A Pilot Controlled Clinical Trial of ABT-418, a Cholinergic Agonist, in the Treatment of Adults With Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Despite the increasing recognition of attention deficit hyperactivity disorder (ADHD) in adults, there is a paucity of controlled pharmacological trials. Recent reports have suggested the potential usefulness of cholinergic agents for ADHD. To this end, the authors completed a controlled study of ABT-418, a novel cholinergic activating agent, for the treatment of adults with ADHD. METHOD: This was a double-blind, placebo-controlled, randomized, crossover trial that compared a transdermal patch of ABT-418 (75 mg/day) to placebo in adults who met DSM-IV criteria for ADHD. There were two 3-week treatment periods separated by 1 week of washout. RESULTS: Of the 32 subjects enrolled in the study (88% were men; mean age=40 years, SD=9), 29 completed the study. At the endpoint of each active arm (last observation carried forward), a significantly higher proportion of subjects was considered improved while receiving ABT-418 than while receiving placebo (40% versus 13%). Similarly, at endpoint there was a significantly greater reduction in ADHD symptom checklist scores (28% versus 15%). Symptoms reflective of attention, and subjects with less severe ADHD, responded more robustly to ABT-418. Treatment with ABT-418 was relatively well tolerated; dizziness and nausea were the most frequently reported adverse effects. CONCLUSIONS: The results of this investigation indicate that ABT-418, a nicotinic analog, may be a potentially useful agent for the treatment of ADHD.
Attention deficit hyperactivity disorder (ADHD) is a prevalent disorder estimated to affect at least 5% of schoolchildren (1). While its prevalence in adults is less well-known (2), longitudinal follow-up studies have documented a persistent course in a substantial number of affected youth (3–6). Characterized by high degrees of symptoms of inattentiveness (7), adults with ADHD have been documented in controlled studies to have more psychopathology, substance abuse, occupational, academic, and relationship difficulties than adults without ADHD (3, 4, 8, 9).
Although central noradrenergic and dopaminergic neurotransmission dysregulation has been hypothesized as the underlying pathophysiology of this disorder (10), in recent years, evidence has suggested that nicotinic dysregulation may also play a role in the pathophysiology of ADHD. Independent lines of investigation have documented that subjects with ADHD have a greater risk, and earlier age at onset, of cigarette smoking than comparison subjects without ADHD (11, 12). In addition, maternal smoking during pregnancy appears to increase the risk for ADHD in the offspring (13), and in utero exposure to nicotine in animals confers a heightened risk for an ADHD-like syndrome in the newborn (14, 15). That nicotine dysregulation could play an important role in the pathophysiology of ADHD is not surprising, considering that nicotinic activation enhances dopaminergic neurotransmission (16, 17).
Further support for a “nicotinic hypothesis” of ADHD can be derived from a recent study that evaluated the therapeutic effects of nicotine in the treatment of adults with ADHD (18). That controlled clinical trial in a small sample of adults with ADHD documented that commercially available transdermal nicotine resulted in significant improvement in ADHD symptoms and neuropsychological functioning (18). Although this finding is promising, the short 2-day duration of the study necessitates the reexamination of the therapeutic role of nicotinic agents in ADHD.
One such promising agent is ABT-418. ABT-418 is a prototype of a new class of compounds referred to as selective cholinergic channel activators. ABT-418 is a potent and selective agonist for α-4 β-2 subtype central nervous system (CNS) neuronal nicotinic receptors (19, 20). In addition to sharing some structural similarities to nicotine (21), ABT-418 is equipotent to (‐)‐nicotine in enhancing cognitive performance in animal models (20). However, because of its selectivity for CNS neuronal nicotinic receptor subtypes, ABT-418 is less potent than (-)-nicotine in eliciting EEG activation, hypothermia, seizures, and locomotor reduction (22). ABT-418 also had markedly lower effects on mean arterial pressure and heart rate than (-)-nicotine (22). For example, in animal (canine) testing, intravenous (‐)‐nicotine (500 nmol/kg) resulted in a rapid, dose-related biphasic response characterized as a short-lived depressor response followed by a 2–5-minute pressor increase of 50 mm Hg. In contrast, ABT-418 at the same dose had no pressor effects and only a modest depressor response in dogs and had no effect on either systolic or diastolic blood pressure or heart rate in primates (22). Moreover, in a study of 251 normal volunteers and elderly subjects with Alzheimer’s disease, at presumed therapeutic doses (75 mg/day or less), ABT-418 had no clinically meaningful cardiovascular abnormalities (Abbott Laboratories, unpublished data).
Because central nicotinic activation can improve temporal memory (23), attention (24), cognitive vigilance (25–27), and executive function (26) and because phase 1 and 2 studies of this compound in humans indicated its low abuse liability, safety, and tolerability in elderly adults (22) (Abbott Laboratories, unpublished data), we hypothesized that ABT-418 would be effective and well tolerated in the treatment of ADHD. We now report the results of a placebo-controlled, double-blind study of ABT-418 in adults with ADHD.
METHOD
Subjects
Subjects were outpatient adults between 19 and 60 years of age who were recruited from advertisements and clinical referrals to a psychopharmacology clinic and who satisfied DSM-IV criteria for ADHD. We excluded potential subjects if they had any clinically significant chronic medical conditions, history of cardiac arrhythmias, mental retardation (IQ less than 75), organic brain disorders, clinically unstable psychiatric conditions, bipolar disorder, drug or alcohol abuse or dependence within the 6 months before the study, or current use of psychotropics. We also excluded any women of childbearing potential. This study was approved by the institutional review board, and all subjects completed a written informed consent form before inclusion in the study.
Assessment Measures
Patients underwent a standard clinical assessment comprising a psychiatric evaluation, a structured diagnostic interview, a cognitive battery, a medical history, a physical and neurological examination, ECG, and a complete laboratory assessment (CBC, liver function tests, renal function tests, electrolytes, and thyroid function tests). The structured diagnostic interview used was the Structured Clinical Interview for DSM-III-R and DSM-IV, supplemented for childhood disorders by unmodified modules from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (28). To obtain a full diagnosis of adult ADHD, the subject must have 1) met full DSM-IV criteria for a diagnosis of ADHD by the age of 7 as well as currently (within the past month), 2) described a chronic course of ADHD symptoms from childhood to adulthood, and 3) endorsed a moderate or severe level of impairment attributed to the ADHD symptoms. Diagnostic reliability between raters and board-certified psychiatrists was excellent. A kappa of 1.0 was obtained for ADHD, with a 95% confidence interval of 0.8–1.0.
To assess intellectual functioning, we administered subtests of the WAIS-R and the Wide-Range Achievement Test III. Socioeconomic status was measured by the Hollingshead Four-Factor Index of Social Status (29); low values indicated high socioeconomic status.
To assess change during treatment, we examined ADHD, depression, and anxiety symptoms. Similar to previous reports (30, 31), overall severity in each of these domains was assessed with the Clinical Global Impression (CGI) scale (32). The CGI includes scales for global severity (1=not ill, 7=extremely ill) and global improvement (1=very much improved, 7=very much worse). The intraclass correlation coefficient for the CGI was 0.91. In addition, the following domain-specific rating scales were used. To assess improvement in ADHD symptoms, we used the ADHD Rating Scale (33, 34), which has been shown to be sensitive to drug effects in pediatric (33) and adult (30, 31) groups. This scale, updated for DSM-IV, assesses each of the 18 individual criterion symptoms through use of an identical severity grid (0=not present; 3=severe; overall minimum score=0; maximum score=54) that has been shown to be correlated with ADHD in adults (35, 36) and is medication sensitive (37). An intraclass correlation of 0.85 was obtained for the ADHD symptom checklist. To assess depression, we used the Hamilton Depression Rating Scale (minimum score=0, maximum=64) (38) and the Beck Depression Inventory (minimum=0, maximum=63) (39). To measure anxiety, we used the Hamilton Anxiety Rating Scale (minimum score=0, maximum=56) (40). In addition, adverse experiences were systematically recorded at each visit. While the ADHD symptom checklists and CGI were administered at baseline and at each follow-up visit of the study, the Hamilton anxiety scale, Hamilton depression scale, and the Beck Depression Inventory were administered at baseline and at the end of each treatment arm (end of weeks 3 and 7).
Procedure
This was a double-blind, placebo-controlled, randomized, crossover trial comparing 75 mg of ABT-418 to placebo. There were two 3-week treatment periods separated by 1 week of washout. The order of treatment (ABT-418–placebo or placebo–ABT-418) was randomized. Weekly supplies of ABT-418 or placebo were dispensed by the pharmacy in identically appearing 37.5-mg patches. All subjects were instructed on how to apply their patches and to rotate application sites daily. Subjects were instructed to apply two patches every morning and to remove the patches just before retiring. Compliance was monitored by diary and by patch counts at each physician visit. Study medication was begun at two 37.5-mg patches applied simultaneously and continued throughout each phase unless adverse effects emerged, in which case one 37.5-mg patch was applied. Vital sign monitoring and ECG were performed at baseline and were repeated each week.
Statistical Analysis
Improvement in ADHD was defined as a reduction of 30% or better in the ADHD rating scale score or a CGI score of 1–2 (much to very much improved) (37, 41). For analyses of CGI scores and a 30% or more reduction in ADHD symptom checklist scores, we used the intent to treat with the last observation carried forward. To compare the proportion of subjects who improved with ABT-418 versus placebo, we used the McNemar test for correlated proportions with the continuity correction. To compare ordinal data between two time points, we used the Wilcoxon signed rank test for paired data. For continuous variables, we tested for group differences through use of a generalized estimation equation that estimated main effects of drug (ABT-418 versus placebo), time (week in study), and order (ABT-418 first versus placebo first) over time, as well as any interactions. The model assumed a subject-specific residual that differed among subjects but that was constant over time (42, 43). All statistical tests were performed by using Stata (Stata Corp., College Park, Tex.). We set statistical significance at the 5% level (p≥0.05, n.s.). Data are expressed as mean and standard deviation unless otherwise specified.
RESULTS
Of 130 subjects screened, 32 subjects met inclusion and exclusion criteria and were enrolled in the study. Ninety-eight subjects were screened out: 28 had a history of bipolar disorder, 27 were women of child-bearing potential, 22 did not meet criteria for ADHD, nine had major depression, eight were receiving concurrent exclusionary medications, and four had a substance use disorder. The final group consisted of four women and 28 men who ranged in age from 25 to 60 years (mean age=40.3 years, SD=9.4). Twenty-nine subjects (91%) completed the protocol; of the three subjects who dropped out, one did so because of adverse effects, and two because of noncompliance.
Subjects were most frequently diagnosed with the combined subtype of ADHD (N=24, 75%), followed by the inattentive (N=5, 16%) and hyperactive/impulsive (N=3, 9%) subtypes. As depicted in table 1, 91% of subjects with ADHD had at least one past comorbid psychiatric disorder, and for 44% the comorbid disorder was also present within the past month. The two groups did not differ on these variables (placebo to ABT-418 versus ABT-418 to placebo: χ2=0.4, df=1, p=0.5 for past disorders, χ2=0.0, df=1, p=1.0 for current disorders). Baseline ratings of depression (mean Hamilton depression score=4.3, SD=2.3; mean Beck depression score=7.6, SD=4.6) and anxiety (mean Hamilton anxiety score=7.3, SD=4.6) were relatively low and did not differ between groups. Only two subjects had scores above standard cutoff points for depression (Hamilton depression score greater than 16, Beck depression score greater than 19), and only one subject had scores indicative of meaningful anxiety (Hamilton anxiety score greater than 21). Before entering this study, only two subjects had received medications for ADHD, and 12 others had received medications for non-ADHD psychiatric disturbance. This group of adults with ADHD had average to above average intelligence (mean IQ=108.9, SD=11.1).
Average daily doses of ABT-418 at the end of each weekly assessment period were 70 mg/day for week 1, 69 mg/day for week 2, and 67 mg/day for week 3. Of the 32 patients enrolled in the study, 31 were exposed to ABT-418; of these, 25 received the full daily dose of 75 mg (two patches), and six received 37.5 mg/day (one patch).
Outcome Assessment
On the basis of categorical definitions of improvement in ADHD with the last observation carried forward, ABT-418 was found to be clinically and statistically superior to placebo (figure 1). On the basis of a predefined criterion of a CGI improvement of 1 or 2 (much to very much improved) for ADHD (37, 41), a significantly higher proportion of subjects were considered improved while receiving ABT-418 than while receiving placebo (40% versus 13%; p=0.03) (figure 1). A similar trend was found by using a preestablished definition of improvement of a 30% or more reduction in the DSM-IV ADHD symptom checklist scores (37, 41); more subjects improved during the ABT-418 phase than during the placebo phase (47% versus 22%) (χ2=3.5, df=1, p=0.06; McNemar test).
To test for the presence of a medication carryover effect, an order effects analysis was performed on the ADHD symptom checklist by random regression. This analysis failed to reveal a significant order effect (ABT-418 first versus placebo first). Results from the random regression model indicated a significant effect of time (z=–2.7, p=0.006), no significant main effect of drug (ABT-418 or placebo), and a trend toward a drug-by-time interaction for ADHD symptoms (z=–1.9, p=0.06). Endpoint analysis (weeks 3 and 7, last observation carried forward) revealed that the ADHD symptom checklist scores were reduced by 28% with ABT-418, compared to 15% with placebo (by linear regression, t=–2.2, df=31, p=0.04).
To evaluate what ADHD characteristics might have accounted for our findings on the ADHD symptom checklist, we stratified our group both by ADHD DSM-IV subtype and ADHD severity. Response to treatment was not significantly related to ADHD subtype (inattentive versus combined). However, when we stratified the group by severity of ADHD symptoms (using the median value on the ADHD symptom checklist), we found a significant drug effect for the less severe subgroup (by random regression, z=–2.25, p=0.03).
We also evaluated the effect of ABT-418 on DSM-IV ADHD symptoms at the end of each treatment phase (last observation carried forward). Subjects treated with ABT-418 had significantly greater overall improvement than placebo-treated subjects in ADHD symptoms (McNemar χ2=8.1, df=1, p=0.004). The improvement was particularly notable in the inattentive subgroup (McNemar χ2=5.2, df=1, p=0.02), compared to the hyperactivity/impulsivity subgroup (McNemar χ2=1.3, df=1, p=0.25). These analyses also showed that compared to baseline, 15 of 18 DSM-IV ADHD symptoms improved significantly during the ABT-418 phase (all nine DSM-IV symptoms of inattention and six of nine symptoms of hyperactivity/impulsivity), whereas only five of 18 DSM-IV ADHD symptoms improved during the placebo phase.
ABT-418 resulted in neither improvement nor deterioration in symptoms of depression and anxiety. There was no effect of gender, socioeconomic status, or current or lifetime history of smoking, anxiety, or depressive disorders on response to ABT-418.
Adverse Effects
No serious adverse drug effects were observed during the trial. With the exception of one subject who dropped out during the ABT-418 phase of treatment because of nausea, ABT-418 was well tolerated. The rate of individuals with at least one adverse effect was high in both groups, but there were no statistically significant differences observed between ABT-418 and placebo arms (73% versus 61%) (McNemar χ2=2.3, df=1, p=0.13). As depicted in table 2, the most common adverse effects during ABT-418 treatment were dizziness, skin irritation, nausea, and headaches. Six subjects taking ABT-418 and one subject taking placebo had the dose lowered because of adverse effects. No clinically meaningful heart rate, blood pressure, or ECG findings were observed in either phase. There were no significant changes in laboratory studies, including liver, renal, and thyroid function tests and electrolytes, during this short-term study. Similarly, according to detailed medical and neurological examinations, there were no clinically significant changes in neurological or general medical status at the end of the study.
DISCUSSION
Results of this randomized, placebo-controlled pilot investigation of ABT-418 showed clinical efficacy of a cholinergic-nicotinic analog for treatment of ADHD in adults. A preferential improvement with this treatment was found in adults with the inattentive symptom cluster of ADHD, as well as in those adults with less severe ADHD symptoms. ABT-418 was relatively well tolerated, with predictable cholinergic adverse events. Further controlled trials of ABT-418, with higher dosing and a longer duration of treatment, are warranted.
Our results with this nicotinic analog are consistent with those obtained by Levin et al. (18) with the nicotine patch. In that brief controlled trial, nicotine patch administration to 17 adults with ADHD resulted in a modest reduction in ADHD symptoms, with concomitant reduction in reaction time on the Continuous Performance Test (18). Other work has also demonstrated an improvement in attention associated with nicotine administration in subjects without ADHD (for review, see reference 44). For example, improvement in cognition has been reported in studies of adults receiving nicotine by gum (2 and 4 mg) (27), cigarette consumption (26), and subcutaneous injection (25). Nicotine has also been shown to improve rapid visual information processing (26), reaction time (25), and vigilance (45). Consistent with cholinergic activating agents’ broad effects on attention (44), ABT-418 resulted in significantly larger improvement in the inattentive cluster of symptoms than in the hyperactive/impulsive symptoms.
Although the 47% response rate for ABT-418 (30% or greater reduction in DSM-IV ADHD symptoms) is quite consistent with our findings in a controlled investigation of pemoline (37) and tomoxetine (41) in adults with ADHD that used identical methodology, the response was lower than that observed in our prior trials of methylphenidate (30) and desipramine (31). Moreover, whereas methylphenidate had a more rapid onset of action, ABT-418 was similar to pemoline, tomoxetine, and desipramine in the time course of effect (30, 31, 37, 41). Unlike the stimulants and antidepressants that result in comparable reductions in hyperactive, impulsive, and attentional symptom clusters of ADHD, ABT-418 appears to more selectively affect the attentional symptoms. While the apparent difference in the magnitude of effect could suggest that ABT-418 may have a weaker anti-ADHD effect than some stimulants or tricyclic antidepressants, it could also be due to nonspecific cognitive-enhancing properties of this cholinergic agent (44) or insufficient dosing and duration of the trial. In support of the latter notion is the observation that there was continued improvement in both the ADHD symptom checklist and the CGI at the completion of each active phase, and the effect of higher dosing of ABT-418 on ADHD symptoms remains unknown. Although nicotine has a rapid habituation period (44, 46, 47), ABT-418 may not have a similar pharmacodynamic time course. Moreover, studies of other compounds with presumably rapid onset of action, such as pemoline (48), suggest the need for at least 3 weeks to obtain maximal response in adults with ADHD (37). Clearly, more information, based on a longer study using variable doses fixed over time, is needed to establish a dose-response and time-course profile of ABT-418 in the treatment of ADHD in adults.
Consistent with our previous controlled pharmacological studies of ADHD, response to ABT-418 was not affected by gender, socioeconomic class, or psychiatric comorbidity. Moreover, past or current smoking status did not influence outcome; however, there were only a small number of current smokers. We also found that adults with less severe ADHD symptoms manifested more robust improvement with ABT-418 than did subjects with more severe symptoms. Our finding of a preferential improvement in attentional symptoms, coupled with reports suggesting less overall severity in the inattentive subtype of ADHD (7, 49), led us to speculate that this compound may have more efficacy in the inattentive subtype of ADHD. Contrary to our expectation, our analysis found that response was independent of ADHD subtype. It may be that ABT-418, by nature of its cholinergic agonistic properties, may nonspecifically improve attention independent of ADHD status. Clearly, more work needs to be completed to elaborate the specific neuropsychological and behavioral outcomes of cholinergic agents on ADHD.
ABT-418 was relatively well tolerated, and most subjects achieved the targeted dose. Adverse effects of ABT-418 reported in the current study were similar to those described with other cholinergic agents, namely, dizziness, nausea, headaches, and dysphoria (44, 50). Our observations of the generally transient nature of adverse effects of ABT-418 are consistent with findings that repeated exposure to nicotine results in habituation to its acute effects (50). In the current study, there were no reports of withdrawal symptoms or cravings for cigarettes or other forms of nicotine or drugs of abuse, consistent with the low abuse liability of transdermal application of nicotine (51). No cardiovascular or other laboratory abnormalities occurred during the study; yet, our ability to detect infrequent and idiosyncratic reactions was limited by the relatively small number of subjects and short-term duration of the study. Given that cardiovascular changes have been associated with chronic nicotine administration (21), longer-term studies of ABT-418 are necessary.
The combined positive findings of a nicotinic agent for reduction of ADHD symptoms provide further support for the hypothesis linking cholinergic influence on catecholaminergic neurotransmission in ADHD (18, 44). Data from laboratory studies showed that nicotine stimulates dopaminergic neurotransmission (16, 17). For example, administration of nicotine resulted in a concentration-dependent release of dopamine from superfused slices of rat striatum (17). Moreover, specificity in dopamine release appears dependent on specific subtypes of cholinergic agents administered (17, 52). Whereas muscarinic antagonism has no apparent effect on dopamine release, nicotinic receptor antagonism results in diminished dopamine release (17) and a blunted effect of the indirect-acting sympathomimetic amine methylphenidate on reticular formation firing in rat brain (52).
The results of this study should be viewed in light of methodological limitations. The majority of subjects were from relatively higher socioeconomic strata; hence, the results of the current study may not generalize to subjects from lower socioeconomic strata. Other limitations include the use of a crossover design, a relatively short exposure to medication, and dosing restrictions. Further controlled dose-response trials, employing a parallel design, longer treatment duration, and higher dosing, are needed.
While our results are based on self-reports from affected individuals, it has been suggested that subjects with ADHD may not be ideal reporters of their disorder (33). Although this places some limits on the interpretation of our results, the highly significant effects on ADHD symptoms observed in this and previous studies (30, 37, 41, 53) suggest that adults with ADHD are acceptable reporters of their own condition. In addition, self-report of ADHD symptoms has been shown to be a reliable and valid method of assessing ADHD in adults (54, 55).
Despite these limitations, the results of this pilot study show that ABT-418 was well tolerated and improved ADHD symptoms in adults. This nicotinic analog appeared to have preferential efficacy for the attentional symptom cluster of the disorder. These pilot findings add to a growing clinical and basic science literature linking nicotinic-cholinergic agents to catecholamine function and improvement in ADHD. Given the continuity of pharmacological responsivity across the life cycle in subjects with ADHD (56), further study that evaluates the efficacy and tolerability of novel agents in the treatment of ADHD in adults, before use of these agents in youth with ADHD, appears to be a promising concept.
Presented at a meeting of the New Clinical Drug Evaluation Unit program, June 9–13, 1998. Received Oct. 28, 1998; revisions received Feb. 22 and April 8, 1999; accepted May 17, 1999. From the Pediatric Psychopharmacology Clinic, Massachusetts General Hospital, Harvard Medical School, Boston. Address correspondence to Dr. Wilens, Pediatric Psychopharmacology Clinic, ACC 725, Massachusetts General Hospital, Boston, MA 02114; [email protected] (e-mail). Funded by a grant from Abbott Laboratories and by NIH Scientist Development Award MH-01175 (to Dr. Wilens). The authors thank John Vetrano and Harold Demonaco and the other pharmacy staff at the Massachusetts General Hospital for their assistance with this project.
 |
 |
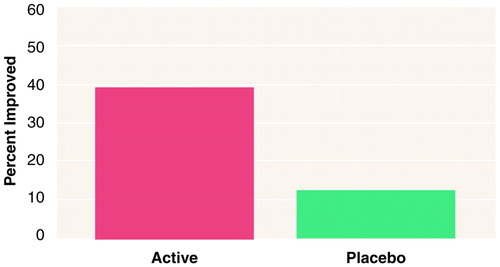
FIGURE 1. Adults With ADHD (N=32) Who Had CGI Global Improvement Scores of 1 or 2a at Treatment Endpoint While Receiving ABT-418, a Selective Cholinergic Channel Activator, or Placebo
aMuch to very much improved. A significantly higher proportion of subjects were considered improved while receiving ABT-418 than while receiving placebo (40% versus 13%) (χ2=4.9, df=1, p=0.03; McNemar test).
1. Bauermeister JJ, Canino G, Bird H: Epidemiology of disruptive behavior disorders, in Child and Adolescent Psychiatric Clinics of North America, vol 3. Edited by Greenhill L. Philadelphia, WB Saunders, 1994, pp 177–194Google Scholar
2. Murphy K, Barkley RA: Prevalence of DSM-IV symptoms of ADHD in adult licensed drivers: implications for clinical diagnosis. J Attention Disorders 1996; 1:147–161Crossref, Google Scholar
3. Weiss G, Hechtman LT: Hyperactive Children Grown Up. New York, Guilford Press, 1986Google Scholar
4. Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M: Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993; 50:565–576Crossref, Medline, Google Scholar
5. Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, Ouellette C, Moore P, Spencer T: Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry 1996; 35:343–351Crossref, Medline, Google Scholar
6. Fisher M: Persistence of ADHD into adulthood: it depends on whom you ask. ADHD Report 1997; 5(May):8–10Google Scholar
7. Millstein R, Wilens T, Biederman J, Spencer T: Presenting symptoms of ADHD in clinically referred adults with ADHD. J Attention Disorders 1997; 2:159–166Crossref, Google Scholar
8. Biederman J, Faraone SV, Spencer T, Wilens TE, Norman D, Lapey KA, Mick E, Lehman BK, Doyle A: Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993; 150:1792–1798Google Scholar
9. Shekim WO, Asarnow RF, Hess E, Zaucha K, Wheeler N: A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Compr Psychiatry 1990; 31:416–425Crossref, Medline, Google Scholar
10. Zametkin A, Liotta W: The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 1998; 59:17–23Medline, Google Scholar
11. Milberger S, Biederman J, Faraone S, Chen L, Jones J: ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997; 36:37–43Crossref, Medline, Google Scholar
12. Pomerleau O, Downey K, Stelson F, Pomerleau C: Cigarette smoking in adult patients diagnosed with ADHD. J Subst Abuse 1996; 7:373–378Crossref, Medline, Google Scholar
13. Milberger S, Biederman J, Faraone SV, Chen L, Jones J: Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry 1996; 153:1138–1142Google Scholar
14. Fung YK, Lau Y-S: Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacol Biochem Behav 1989; 33:1–6Crossref, Medline, Google Scholar
15. Johns JM, Louis TM, Becker RF, Means LW: Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurobehav Toxicol Teratol 1982; 4:365–369Medline, Google Scholar
16. Mereu G, Yoon K, Gessa G, Naes L, Westfall T: Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol 1987; 141:395–399Crossref, Medline, Google Scholar
17. Westfall T, Grant H, Perry H: Release of dopamine and 5-hydroxytryptamine from rat striatal slices following activation of nicotinic cholinergic receptors. Gen Pharmacol 1983; 14:321–325Crossref, Medline, Google Scholar
18. Levin E, Conners C, Sparrow E, Hinton S, Erhardt D, Meck W, Rose J, March J: Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996; 123:55–63Crossref, Medline, Google Scholar
19. Arneric SP, Sullivan JP, Briggs CA, Donnelly-Roberts D, Anderson DJ, Raszkiewicz JL, Hughes ML, Cadman ED, Adams P, Garvey DS, Wasicak J, Williams M: (S)-3-Methyl-5-(1-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities, I: in vitro characterization. J Pharmacol Exp Ther 1994; 270:310–318Medline, Google Scholar
20. Decker MW, Brioni JD, Sullivan JP, Buckley MJ, Raszkiewicz JL, Kang CH, Kim DJ, Giardina W, Wasicak JT, Radek R, Williams M, Arneric SP: (S)-3-Methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities, II: in vitro characterization. J Pharmacol Exp Ther 1994; 270:319–328Medline, Google Scholar
21. Benowitz NL: Pharmacology of nicotine, in Handbook of Substance Abuse: Neurobehavioral Pharmacology. Edited by Tarter RE, Ammerman RT, Ott P. New York, Plenum, 1998, pp 293–297Google Scholar
22. Arneric SP, Anderson DJ, Bannon AW, Briggs CA, Buccafusco JJ, Brioni JD, Cannon JB, Decker MW, Donnelly-Roberts D, Gopalakrishnan M, Holladay MW, Kyncl J, Marsh KC, Pauly J, Radek RJ, Rodrigues AD, Sullivan JP: Preclinical pharmacology of ABT-418: a prototypical cholinergic channel activator for the potential treatment of Alzheimer’s disease. CNS Drug Reviews 1995; 1:1–26Crossref, Google Scholar
23. Meck W, Church R: Cholinergic modulation of the content of temporal memory. Behav Neurosci 1987; 101:457–464Crossref, Medline, Google Scholar
24. Peeke S, Peeke H: Attention, memory, and cigarette smoking. Psychopharmacology (Berl) 1984; 84:205–216Crossref, Medline, Google Scholar
25. Jones G, Sahakian B, Levy R, Warburton D, Gyra J: Effects of acute subcutaneous nicotine on attention, information and short-term memory in Alzheimer’s disease. Psychopharmacology (Berl) 1992; 108:485–494Crossref, Medline, Google Scholar
26. Wesnes K, Warburton D: The effects of cigarettes of varying yield on rapid information processing performance. Psychopharmacology (Berl) 1984; 82:338–342Crossref, Medline, Google Scholar
27. Parrott AC, Winder G: Nicotine chewing gum (2 mg, 4 mg) and cigarette smoking: comparative effects upon vigilance and heart rate. Psychopharmacology (Berl) 1989; 97:257–261Crossref, Medline, Google Scholar
28. Orvaschel H: Psychiatric interviews suitable for use in research with children and adolescents. Psychopharmacol Bull 1985; 21:737–745Medline, Google Scholar
29. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
30. Spencer T, Wilens TE, Biederman J, Faraone SV, Ablon S, Lapey K: A double blind, crossover comparison of methylphenidate and placebo in adults with childhood onset attention deficit hyperactivity disorder. Arch Gen Psychiatry 1995; 52:434–443Crossref, Medline, Google Scholar
31. Wilens TE, Biederman J, Prince J, Spencer TJ, Faraone SV, Warburton R, Schleifer D, Harding M, Linehan C, Geller D: Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996; 153:1147–1153Google Scholar
32. NIMH: Clinical Global Impressions (CGI) scale. Psychopharmacol Bull 1985; 21:839–843Google Scholar
33. Barkley RA: Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford Press, 1990Google Scholar
34. DuPaul G: The ADHD Rating Scale: Normative Data, Reliability, and Validity. Worcester, University of Massachusetts Medical School, 1990Google Scholar
35. Barkley RA, Biederman J: Towards a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:1204–1210Google Scholar
36. Murphy K, Barkley RA: Preliminary normative data on DSM IV criteria for adults. ADHD Report 1995; 3(3):6–7Google Scholar
37. Wilens TE, Biederman J, Spencer TJ, Frazier J, Prince J, Bostic J, Rater M, Soriano J, Hatch M, Sienna M, Millstein RB, Abrantes A: Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999; 19:257–264Crossref, Medline, Google Scholar
38. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
39. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
40. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
41. Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L: Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998; 155:693–695Link, Google Scholar
42. Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea T, Imber SD, Sotsky SM, Watkins JT: Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Arch Gen Psychiatry 1993; 50:739–750Crossref, Medline, Google Scholar
43. Bailor JC, Mosteller F: Medical Uses of Statistics. Waltham, Mass, New England Journal of Medicine Books, 1986Google Scholar
44. Levin E: Nicotinic systems and cognitive function. Psychopharmacology (Berl) 1992; 108:417–431Crossref, Medline, Google Scholar
45. Wesnes K, Warburton DM: Smoking, nicotine, and human performance. Pharmacol Ther 1983; 21:189–208Crossref, Medline, Google Scholar
46. Marks MJ, Grady SR, Collins AC: Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther 1993; 266:1268–1276Google Scholar
47. Levin ED, Rosecrans JA: Promise of nicotinic-based therapeutic treatments. Drug Dev Res 1994; 31:1–2Crossref, Google Scholar
48. Sallee FR, Stiller RL, Perel JM: Pharmacodynamics of pemoline in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 1992; 31:244–251Crossref, Medline, Google Scholar
49. Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ: Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol 1995; 23:729–749Crossref, Medline, Google Scholar
50. Henningfield JE: Behavioral pharmacology of cigarette smoking. Advances in Behavioral Pharmacol 1984; 4:131–210Crossref, Google Scholar
51. Pickworth WB, Bunker E, Henningfield J: Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology (Berl) 1994; 115:9–14Crossref, Medline, Google Scholar
52. Shih T, Khachaturian Z, Barry H III, Hanin I: Cholinergic mediation of the inhibitory effect of methylphenidate on neuronal activity in the reticular formation. Neuropharmacology 1976; 15:55–60Crossref, Medline, Google Scholar
53. Findling RL, Schwartz MA, Flannery DJ, Manos MJ: Venlafaxine in adults with attention-deficit /hyperactivity disorder: an open clinical trial. J Clin Psychiatry 1995; 57:184–189Google Scholar
54. Ward MF, Wender PH, Reimherr FW: The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 1993; 150:885–890Link, Google Scholar
55. Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z: Psychometric characteristics of the Wender Utah Rating Scale: reliability and factor structure for men and women. Psychopharmacol Bull 1995; 31:423–431Google Scholar
56. Spencer T, Biederman J, Wilens TE, Faraone SV: Adults with attention-deficit/hyperactivity disorder: a controversial diagnosis. J Clin Psychiatry 1998; 59(suppl 7):59–68Google Scholar



