Phenomenology of Mania: Evidence for Distinct Depressed, Dysphoric, and Euphoric Presentations
Abstract
OBJECTIVE: A substantial number of manic episodes include conspicuous depressive symptoms. Manic episodes have been clinically classified a posteriori using preset criteria. The aim of this study was to investigate the possibility that there might be a natural division of manic episodes into clinical types. METHOD: One hundred and five inpatients met Research Diagnostic Criteria and DSM-III-R criteria for manic episodes and were rated before institution of pharmacological treatment. The authors conducted a factor analysis of 37 behavior rating items from the Schedule for Affective Disorders and Schizophrenia. The resulting factors were used as independent variables in a cluster analysis of the patients. RESULTS: This analysis revealed four factors corresponding to manic activation, depressed state, sleep disturbance, and irritability/paranoia. Cluster analysis separated the patients into two groups. One included patients with major depressive disorder and mania. Blind, a priori clinical classification into classic and mixed mania (mania plus depression) showed that all of the patients in the depressed cluster, and about 40% of those in the nondepressed cluster, were in a mixed state according to clinical criteria. Comparison of the clinically mixed and nonmixed patients in the nondepressed cluster revealed that the mixed patients in that cluster had higher scores for items related to anger, worry, dysphoria, and irritability. CONCLUSIONS: These data suggest that manic episodes can be naturalistically classified as classic (predominately euphoric), dysphoric, or depressed. (Am J Psychiatry 1999; 156:426–430)
Manic and depressed states are not mutually exclusive. Their combination in mixed states has been repeatedly described since Kraepelin (1). The mixed state, also called depressed or dysphoric mania, is common, potentially severe, can be difficult to treat, and has the potential to reveal much about the psychopathology and pathophysiology of manic depressive illness (2). Yet, the lack of clear definition and boundaries for mixed states hinders definitive research and clinical work.
Most definitions of mixed states have relied on the application of predetermined criteria for depressed symptoms or depressed syndromes to groups of patients meeting diagnostic criteria for mania (2). These definitions leave two basic problems unsolved: 1) whether there is a naturalistic division of manic episodes into depressed and nondepressed types, as opposed to a random or continuous distribution of depressed symptoms across patients with manic episodes and 2) the specificity of mixed states themselves—for example, whether there are distinct depressed and dysphoric manic states.
To approach the problem of defining mixed states naturalistically, we investigated the pattern of symptoms in a group of 105 patients hospitalized for manic episodes. First, we conducted a factor analysis of the broad range of psychiatric symptoms covered by the Schedule for Affective Disorders and Schizophrenia (SADS) (3). Using the resultant factor scores, we then carried out a cluster analysis to determine whether there were relatively homogeneous groups of patients having different combinations of symptoms.
METHOD
The subjects were 105 patients hospitalized for the treatment of manic episodes at the Harris County Psychiatric Center, the primary inpatient teaching facility of the Department of Psychiatry and Behavioral Sciences at the University of Texas Health Science Center in Houston. Fifty-seven percent of the subjects were women; 56% were Caucasian, 33% were African American, and 9% were Hispanic. All patients gave written informed consent. Patients were evaluated by using the SADS before starting treatment. It was administered by trained doctoral-level personnel who underwent periodic retraining using live and taped interviews. All subjects met Research Diagnostic Criteria (RDC) for primary mania. Subjects judged clinically to have depressive mania also met RDC for a major depressive episode (4).
Thirty-seven variables from the SADS were used in a factor analysis employing the principal components method with varimax rotation (5). Standardized factor scores were calculated for each subject. Factor scores were then used as independent variables in the cluster analysis to attempt to differentiate group members without respect to prior diagnostic impression. Cluster analysis used a standard iterative algorithm for minimizing the sum of the squared differences between the cluster means (6). The groups defined by cluster analysis were compared to those based on the application of clinical criteria. Note that the clinical diagnosis of a mixed state depended only on the presence or absence of rating scale items, whereas factor analysis was based on the severity of symptoms.
Normality of distribution was assessed through the use of the Kolmogorov-Smirnov test, and for multiple group comparisons, homogeneity of variance was assessed by the Levene test. Parametric comparisons used analysis of variance (ANOVA); nonparametric comparisons used the Kruskal-Wallis test (7). Significances of individual differences were evaluated by using the Scheff� test if ANOVA was significant. Repeated measures ANOVA was used to test differences of factor scores among clusters (7, p. 525). Age and gender were used as covariates. Probability statements were two tailed, with the critical value for alpha set at 0.05.
RESULTS
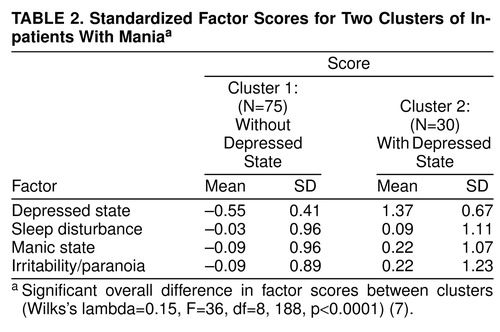
Table 1 shows loadings for the four factors. Only items with loadings greater than 0.5 are shown. There were four orthogonal (i.e., noncorrelated) factors with eigenvalues greater than one. They can be regarded descriptively as depressed state, sleep disturbance, manic state, and irritability/paranoia. Figure 1 shows the distributions of the factor scores. Scores for mania and irritability/paranoia were normally distributed, whereas those for depression and sleep disturbance were not.
The initial cluster analysis was consistent with two clusters of patients. Table 2 summarizes the factor scores for the two clusters. The clusters differed only with respect to depressed mood. It is interesting that although the sleep disturbance factor appeared to be bimodally distributed, this distribution was preserved within the clusters, which had similar sleep disturbance scores. Cluster analysis, therefore, cleanly isolated a group of patients undergoing combined depressed and manic syndromes.
On the basis of a clinical evaluation carried out independently of the statistical analysis of rating scale data, 57 subjects (24 men and 33 women) met the clinical criteria for a mixed state. Unlike the factor analysis, the clinical evaluation was based solely on the presence or absence of symptoms needed for diagnosis of a depressed episode, rather than on the severity of ratings.
We then compared the clinical diagnosis of a mixed state on the basis of the presence of a depressed syndrome according to the SADS and RDC and the initial cluster analysis. The first cluster contained 45 of the 48 patients with nonmixed mania and 30 of the patients judged clinically to be in a mixed state. The second cluster, as expected, was composed nearly exclusively of patients who were clinically judged to be in a mixed state (N=27), as opposed to a nonmixed state (N=3) (χ2=19.6, df=1, p<0.001). Classification was blind with respect to the cluster analysis.
We next compared the patients in cluster 1, divided according to whether the clinical impression was mixed mania, to those in cluster 2. Clinically mixed patients in cluster 1 differed from those who were not mixed by having lower scores for manic activation and higher scores for depressed state and irritability/paranoia, as shown in table 3. Clinically mixed cluster 1 patients differed from cluster 2 patients by having lower scores for depressed state and manic hyperactivity. There appeared to be three groups of patients: clinically nonmixed cluster 1 patients (pure or euphoric mania), clinically mixed cluster 1 patients (dysphoric mania, whose depression scores fell between those of the other two groups and whose mania scores were lower), and clinically mixed cluster 2 patients (depressive mania). The last group, as noted, met RDC for a major depressive episode. Patients having depressive manic episodes were significantly younger (mean=28.9 years, SD=6.6, N=27) than the other two groups (mean=34.9 years, SD=9.9, N=45, for euphoric patients and mean=35.3 years, SD=7.5, N=30, for dysphoric patients) (p<0.05 by Scheff� test). There were no significant differences in gender or ethnic composition.
Figure 2 shows the factor scores for the three groups of patients. MANCOVA (age and gender as covariates, factor score as a nested measure) showed that the groups differed by factor scores (F=36, df=8,188, p<0.0001, N=102); gender and age effects were not significant. Members of cluster 1 clinically diagnosed as nonmixed had low scores for depressed state and irritability/paranoia. These patients appeared to be experiencing predominantly euphoric-grandiose manic episodes. The patients in the dysphoric group were those from cluster 1 who were clinically categorized as mixed; they had depressed state scores between those with pure and depressive mania, manic hyperactivity scores that were lower, and irritability/paranoia scores that were higher than those with pure mania and comparable to those with depressive mania. They appeared to correspond to patients who had predominately irritable and hostile mania with more worry and dysphoria than the euphoric group. Patients in original cluster 2 had high scores for all factors, especially depressed state. They appeared to be experiencing episodes similar to those described by Kraepelin as depressive mania (1).
DISCUSSION
These data suggest the presence of three types of manic episodes. All patients met diagnostic criteria for mania, but the groups differed regarding severity of associated symptoms of depression. One group had minimal depressive symptoms, one group appeared to have superimposed depressive and manic syndromes (resembling patients described by Kraepelin as depressive-manic) (2), and a third group was intermediate, with depression factor scores that differed significantly from both other groups. In terms of the questions raised in the beginning of this article, 1) there appeared to be a naturalistic division between patients with depressive and other types of mania, while dysphoric mania appeared to be part of a continuum (figure 1, figure 2, and table 3), and 2) depressed and dysphoric mania were separated by the cluster analysis.
Classification of manic episodes according to predetermined criteria has provided strong evidence for subtypes of manic episodes, but the logic was potentially circular because patients were fit into previously defined classifications. Moreover, the terminology used to define manic episodes with conspicuous depression has been inconsistent. Depressed or “anxious mania,” the term coined by Kraepelin (1), describes those patients with combined depressed and manic syndromes. “Dysphoric mania” and “mixed states” have also been used to describe the same patients, but these terms lack precision. Dysphoric mania can also describe patients regarded as primarily irritable and paranoid as opposed to those who are euphoric and grandiose; the relationship to depression during mania is not always clear (8). Bauer et al. (9), using depression item scores in outpatients with bipolar disorder, found a continuum of dysphoria among manic and hypomanic patients that was not stable across episodes. The smaller number of subjects and milder illness would have made a group of patients analogous to our cluster 2 hard to detect. The data in this report suggest that mixed states can be divided into dysphoric and depressed manias.
There were limitations in the strategies of this study. First, the division into three groups of patients with manic episodes was based on a combination of factor-analytic and clinical strategies. The results show that classification using clinical criteria (on the basis of presence of symptoms) differed from that based on factor analysis of rating scale scores (where severity was taken into account). As discussed previously, however, this strategy is directly relevant to previously reported inconsistencies in classification of manic episodes, demonstrating 1) a relatively sharply demarcated Kraepelinian depressive mania and 2) a dysphoric mania based more on a continuum of symptoms. Second, the study was based entirely on symptoms exhibited during the brief period of a single episode. Consideration of relationships between subtypes of mania and the course of a single episode and of the lifetime illness are critical problems for further study.
Dividing manic episodes into subgroups might atomize the syndrome unnecessarily. Subjects meeting various criteria for mixed states differ from those with classic mania, however, regarding treatment response (2, 10, 11), suicidality (12), panic disorder (13), elevated hypothalamic-pituitary-adrenocortical axis function (14), and increased noradrenergic system function (15); where the dysphoric group would fit is unknown.
Yet, viewing mania as a syndrome with many subtypes may be too fragmentary. It may be more parsimonious to view affective states as orthogonal combinations of elemental behavioral disturbances, each with specific biological substrates and pharmacologic sensitivities, rather than as many fragmented syndromes. The underlying disturbances could be related to constructs such as reward, activity, and arousal (16–18). Kraepelin proposed a system based on mood, activity, and thought (1). Carroll developed a more biologically oriented system based on two types of reward—“psychic pain” and hyperactivity (19). Neither system was based on systematic quantitative observations of patients. A recent factor analysis of manic episodes, based on a briefer, nonstandard rating instrument, also found a prominent orthogonal depression factor that did not appear normally distributed (20). Our data, like the earlier data, are based on symptom rating scales and would therefore not be directly relevant to a model of underlying causes. The results suggest, however, that essentially orthogonal combinations of behavioral disturbances may underlie subtypes of the manic syndrome.
Received Nov. 24, 1997; revisions received May 4 and Sept. 2, 1998; accepted Sept. 24, 1998. From the Harris County Psychiatric Center and the Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center, Houston. Address reprint requests to Dr. Swann, Department of Psychiatry, University of Texas Medical School at Houston, 1300 Moursund, Rm. 270, Houston, TX 77030. The authors thank Abbott Laboratories and the administration of the Harris County Psychiatric Center for their support of this research and the nursing staffs of units 3D and 3E for patient care.
 |
 |
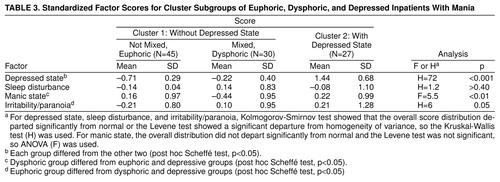 |
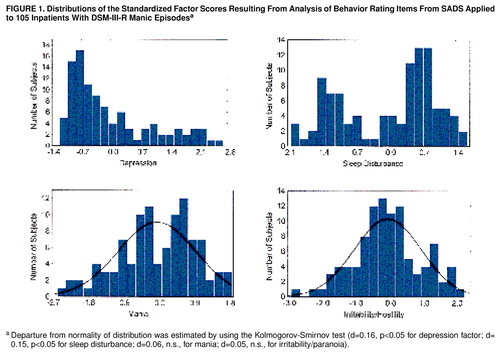
FIGURE 1. Distributions of the Standardized Factor Scores Resulting From Analysis of Behavior Rating Items From SADS Applied to 105 Inpatients With DSM-III-R Manic Episodesa
aDeparture from normality of distribution was estimated by using the Kolmogorov-Smirnov test (d=0.16, p<0.05 for depression factor; d=0.15, p<0.05 for sleep disturbance; d=0.06, n.s., for mania; d=0.05, n.s., for irritability/paranoia).
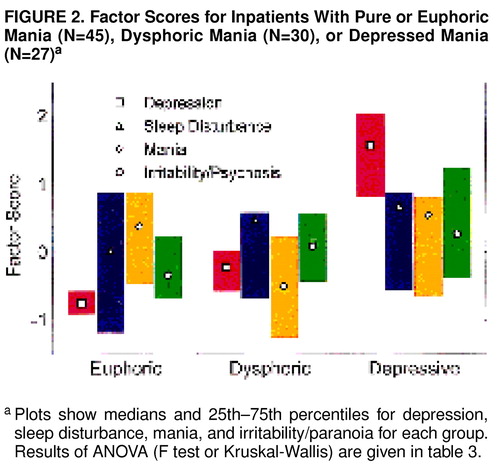
FIGURE 2. Factor Scores for Inpatients With Pure or Euphoric Mania (N=45), Dysphoric Mania (N=30), or Depressed Mania (N=27) a
aPlots show medians and 25th–75th percentiles for depression, sleep disturbance, mania, and irritability/paranoia for each group. Results of ANOVA (F test or Kruskal-Wallis) are given in table 3.
1. Kraepelin E: Manic-Depressive Insanity and Paranoia. Translated by Barclay RM, edited by Robertson GM. Edinburgh, E & S Livingstone, 1921Google Scholar
2. McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI, Faedda GL, Swann AC: Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry 1992; 149:1633–1644Link, Google Scholar
3. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
4. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
5. Tabachnick BG, Fidell LS: Using Multivariate Statistics. New York, HarperCollins, 1989Google Scholar
6. Hartigan JA: Clustering Algorithms. New York, John Wiley & Sons, 1975Google Scholar
7. Winer BJ: Statistical Principles in Experimental Design, 2nd ed. New York, McGraw-Hill, 1971Google Scholar
8. Taylor MA, Abrams R: The phenomenology of mania: a new look at some old patients. Arch Gen Psychiatry 1973; 29:520–522Crossref, Medline, Google Scholar
9. Bauer MS, Whybrow PC, Gyulai L, Gonnel J, Yeh H-S: Testing definitions of dysphoric mania and hypomania: prevalence, clinical characteristics and inter-episode stability. J Affect Disord 1994; 32:201–211Crossref, Medline, Google Scholar
10. Secunda SK, Swann A, Katz MM, Koslow SH, Croughan J, Chang S: Diagnosis and treatment of mixed mania. Am J Psychiatry 1987; 144:96–98Link, Google Scholar
11. Prien RF, Himmelhoch JM, Kupfer DJ: Treatment of mixed mania. J Affect Disord 1988; 15:9–15Crossref, Medline, Google Scholar
12. Dilsaver SC, Chen Y-W, Swann AC, Shoaib AM, Krajewski KJ: Suicidality in patients with pure and depressive mania. Am J Psychiatry 1994; 151:1312–1315Link, Google Scholar
13. Chen Y-W, Dilsaver SC: Comorbidity of panic disorder in bipolar illness: evidence from the Epidemiologic Catchment Area survey. Am J Psychiatry 1995; 152:280–282Link, Google Scholar
14. Swann AC, Stokes PE, Casper R, Secunda SK, Bowden CL, Berman N, Katz MM, Robins E: Hypothalamic-pituitary-adrenocortical function in mixed and pure mania. Acta Psychiatr Scand 1992; 85:270–274Crossref, Medline, Google Scholar
15. Swann AC, Stokes PE, Secunda S, Maas JW, Bowden CL, Berman N, Koslow SH: Depressive mania vs agitated depression: biogenic amine and hypothalamic-pituitary-adrenocortical function. Biol Psychiatry 1994; 35:803–813Crossref, Medline, Google Scholar
16. El-Mallakh RS: The Na,K-ATPase hypothesis for manic-depression, I: general considerations. Med Hypotheses 1983; 12:253–268Crossref, Medline, Google Scholar
17. Bunney WE, Goodwin FK, Murphy DL: The “switch process” in manic-depressive illness, III: theoretical implications. Arch Gen Psychiatry 1972; 27:312–317Crossref, Medline, Google Scholar
18. Baker NJ, Staunton M, Adler LE, Gerhardt GA, Drebing C, Waldo M, Nagamoto H, Freedman R: Sensory gating deficits in psychiatric inpatients: relation to catecholamine metabolites in different diagnostic groups. Biol Psychiatry 1990; 27:519–528Crossref, Medline, Google Scholar
19. Carroll BJ: Neurobiologic dimensions in depression and mania, in The Origins of Depression. Edited by Berlin AJ. Springer-Verlag, 1983, pp 163–186Google Scholar
20. Cassidy F, Forest F, Murry E, Carroll BJ: A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry 1998; 55:27–32Crossref, Medline, Google Scholar



