Enhancement of CO2-Induced Anxiety in Healthy Volunteers With the Serotonin Antagonist Metergoline
Abstract
OBJECTIVE: The mechanism of action of CO2-induced anxiety is unknown and has been little studied. The authors studied healthy volunteers for the possible influence of serotonin (5-HT) on CO2-induced anxiety. METHOD: Fourteen healthy volunteers received two vital capacity inhalations each of 35% CO2 and of air, preceded once by placebo and once by the 5-HT antagonist metergoline in a double-blind, randomized crossover design. RESULTS: Mean National Institute of Mental Health self-rating anxiety subscale scores increased nonsignificantly after CO2 inhalation; this effect was significantly enhanced by the administration of metergoline. CONCLUSIONS: The authors hypothesize that 5-HT may inhibit CO2-induced anxiety, a function that is lessened by metergoline.
CO2 causes anxiety in patients with panic disorder (1, 2) and, to some extent, in healthy volunteers (3, 4). The mechanism of action is unknown; stimulation of noradrenergic pathways by CO2 has been suggested in preclinical studies (reviewed in reference 4), but peripheral measures of noradrenergic activity in human subjects receiving CO2 have been equivocal (4). To our knowledge, only two studies have investigated the possible influence of serotonin (5-HT) on CO2-induced anxiety. Kent et al. (5) reported that tryptophan depletion, which putatively lowers central 5-HT activity, increased ventilation during CO2 inhalation in patients with panic disorder. Klaassen et al. (6) reported increases in some anxiety measures during CO2 inhalation after tryptophan depletion in healthy volunteers. Therefore, we performed a placebo-controlled study of the acute influences of the nonspecific 5-HT antagonist metergoline (7, 8) on the effects of inhalations of air and of CO2 in healthy volunteers.
METHOD
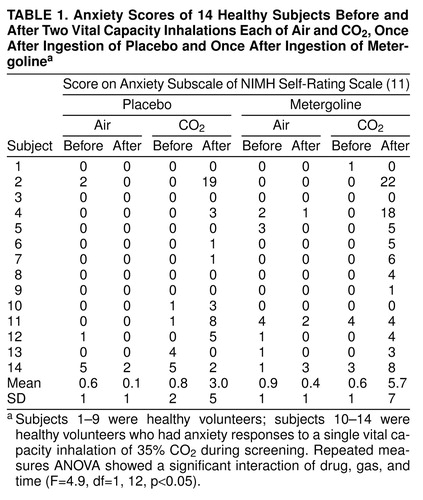
Fourteen healthy volunteers (six at the National Institute of Mental Health [NIMH], Bethesda, Md., and eight at the Soroka Medical Center, Beer-sheba, Israel) underwent psychiatric screening with the overview section of the Structured Clinical Interview for DSM-III-R (9) and a standard medical interview, examination, and laboratory tests to exclude medical illness, drug or medication use, and pregnancy. During the course of the study, an additional inclusion criterion was imposed, to be described later. After complete description of the study to the subjects, including demonstration of the equipment, written informed consent was obtained.
The study used a crossover design; each subject received a capsule containing metergoline (4 mg) once and placebo once; previous experience (7, 10) suggested that this dose was sufficient to exert an effect. Inhalations were separated by at least 1 week; the order of drug and placebo administration was randomized and double-blind.
On test days, 90 minutes were allowed after administration of the capsule for habituation to the setting and for the drug to take effect, as indicated by previous assessments of physiological and neuroendocrine measures (7, 10). Then two vital capacity inhalations of compressed room air (once) and of 35% CO2 (once) were administered, separated by 20 minutes. The order of gas inhalation was random and single-blind.
Assessments with the anxiety subscale of the NIMH self-rating scale of physical and mental symptoms (11) were performed 30 minutes before the first inhalation and immediately before and after each inhalation.
The first nine subjects who satisfied the inclusion and exclusion criteria were included without regard to their potential responses to CO2. However, after assessment of nine subjects, we observed that only two of them (22%) had anxiogenic responses to CO2 (defined as an increase of 2 or more points on their anxiety subscale scores). We considered that metergoline might enhance or attenuate CO2-induced anxiety; however, seven of these nine subjects could not have shown any attenuation of anxiety because they had little or none after CO2 inhalation. Therefore, we selected additional subjects likely to show anxiety responses to CO2. Subsequent potential subjects who had completed screening procedures identical to those employed with the first nine subjects received a single vital capacity inhalation of 35% CO2 while being shown the experimental equipment; those with anxiety responses were included for further study. Fifty potential subjects underwent this screening, and five (10%) were selected.
Analysis employed repeated measures analysis of variance (ANOVA), with the NIMH self-rating anxiety subscale score as the repeated measure; drug (placebo or metergoline), gas (air or CO2) and time relative to inhalation (before or after) as within-subjects factors; and the following between-groups factors, as appropriate: order of drug administration, site (Bethesda or Beer-sheba), selection procedure (whether screened for CO2 response during selection or not), and responder status (response to CO2 alone during the placebo inhalation or not). Eight subjects received placebo first, and six subjects received metergoline first.
RESULTS
The order of administration of the medication and placebo had no effect. The site had no effect on scores on the NIMH self-rating anxiety subscale; however, because it did affect other outcome measures (reported elsewhere), we performed this analysis with the site as a between-group factor. There were no time or drug effects during the 90-minute habituation phases. The physician (I.Z.B.-Z., G.M., or J.B.) administering the procedure guessed the order of drug and placebo administration incorrectly for five out of 11 subjects for whom this information was available.
The drug, gas, and time three-way interaction was statistically significant (Table 1). The Bonferroni t type I 5% post hoc test indicated that the only statistically significant difference within this interaction occurred after metergoline administration and between anxiety readings before and after CO2 inhalation. The difference was 5.1 anxiety units, with the elevated reading occurring after inhalation (Table 1). Anxiety readings after placebo administration and after CO2 inhalation were also higher than readings before CO2 inhalation, but with a statistically insignificant difference of only 2.2 anxiety units. All differences between before and after air inhalations, with metergoline and placebo, were less than 0.5 anxiety units and statistically insignificant.
DISCUSSION
Metergoline enhanced CO2-induced increases in NIMH self-rating anxiety subscale scores in two out of five subjects who responded to CO2 alone and also provoked anxiety responses to CO2 in six out of nine subjects who did not respond to CO2 alone. The absence of effects during the 90 minutes between ingestion of the capsules and the first inhalation suggests that the metergoline dose was small enough to maintain the blind; this is further supported by the failure of physicians to guess the correct order of drug and placebo administration in approximately half of the cases. Therefore, we hypothesize that 5-HT may act to inhibit CO2-induced anxiety and that this inhibition was reduced by metergoline. This is consistent with the finding that the lowering of 5-HT activity by tryptophan depletion increases ventilation, which typically parallels increases in anxiety in patients with panic disorder during CO2 inhalation (5) and also increases some anxiety measures in healthy volunteers during CO2 inhalation (6).
Further study with 5-HT agonists and antagonists is required to elucidate the role of 5-HT in CO2-induced anxiety.
Received March 27, 1998; revisions received Nov. 5, 1998, and Feb. 2, 1999; accepted April 12, 1999. From the Division of Psychiatry, Soroka Medical Center; Ben Gurion University of the Negev, Beer-sheba, Israel; and the Laboratory of Clinical Science, NIMH, Bethesda, Md. Address reprint requests to Dr. Benjamin, Division of Psychiatry, Soroka Medical Center, P.O. Box 151, Beer-sheba 84101, Israel; [email protected] (e-mail)
 |
1. Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF: Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry 1984; 141:857–861Link, Google Scholar
2. Griez E, Lousberg H, van den Hout M, van der Molen G: CO2 vulnerability in panic disorder. Psychiatry Res 1987; 20:87–95Crossref, Medline, Google Scholar
3. Gorman J, Papp L, Martinez J, Goetz RR, Hollander E, Liebowitz MR, Jordan F: High-dose carbon dioxide challenge test in anxiety disorder patients. Biol Psychiatry 1990; 28:743–757Crossref, Medline, Google Scholar
4. Woods S, Charney D, Goodman W, Heninger G: Carbon dioxide-induced anxiety: behavioral, physiologic, and biochemical effects of carbon dioxide in patients with panic disorder and healthy subjects. Arch Gen Psychiatry 1988; 45:43–52Crossref, Medline, Google Scholar
5. Kent J, Coplan J, Martinez J, Karmally W, Papp L, Gorman J: Ventilatory effects of tryptophan depletion in panic disorder: a preliminary report. Psychiatry Res 1996; 64:83–90Crossref, Medline, Google Scholar
6. Klaassen T, Klumperbeek J, Deutz N, van Praag H, Griez E: Effects of tryptophan depletion on anxiety and on panic provoked by carbon dioxide challenge. Psychiatry Res 1998; 77:167–174Crossref, Medline, Google Scholar
7. Benkelfat C, Murphy D, Zohar J, Hill J, Grover G, Insel T: Clomipramine in obsessive-compulsive disorder: further evidence for a serotonergic mechanism of action. Arch Gen Psychiatry 1989; 46:23–28Crossref, Medline, Google Scholar
8. Pigott T, Hill J, Grady T, L’Heureux F, Bernstein S, Rubenstein CS, Murphy DL: A comparison of the behavioral effects of oral versus intravenous mCPP administration in OCD patients and the effect of metergoline prior to IV mCPP. Biol Psychiatry 1993; 33:3–14Crossref, Medline, Google Scholar
9. Spitzer RL, Williams JBW, Gibbon M, First MB: User’s Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
10. Greenberg B, Benjamin J, Martin J, Keuler D, Huang S-J, Altemus M, Murphy DL: Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl) 1998; 140:434–444Crossref, Medline, Google Scholar
11. Murphy D, Mueller E, Hill J, Tolliver T, Jacobsen F: Comparative anxiogenic, neuroendocrine, and other physiologic effects of m-chlorophenylpiperazine given intravenously or orally to healthy volunteers. Psychopharmacology (Berl) 1989; 98:275–282Crossref, Medline, Google Scholar



