Prenatal Exposure to Maternal Genital and Reproductive Infections and Adult Schizophrenia
Abstract
Objective: In this birth cohort study, the authors examined the relation between prenatal exposure to maternal genital/reproductive (G/R) infections and schizophrenia in offspring. Method: The birth cohort consisted of 7,794 offspring of pregnancies with prospectively acquired data on maternal G/R infections from obstetric records. The authors diagnosed 71 cases of schizophrenia and other schizophrenia spectrum disorders in this cohort. The relationship between maternal G/R infections and schizophrenia risk was modeled. Results: Exposure to G/R infections during the periconceptional period was associated with a significantly increased risk of schizophrenia and other schizophrenia spectrum disorders, with adjustment for maternal race, education, age, and mental illness. Conclusions: Maternal G/R infection during periconception appears to increase the risk of schizophrenia in offspring.
Previous studies have shown associations between schizophrenia and prenatal exposure to several infections (1 – 3) . Maternal genital/reproductive (G/R) infections are known to increase the risk of congenital neurological disorders in offspring (4 , 5) , and elevated herpes simplex virus type 2 antibody has been associated with psychoses, including schizophrenia (6) . In this study, the authors examined the relationship between exposure to maternal G/R infections during pregnancy and the risk of schizophrenia in adult offspring. For this purpose, the authors used prospectively collected data on physician-diagnosed maternal G/R infections from the Prenatal Determinants of Schizophrenia study (7) .
Method
The methods of the Prenatal Determinants of Schizophrenia study are described in full in a previous publication (7) . Briefly, the analytical sample (N=7,794) was derived from the Child Health and Development Study, a large birth cohort investigation that enrolled pregnant mothers who received prenatal care from the Kaiser Permanente Medical Care Plan in Alameda County, Calif., between 1959 and 1966. All subjects provided written informed consent for human investigation. The study protocol was approved by the institutional review boards of the New York State Psychiatric Institute and the Kaiser Foundation Research Institute.
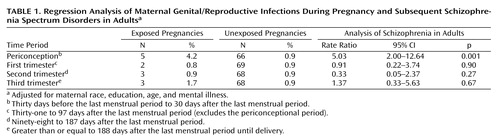
The data set on maternal G/R conditions was derived from physician diagnoses made during obstetric and medical visits to the Kaiser Permanente Medical Care Plan. These conditions were systematically abstracted from obstetric and medical records and entered into a computer by professionally trained staff. The gestational timing of each infection over the period from 6 months before pregnancy until delivery was coded. The authors included all maternal G/R infections present in the database; these consisted of the following: endometritis, cervicitis, pelvic inflammatory disease, vaginitis, syphilis, condylomata, “venereal disease,” and gonorrhea. Given the lack of a previous precedent for the period of pregnancy that would confer the greatest vulnerability to schizophrenia, the authors separately examined whether exposure during four pregnancy intervals would be associated with this disorder. These included the following:
Periconception (30 days before the last menstrual period to 30 days after the last menstrual period)
The first trimester, excluding periconception (31–97 days after the last menstrual period)
The second trimester (98–187 days after the last menstrual period)
The third trimester (>188 days after the last menstrual period until delivery)
For each time period, a subject was considered exposed if the mother was diagnosed with any G/R infection at least once. If exposure occurred more than once during pregnancy, then the gestational timing of infection was defined based on the first infection.
Potential cases of schizophrenia and other schizophrenia spectrum disorders were ascertained by linking Child Health and Development Study and Kaiser Permanente Medical Care Plan registries for inpatient or outpatient psychiatric treatment for ICD-9 295–299 diagnoses or treatment with antipsychotic medications. Of 183 potential cases of schizophrenia spectrum disorder, 13 were deceased. The 170 living potential cases were targeted for face-to-face diagnostic interviews. The Diagnostic Interview for Genetic Studies (8) was administered to 107 of the targeted subjects. Consensus DSM-IV diagnoses were reached after review of the interview by three research psychiatrists. For the noninterviewed potential cases (N=76), DSM-IV diagnoses were obtained after chart reviews by trained mental health professionals. The authors defined schizophrenia spectrum disorders as DSM-IV schizophrenia, schizoaffective disorder, schizotypal personality disorder, delusional disorder, and other schizophrenia spectrum psychosis. The diagnostic procedures yielded 71 diagnosed cases of schizophrenia spectrum disorders, 44 assessed with the Diagnostic Interview for Genetic Studies and 27 by chart review only; 85% had either schizophrenia or schizoaffective disorder (7) . The remaining subjects in the analytical cohort (N=7,723) were considered to be noncases.
To adjust for loss to follow-up, data were analyzed with Cox proportional hazards regression (7) . The authors identified potential confounders based on variables previously associated with maternal G/R infection and with schizophrenia spectrum disorders; they included maternal age, race, education, and mental illness.
Results
The demographic characteristics of the sample were reported previously (7) . Offspring exposed to maternal G/R infection during the periconceptional period were five times more likely to develop schizophrenia spectrum disorders than those who were not, after adjustment for maternal age, race, education, and mental illness. Associations between G/R infection and schizophrenia spectrum disorders were not found for exposure during the remainder of the first trimester and the second and third trimesters ( Table 1 ). Effects of periconceptional G/R infection on the risk of schizophrenia spectrum disorders were found for both interviewed (rate ratio=3.30, 95% confidence interval [CI]=0.74–12.50, p=0.10) and chart-reviewed (rate ratio=7.96, 95% CI=2.33–27.30, p=0.001) cases.
Discussion
In a birth cohort investigation, the authors demonstrated an association between periconceptional exposure to G/R infections and the risk of schizophrenia spectrum disorders in adult offspring. The strengths of the study include prospective diagnosis and documentation of these infections by physicians and diagnosis of schizophrenia spectrum disorders by face-to-face assessments. Our finding is biologically plausible, given the physical proximity of the infections to the fetus and the fact that in utero exposure to G/R infections is known to increase the risk of neurological abnormalities in offspring (4) . The findings are also consistent with previous studies of schizophrenia. Early pregnancy exposure to influenza infection (1) and periconceptional nutritional deficiency (9) have each been associated with schizophrenia in offspring. Furthermore, a previous study demonstrated that elevated maternal immunoglobulin G antibody to herpes simplex virus type 2 was associated with an increased risk of psychotic disorders, including schizophrenia (6) . One possible mechanism may involve ascension of the G/R microbes from the perineum, vagina, or cervix and subsequent infection of the fetus. Alternatively, the pathogenic organism may be transmitted by traversing the placental tissues, gaining access to the fetal bloodstream, and crossing into the developing brain. The limitations of the study included a modest sample size, a definition of infection that included a variety of G/R microbes, and a lack of serological confirmation of infection.
We provide preliminary evidence that exposure to G/R infections during the periconceptional period is associated with an increased risk of schizophrenia and other schizophrenia spectrum disorders. This work may have several implications for public health. First, because many of these infections are treatable with antibiotics, early surveillance of these infections before pregnancy and institution of medications can prevent them during pregnancy. A second method for preventing at least some of these infections is through consistent use of condoms. Thus, if the association can be replicated, it is conceivable that a reduction in the incidence of schizophrenia may be brought about by readily available pharmacotherapeutic and other public health measures. The potential public health implications of this work should also be considered in light of other prenatal infectious exposures, including influenza and rubella (1 , 2) , that our group has linked to the risk of schizophrenia. Unlike G/R infections, these viruses can be prevented by immunization of populations or at-risk subpopulations. Thus, future prevention efforts will need to tailor the appropriate strategy to the targeted infection.
1. Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES: Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 2004; 61:774–780Google Scholar
2. Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, Gorman JM, Susser ES: AE Bennett Research Award: prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry 2001; 49:473–486Google Scholar
3. Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES: Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 2005; 162:767–773Google Scholar
4. Corey L, Whitley RJ, Stone EF, Mohan K: Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet 1988; 1:1–4Google Scholar
5. Ingall D, Sanchez PJ: Syphilis, in Infectious Diseases of the Fetus & Newborn. Edited by Remington JS, Klein JO. Philadelphia, WB Saunders, 1995, p 652Google Scholar
6. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH: Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001; 58:1032–1037Google Scholar
7. Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ: The design of the Prenatal Determinants of Schizophrenia Study. Schizophr Bull 2000; 26:257–273Google Scholar
8. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T: Diagnostic Interview for Genetic Studies: rationale, unique features, and training: NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51:849–859Google Scholar
9. Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM: Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry 1996; 53:25–31Google Scholar