corrected for multiple comparisons using Gaussian random field theory) and a cluster-level extent threshold of 100 contiguous voxels. Coordinates for foci of maximal white matter change within each suprathreshold cluster were produced as Montreal Neurological Institute coordinates. To facilitate interpretation of results relative to previous studies, we transformed these Montreal Neurological Institute coordinates into Talairach (28) coordinates using the “mni2tal.m” Matlab script by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging/Common).
As we have previously documented (16) , the first-episode schizophrenia patients in this study exhibited widespread, progressive gray matter atrophy over the follow-up interval relative to the healthy comparison subjects throughout the parietal and temporal cortices. This raised the question as to whether the longitudinal white matter changes observed in the present study resulted from or were secondary to these gray matter changes. To control for this possibility, we extracted as binary masks the region of interest in Montreal Neurological Institute space where the first-episode schizophrenia patients exhibited gray matter reductions over the follow-up interval (using a height threshold of p<0.05 corrected for multiple comparisons and an extent threshold of 100 voxels). These gray matter reductions were over and above any reductions experienced by the healthy comparison subjects. Using a procedure that we have described previously (25) , we then calculated the volume of this region of interest for each subject, by convolving the mask with subjects’ preprocessed baseline and follow-up gray matter images. Any longitudinal change in the gray matter volume of this region of interest was thereby statistically controlled for in the white matter analysis by being included as a nuisance covariate.
Statistical Analysis: Longitudinal Study
The preprocessed images were entered into the design matrix in four conditions: comparison product images, comparison white matter segments, schizophrenia product images, and schizophrenia white matter segments. Fifty-one subject-specific dummy covariates (i.e., 25 schizophrenia and 26 comparison subjects) modeled the variance attributable to repeated measures within subject. The interaction between subjects’ diagnosis (i.e., comparison or schizophrenia) and image type (i.e., product or white matter segment) were examined with a (–1 1 1 –1) contrast of parameter estimates for each voxel using two-tailed t statistics. Subjects’ age, gender, handedness, and follow-up interval were entered as nuisance covariates in the analysis. Output was again in the form of SPMs, with a height threshold of p<0.05 corrected and an extent threshold of 100 contiguous voxels.
Results
Baseline Study
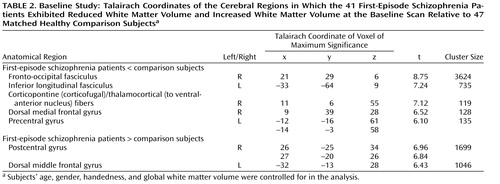
The 41 first-episode schizophrenia patients showed reduced white matter volume in a number of regions at baseline relative to healthy comparison subjects after controlling for age, gender, handedness, and global white matter volume ( Table 2 , Figure 1 ). These regions of reduction were mainly located in the white matter of the frontal and posterior temporal lobe. The first-episode schizophrenia patients also showed a bilateral region of increased white matter volume at the frontoparietal junction at baseline relative to the comparison subjects ( Figure 1 , Table 2 ).
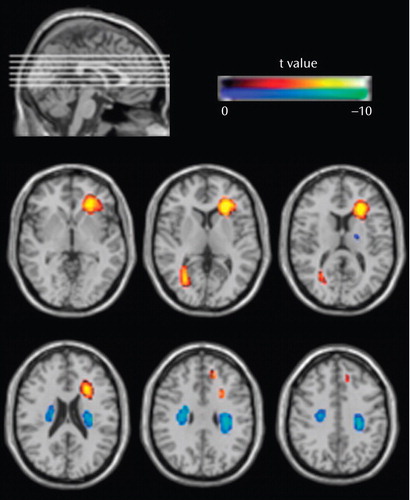
a To aid visualization, the t values for the regions of difference have been overlaid onto the Montreal Neurological Institute T1-weighted single-subject scan. In the analysis, we controlled for participants’ age, gender, handedness, and whole brain white matter volume.
Longitudinal Study
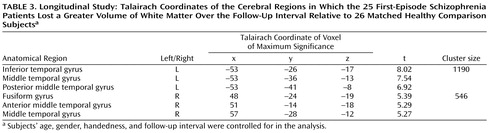
The 25 first-episode schizophrenia subjects showed volumetric white matter reductions in the temporal lobe bilaterally over the first 2 to 3 years of their illness over and above the longitudinal changes experienced by the matched healthy comparison subjects ( Table 3 , Figure 2 ). These regions of longitudinal white matter loss were confined to the middle and inferior temporal cortices. There were no regions in which the comparison subjects were observed to lose more white matter over the follow-up interval than the first-episode schizophrenia patients. There was no change to the pattern or extent of the regions of longitudinal white matter atrophy exhibited by the first-episode schizophrenia patients when subjects’ longitudinal change in gray matter volume was controlled for in the analysis.
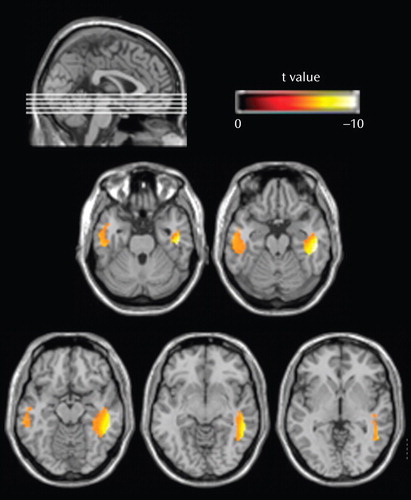
a To aid visualization, the t values of the regions of longitudinal reduction were overlaid onto the Montreal Neurological Institute T1-weighted single-subject scan. In the analysis, we controlled for participants’ age, gender, handedness, and follow-up interval.
Discussion
In spite of the fact that schizophrenia has long been thought of as a disorder of brain connectivity, few studies have investigated for evidence of white matter abnormalities in patients with the disease, and almost none have examined whether white matter pathology is static or progressive over time. In this study, we used voxel-based morphometry to identify volumetric white matter reductions in the frontal and temporal lobes and white matter increases at the frontoparietal junction bilaterally in 41 first-episode schizophrenia patients relative to 47 matched healthy comparison subjects (baseline study). Furthermore, we also used tensor-based morphometry to identify abnormal progressive volumetric white matter reductions in the temporal cortex bilaterally over the first 2 to 3 years of illness in 25 first-episode schizophrenia patients relative to 26 matched comparison subjects (follow-up study). These results suggest that white matter abnormalities are present in patients with schizophrenia at the time of their first presentation to mental health services with psychotic symptoms and that these abnormalities progress over at least the first few years of illness.
The baseline results are consistent with a number of recent studies that have inferred the existence of white matter abnormalities in patients with first-episode schizophrenia. Szeszko et al. (9) , for example, used diffusion tensor imaging and found that first-episode schizophrenia patients exhibited reduced fractional anisotropy, which has been shown to be reduced in patients with the demyelinating disease multiple sclerosis (29) , in the internal capsule and in the white matter of the middle frontal gyrus and superior temporal gyrus. Similar results were reported by Hao et al. (30) , who observed that first-episode schizophrenia patients exhibited globally reduced fractional anisotropy relative to comparison subjects, which was most significant in the white matter of the inferior frontal gyrus, cerebral peduncle, and orbitofrontal cerebrum. Furthermore, Bagary et al. (10) used magnetization transfer imaging and observed abnormal hypointensities (which is a putative measure of demyelination) in patients with first-episode schizophrenia in the white matter of the prefrontal cortex, insula, and fasciculus uncinatus.
In contrast, while a number of studies have reported volumetric white matter abnormalities in patients with chronic schizophrenia (31 , 32) , to our knowledge, this is the first study to report volumetric white matter abnormalities in patients with first-episode schizophrenia. The majority of previous volumetric studies have investigated patients’ white matter volumes at either the level of the whole brain or the level of the brain lobe, and they have failed to observe any significant volumetric abnormalities in patients with first-episode schizophrenia (11 – 13) . Thus, it appears as though the white matter abnormalities present in patients with first-episode schizophrenia are subtle and limited in extent and possibly only detectable via a voxel-based analysis.
In addition to the baseline white matter abnormalities, the first-episode schizophrenia patients also exhibited abnormal, progressive white matter atrophy in the inferior temporal lobe over the first 2 to 3 years of their illness. While there have been several studies in the literature that have reported longitudinal gray matter atrophy over the first few years of illness in patients with first-episode schizophrenia (33 , 34) , there has been (to the best of our knowledge) only one previous study that has reported longitudinal white matter change over this period. Ho et al. (14) used an automated masking procedure to segment patients’ MRI into the frontal, temporal, and parietal lobes. They found that patients with recent-onset schizophrenia lost a greater amount of frontal-lobe white matter over a 3-year interval relative to a group of matched healthy comparison subjects, who actually gained white matter over this period. Thus, the results of our study, when considered in combination with the findings of Ho et al. (14) , support the possibility of progressive frontotemporal white matter atrophy in patients with first-episode schizophrenia over at least the first few years of their illness.
Given the role that white matter plays in connecting disparate regions of neural tissue and in modulating the transmission velocities of action potentials, volumetric white matter abnormalities (be they volumetric increases or decreases), as we have reported in the present study, could be expected to result in a dysfunction in neural communication and a disintegration of neural activity (4) . Such disintegration in neural activity has previously been argued to underlie the cognitive disorganization that is characteristic of schizophrenia (1 , 4) . Furthermore, others have argued that certain specific symptoms of psychosis (such as delusions of control) could arise from disintegration between the neural correlates of actions and the neural correlates of perceiving their intended consequences (35) . The fact that we observed the white matter of the frontal and temporal lobes to be especially affected in patients with first-episode schizophrenia adds support to those theories that have argued for a breakdown in frontotemporal connectivity as being the underlying cause of schizophrenia (3 , 36 , 37) .
In a previous study using the same participant cohort, we observed evidence of substantial abnormal gray matter atrophy in the first-episode schizophrenia patients over the 2- to 3-year follow-up interval. This raised the question of whether the white matter abnormalities reported in the present study were secondary to these gray matter changes. We suggest that this scenario is unlikely for two reasons. First, the regions of longitudinal white matter abnormality in the first-episode schizophrenia patients remained when we controlled for the degree of subjects’ gray matter loss over the follow-up interval. Second, relative to the widespread gray matter atrophy exhibited by the first-episode schizophrenia patients over the follow-up interval (which engulfed most of the parietal cortex), there was comparatively little white matter atrophy over the same period, and it was confined to the inferior temporal lobe. Given that oligodendrocytes (which constitute the bulk of the white matter) depend on their corresponding neuronal axons for survival (38) , the fact that we observed widespread progressive gray matter loss but relatively circumscribed white matter loss suggests that the first-episode schizophrenia patients did not experience widespread neuronal death over the follow-up interval. This suggestion is consistent with previous stereological studies that have failed to observe a reduction in neuron number in the neocortex of patients with schizophrenia (39) . Instead, the results of the present study are consistent with the “reduced neuropil hypothesis” (40) , which argues that the characteristic gray matter loss exhibited by patients with schizophrenia is underpinned by a reduction in the numbers of dendrites, dendritic spines, and glial cells (all of which can occur without a corresponding reduction in white matter), rather than the death of neuronal cell bodies and their associated axons.
In summary, this study is the first (to our knowledge) to report volumetric white matter abnormalities in patients with first-episode schizophrenia. Furthermore, we also observed evidence of abnormal, progressive white matter atrophy over the first 2 to 3 years of illness in these patients. Given the role that white matter plays in neural communication, we suggest that these white matter abnormalities may underlie the dysfunctional neural connectivity that has been proposed as being a fundamental cause of schizophrenia.
1. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M: Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908–920Google Scholar
2. Friston K: The disconnection hypothesis. Schizophr Res 1998; 30:115–125Google Scholar
3. McGuire PK, Frith CD: Disordered functional connectivity in schizophrenia. Psychol Med 1996; 26:663–667Google Scholar
4. Bartzokis G: Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology 2002; 27:672–683Google Scholar
5. Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V: White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 2003; 60:443–456Google Scholar
6. Madsen AL, Karle A, Rubin P, Cortsen M, Andersen HS, Hemmingsen R: Progressive atrophy of the frontal lobes in first-episode schizophrenia: interaction with clinical course and neuroleptic treatment. Acta Psychiatr Scand 1999; 100:367–374Google Scholar
7. Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG: Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry 2003; 8:811–820Google Scholar
8. Frumin M, Golland P, Kikinis R, Hirayasu Y, Salisbury DF, Hennen J, Dickey CC, Anderson M, Jolesz FA, Grimson WE, McCarley RW, Shenton ME: Shape differences in the corpus callosum in first-episode schizophrenia and first-episode psychotic affective disorder. Am J Psychiatry 2002; 159:866–868Google Scholar
9. Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO: White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005; 162:602–605Google Scholar
10. Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA: Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry 2003; 60:779–788Google Scholar
11. Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW: Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex 2001; 11:374–381Google Scholar
12. Salokangas RK, Cannon T, Van Erp T, Ilonen T, Taiminen T, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Kaljonen A, Syvalahti E, Vilkman H, Alanen A, Hietala J: Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy controls: results of the Schizophrenia and Affective Psychoses (SAP) project. Br J Psychiatry Suppl 2002; 43:58–65Google Scholar
13. Cahn W, Hulshoff Pol HE, Bongers M, Schnack HG, Mandl RC, van Haren NE, Durston S, Koning H, van der Linden JA, Kahn RS: Brain morphology in antipsychotic-naive schizophrenia: a study of multiple brain structures. Br J Psychiatry Supp 2002; 43:s66–72Google Scholar
14. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M: Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60:585–594Google Scholar
15. Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A: Progressive cortical change during adolescence in childhood-onset schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:649–654Google Scholar
16. Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AWF, Gordon E, Williams LM: Progressive grey matter atrophy over the first 2–3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage 2006; 32:511–519Google Scholar
17. Harris A, Brennan J, Anderson J, Taylor A, Sanbrook M, Fitzgerald D, Lucas S, Redoblado-Hodge A, Gomes L, Gordon E: Clinical profiles, scope and general findings of the Western Sydney First Episode Psychosis Project. Aust NZ J Psychiatry 2005; 39:36–43Google Scholar
18. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 1994Google Scholar
19. Kay SR, Opler LA, Lindenmayer J-P: The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry 1989; 155:59–65Google Scholar
20. Hickie I, Hadzi-Pavlovic D, Scott E, Davenport T, Koschera A, Naismith S: SPHERE: A national depression project. Australas Psychiatry 1998; 6:248–250Google Scholar
21. Lovibond PF, Lovibond SH: The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995; 33:335–343Google Scholar
22. Wilkinson GS: The Wide Range Achievement Test-Administration Manual. Wilmingham, Del, Wide Range Inc, 1993Google Scholar
23. Baddeley A, Emslie H, Nimmo-Smith I: The Spot-the-Word Test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol 1993; 32:55–65Google Scholar
24. Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AWF: Diagnosis-related regional grey matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry 2005; 58:713–723Google Scholar
25. Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM: Grey matter deficits and symptom profile in first episode schizophrenia. Psych Res Neuroimag 2005; 139:229–238Google Scholar
26. Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA: Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J Neurol Neurosurg Psychiatry 2005; 76:650–655Google Scholar
27. Ashburner J, Andersson JL, Friston KJ: Image registration using a symmetric prior—in three dimensions. Hum Brain Mapp 2000; 9:212–225Google Scholar
28. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
29. Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G: Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 2001; 56:304–311Google Scholar
30. Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z: White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport 2006; 17:23–26Google Scholar
31. McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM: Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 2005; 186:369–377Google Scholar
32. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049–1055Google Scholar
33. Cahn W, Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS: Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry 2002; 59:1002–1010Google Scholar
34. Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R: Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001; 49:487–499Google Scholar
35. Frith CD, Blakemore S, Wolpert DM: Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Rev 2000; 31:357–363Google Scholar
36. Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR: Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biol Psychiatry 2003; 54:1181–1192Google Scholar
37. Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC: Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 2002; 51:1008–1011Google Scholar
38. Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC: Does oligodendrocyte survival depend on axons? Curr Biol 1993; 3:489–497Google Scholar
39. Pakkenberg B: Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry 1993; 34:768–772Google Scholar
40. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Google Scholar