Fragile X Syndrome: Molecular Mechanisms of Cognitive Dysfunction
Fragile X syndrome , the most common inherited form of mental retardation, is caused by a trinucleotide repeat expansion on the X chromosome; the affected gene prevents the expression of FMRP, a protein important to synaptic development and plasticity. Symptoms of fragile X are broad and include moderate to severe mental retardation, attention deficit, and developmental delay along with autistic symptoms. Although the overall brain is normal, the dendritic spines of neurons in many brain regions, including the cerebellum, are long and appear immature. Research into fragile X pathophysiology developed rapidly after the discovery of its gene and the generation of a genetic mouse mutant (the Fmr1 knockout mouse). The machinery for protein synthesis is present in the dendrites of cortical neurons near synapses; activation of Group 1 mGluR1 or mGluR5 receptors, mediated through local protein synthesis, stimulates the loss of surface-expressed synaptic AMPA and NMDA receptors. This mGluR activation in the hippocampus and cerebellum mediates LTD (long-term synaptic depression), a mechanism for synaptic pruning; this action is modulated (inhibited) by FMRP. FMRP is a repressor of translation and functions to inhibit the mGluR effects and to put a brake on LTD. Since proper cognitive function depends on the generation of synaptic plasticity within a finite dynamic range, mutations that cause this range to be exceeded in either direction (i.e., too much or too little) can lead to an impairment of learning and memory. Brain regions involved in synaptic plasticity and cognition are modulated by LTD and FMRP, including the hippocampus and cerebellum. Several synaptic proteins in dendrites mediate the translation of mRNA to protein directly in the dendritic shaft, stimulated by activation of the mGluR receptor and balanced by FMRP action. Here (and elsewhere in the fragile X brain) exaggerated LTD could slow net synaptic maturation during the critical period of synaptogenesis, contributing to developmental delay and cognitive impairment. Fragile X pathophysiology is one model for how a single protein loss can result in abnormal synaptic function and plasticity, associated with cognitive abnormalities in many brain areas, including the cerebellum.
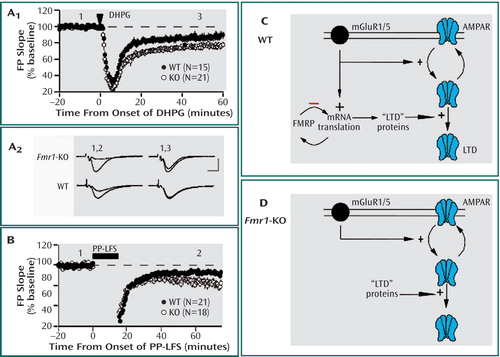
Figure 1. The figure depicts the proposed mechanism for the role of fragile X mental retardation protein (FMRP) in mGluR-dependent long-term synaptic depression (LTD). Brief application of the mGluR agonist DHPG (100 mM for 5 minutes) induces greater LTD of synaptic responses in hippocampus of knockout ( Fmr1 -KO) mice as compared with wildtype (WT) littermate control mice (A1). Plotted are average field potential (FP) slope values over the course of the experiment. Graph A2 shows representative synaptic responses (2 min average) taken at the times indicated (scale bar: 1 mV; 5 msec). Synaptic induction of mGluR-LTD (B) using paired-pulse low-frequency stimulation (PP-LFS) is significantly enhanced in hippocampus of Fmr1 -KO mice as compared with WT control mice. A working model of FMRP function in mGluR-LTD for WT control mice (C) shows Group 1 mGluR stimulation inducing endocytosis of AMPA receptors and stimulating translation of FMRP as well as proteins, which increase the persistence of LTD or AMPA receptor internalization, called “LTD” proteins. FMRP then feedbacks (-) to inhibit or terminate synthesis of “LTD” proteins. In Fmr1 -KO mice (D), the absence of translational suppression leads to enhanced steady state levels of “LTD proteins,” which in turn leads to elevated levels of LTD as well as LTD that no longer requires de novo protein synthesis. (Figure adapted from “Altered Synaptic Plasticity in a Mouse Model of Fragile X Mental Retardation” by K. Huber et al [Proc Natl Acad Sci U S A 2002; 99:7746–7750]).



