In Vivo 1 H-Magnetic Resonance Spectroscopy Study of Amygdala-Hippocampal and Parietal Regions in Autism
Abstract
Objective: The neural basis for autistic spectrum disorders is unclear, but abnormalities in the development of limbic areas and of glutamate have been suggested. Proton magnetic resonance spectroscopy ( 1 H-MRS) can be used to measure the concentration of brain metabolites. However, the concentration of glutamate/glutamine in brain regions implicated in autistic spectrum disorders has not yet been examined in vivo. Method: The authors used 1 H-MRS to investigate the neuronal integrity of the amygdala-hippocampal complex and a parietal control region in adults with autistic spectrum disorders and healthy subjects. Results: People with autistic spectrum disorders had a significantly higher concentration of glutamate/glutamine and creatine/phosphocreatine in the amygdala-hippocampal region but not in the parietal region. Conclusions: Abnormalities in glutamate/glutamine may partially underpin the pathophysiology of autistic spectrum disorders, and the authors confirm earlier reports that limbic areas are metabolically aberrant in these disorders.
Autistic spectrum disorders, comprising autism, Asperger’s syndrome, and pervasive developmental disorder (not otherwise specified), are highly genetic, neurodevelopmental conditions that are characterized by impaired communication, deficits in social reciprocity, and obsessional/repetitive behavior. These symptoms are associated with considerable burden—the prevalence of autistic spectrum disorders is approximately 60 per 10,000 children (1) , and the annual cost exceeds £1 billion in the United Kingdom alone (2) . However, the neural basis of autistic spectrum disorders is poorly understood, and although people with these disorders have functional abnormalities in amygdala-hippocampal (limbic) regions (3) , the causes of these differences are unknown.
Glutamate is the major excitatory neurotransmitter and is of crucial importance to brain development, leading some to propose that autistic spectrum disorders are caused by abnormalities in glutamate transmission (4) . Preliminary genetic studies have reported an association between autistic spectrum disorders and alleles encoding for kainite and metabotropic glutamate receptors (5 – 7) , the mitochondrial aspartate/glutamate carrier (8) , and astrocytic glutamate transporter proteins (9) . However, we know of only one previous preliminary report (an abstract reporting a study of eight autistic children) that has measured the concentration of glutamate/glutamine in vivo in brain regions implicated in autistic spectrum disorders (10) , and there are few in vivo studies of neuronal integrity (defined as neuronal density/mitochondrial metabolism, membrane turnover, and cellular energy metabolism).
Proton magnetic resonance spectroscopy ( 1 H-MRS) can be used to quantify a range of brain metabolites, including glutamine/glutamate (Glu+Gln); N -acetylaspartate (NAA), a marker of neuronal density and/or mitochondrial function; choline-containing compounds (Cho), a measure of membrane synthesis/turnover; creatine and phosphocreatine (Cr+PCr), a measure of cellular energy metabolism; and myo -inositol, a major osmolite and precursor to several brain metabolites. Previous 1 H-MRS studies of autistic spectrum disorders have mostly assessed children and were confounded by the inclusion of learning disabled subjects with physical illnesses. In the only previous study of normal intelligence adults with autistic spectrum disorders, Murphy et al. reported a significant increase in frontal (but not parietal) NAA, Cho, and Cr+PCr in adults with Asperger’s syndrome (11) . However, to our knowledge there are no prior in vivo studies of the neuronal integrity of amygdala-hippocampal regions or of Glu+Gln in normal-intelligence adults with autistic spectrum disorders.
Method
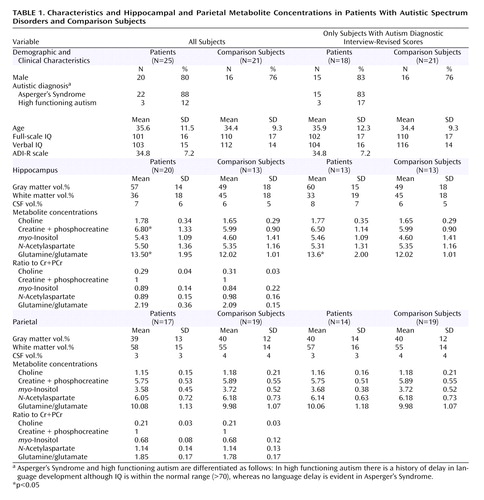
We used 1 H-MRS to investigate the neuronal integrity of the amygdala-hippocampal complex and a parietal region in 25 normal-intelligence adults with autistic spectrum disorders (full-scale IQ>70, measured using Wechsler Adult Intelligence Scale-III) and 21 healthy comparison subjects who did not differ significantly in age or sex. We obtained measures of the amygdala-hippocampal region in 20 subjects, the parietal region in 17 subjects, and both regions in 12 subjects with autistic spectrum disorders. All volunteers were diagnosed using ICD-10 criteria and the Autism Diagnostic Interview-Revised where possible. Seven (28%) subjects with an autistic spectrum disorder did not have an Autism Diagnostic Interview-Revised score. Exclusion criteria were a history of physical or psychiatric disorder affecting brain function (e.g., epilepsy or psychosis), a genetic disorder putatively associated with autistic spectrum disorders (e.g., Fragile X syndrome), or use of psychotropic medication. The study had ethical approval, and written informed consent was obtained from all subjects.
Volunteers were scanned using a 1.5-T GE Signa neurovascular magnetic resonance system. Initially, a coronal three-dimensional spoiled-gradient-recalled acquisition in a steady-state pulse sequence was acquired with whole-brain coverage. A voxel of interest was positioned in the right amygdala-hippocampal complex (6 ml) and in the right parietal lobe (8 ml) using a standard protocol, and a PRESS spectrum (time to repetition/time to echo=3000/35 msec, 160 data averages) was obtained after chemical shift selective water suppression.
MRS metabolite concentrations may be confounded by differences in the proportion of gray and white matter and by CSF contained in the voxel of interest. Thus, the three-dimensional spoiled-gradient-recalled volume was segmented into white matter, gray matter, and CSF using statistical parametric mapping software and the tissue composition of the voxels determined. Metabolite concentrations were corrected for the proportion of cerebral spinal fluid within the voxel. We measured concentrations of Glu+Gln, NAA, myo -inositol, Cho, and Cr+PCr using LC-model and in-house software. Some spectra did not yield significant fits and were discarded. Between-group differences in demographic factors, tissue volumes, and mean metabolite concentrations were assessed using a Student’s t test (two-tailed), while equality of variance was assessed using an F value. Gender differences were assessed using chi-square tests.
Results
The voxel of interest proportions of gray and white matter did not differ between groups in either brain region. However, people with an autistic spectrum disorder had significantly higher concentrations of Cr+PCr and Glu+Gln than comparison subjects in the amygdala-hippocampal complex ( Table 1 ). This corresponds to an effect size of 0.71 for Cr+PCr and 0.95 for Glu+Gln. There were no significant differences in any metabolite in the parietal region. There were no significant correlations between metabolite concentrations and IQ or Autism Diagnostic Interview-Revised subscores. The analysis was then repeated, excluding the seven subjects without an Autism Diagnostic Interview-Revised score. There was no longer a significant between-group difference in Cr+PCr (p=0.22, effect size reduced from 0.71 to 0.50); however, the significant between-group difference remained for Glu+Gln (p=0.02, effect size increased from 0.95 to 1.00).
Discussion
People with autistic spectrum disorders had a significantly higher concentration of Glu+Gln and Cr+PCr in amygdala-hippocampal complex than comparison subjects. Our findings may be confounded by type I error; however, they cannot be fully explained by differences in physical health or voxel proportion of gray/white matter. In addition, our negative findings in the parietal lobe are consistent with previous 1 H-MRS studies. One possible interpretation is that people with autistic spectrum disorders have regionally specific differences in Glu+Gln and Cr+PCr. We were unable to acquire voxels of interest in both regions in all subjects, but after restricting our analysis to those in whom we obtained both voxels of interest, the results remained significant for Glu+Gln but not for Cr+PCr.
At 1.5-T, it is not possible to examine which compound (i.e., glutamate or glutamine) is contributing most to the elevation in Glu+Gln. However, glutamate is the most abundant central neurotransmitter and is crucial for many neurodevelopmental processes, including synapse induction, cell migration, and synapse elimination. We suggest that neurodevelopmental differences in autistic spectrum disorders may be partially explained by differences in the glutamatergic system. This proposal is supported by recent postmortem reports of glutamatergic abnormalities in cerebellum (12) of people with autistic spectrum disorders and reports of an association between autistic spectrum disorders and a number of glutamate receptor genes (5 , 7 – 9) . In addition, postmortem studies of autistic spectrum disorders have reported abnormalities in neuronal migration in the amygdala-hippocampal region (13) , and functional brain imaging studies found differences in limbic anatomy and function (3) .
By demonstrating increased Cr+PCr in amygdala-hippocampal regions, this study augments prior work showing that normal-intelligence adults with Asperger’s syndrome have increased Cr+PCr in frontal regions (11) . It is not known whether the changes that we report are caused by the same underlying abnormality, nor is it clear if our findings represent a primary (causal) abnormality or are secondary to the disorder. In summary, differences in amygdala-hippocampal Cr+PCr and Glu+Gln occur in normal-intelligence adults with autistic spectrum disorders, and this may be linked to some of the pathological features of these disorders. Further developmental studies in children are required.
1. Chakrabarti S, Fombonne E: Pervasive developmental disorders in preschool children. JAMA 2001; 285:3093–3099Google Scholar
2. Jarbrink K, Knapp M: The economic impact of autism in Britain. Autism 2001; 5:7–22Google Scholar
3. Critchley H, Daly E, Bullmore E, Williams S, Amelsvoort TV, Robertson D, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy D: The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 2000; 123:2203–2212Google Scholar
4. Polleux F, Lauder J: Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev 2004; 10:303–317Google Scholar
5. Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T, Paris Autism Research International Sibpair S: Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry 2002; 7:302–310Google Scholar
6. Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, Ling YS, Ruan Y, Yang XL, Zhang D: Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios. Am J Med Genet B Neuropsychiatr Genet 2004; 131B:48–50Google Scholar
7. Serajee FJ, Zhong H, Nabi R, Huq AH: The metabotropic glutamate receptor 8 gene at 7q31: partial duplication and possible association with autism. J Med Genet 2003; 40:e42Google Scholar
8. Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD: Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry 2004; 161:662–669Google Scholar
9. Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J: Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 2001; 57:1618–1628Google Scholar
10. O’Neill J, Levitt J, McCracken J, Toga A, Alger J: Anterior cingulate and amygdalar 1H MRS abnormalities in childhood autism, in Abstracts of the 11th Scientific Meeting. Toronto, ISMRM, 2003Google Scholar
11. Murphy D, Critchley H, Schmitz N, McAlonan G, Amelsvoort TV, Robertson D, Daly E, Rowe A, Russell A, Simmons A, Murphy K, Howlin P: Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry 2002; 59:885–891Google Scholar
12. Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR: Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 2002; 52:805–810Google Scholar
13. Kemper T, Bauman M: The contribution of neuropathologic studies to the understanding of autism. Neurol Clin 1993; 11:175–187Google Scholar