Age-Associated Testosterone Decline in Men: Clinical Issues for Psychiatry
Abstract
Objective:The author summarizes current knowledge about the diagnosis and treatment of testosterone decline in healthy aging men and the associated clinical issues for psychiatry. Method:A MEDLINE search was conducted in which the search terms “male climacteric,” “male menopause,” “andropause,” “viropause,” “low-testosterone syndrome,” and “testosterone replacement therapy” were used. Literature published before 1966 was identified by reviewing the reference lists of later publications.Results:Manifestations of testosterone deficiency have included depression, anxiety, irritability, insomnia, weakness, diminished libido, impotence, poor memory, reduced muscle and bone mass, and diminished sexual body hair. Although testosterone levels decline with age, there is great interindividual variability, and the connection between serum testosterone levels and clinical psychiatric signs and symptoms is not clear-cut, since other hormonal changes are implicated as well. Testosterone replacement therapy may offer hypogonadal men benefit, but long-term studies on its efficacy and safety are lacking. Comprehensive biopsychosocial assessment should be a routine part of the evaluation of complaints of low-testosterone syndrome in men.Conclusions:Testosterone decline/deficiency is not a state strictly analogous to female menopause and may exhibit considerable overlap with primary and other secondary psychiatric disorders. Am J Psychiatry 1998; 155: 1310-1318
Menopause—the termination of menstrual flow associated with ovarian failure, accompanied by characteristic signs and symptoms (e.g., hot flashes, skin and reproductive tract atrophy, increased body fat) as well as an increased incidence of cardiovascular disease, cognitive impairment, and osteoporosis—is a well-recognized concomitant of aging in women, with an average age at onset of 51.4 years (1). The endocrinologic, urologic, and geriatric literatures suggest that a possible male equivalent of menopause exists, denoted by such terms as the “male climacteric,” “andropause,” “viropause,” and “low-testosterone syndrome” (1-6).
The testes had been linked with sexual vigor and longevity in antiquity; the Greeks and Romans used “satyricon” preparations, made from goat and wolf testicles, as stimulants and aphrodisiacs (7). Testicular extracts from animals, a form of “organotherapy,” continued to be used into the twentieth century, having been personally endorsed by the French physiologist Brown-Sequard (7, 8) in 1889, when he claimed rejuvenation and reversal of his own impotence. Further, in the early part of this century, men received testicular implants from animals to reverse the aging process; improvements in energy and cognition were noted, although it is probable that these implants were rejected and that the improvements represented a placebo effect (7). In the late 1930s, subsequent to the isolation of various androgens (9), reports were published on treatment of the male climacteric and involutional melancholic symptoms with testosterone. Schmitz (10) noted improvement in a majority of middle-aged and elderly men with complaints of impotence and depression when they were treated with testosterone propionate, while Werner (11) noted the relief of fatigue, anxiety, and depression in two men with symptoms of the “climacteric” treated with the same form of testosterone. The problem in these and other reports (12-14) on the use of testosterone in men with climacteric symptoms or involutional melancholia was that many reports were of open-label treatment, for different periods of time, of patients who had varying mixtures of signs and symptoms. In addition, not all reports were of positive results (15, 16), and as noted by Bharke et al. (9), the American Medical Association’s Council on Pharmacy and Chemistry stated in 1939 that “the involutional melancholia of males, for which testosterone has been suggested, has not been subjected to adequate trials to justify androgenic therapy other than on an experimental basis.” Nevertheless, refinements in the measurement of various hormones (e.g., testosterone, luteinizing hormone [LH], follicle-stimulating hormone [FSH]), and preparations of replacement forms of testosterone have led to an enhanced understanding of the effects of aging on the “well” male, with numerous reports in the nonpsychiatric medical literature on the male climacteric and its treatment (2–6, 17, 18). The purported manifestations of the male climacteric include diminished sexual body hair, lowered libido, fatigue, insomnia, hot flashes, anxiety, depression, poor memory, irritability, impotence, and diminished bone and muscle mass (2–6, 17, 18).
A MEDLINE search that used the search terms “male climacteric,” “male menopause,” “andropause,” “viropause,” “low-testosterone syndrome,” and “testosterone replacement therapy” was conducted to identify English-language reports on testosterone and aging. This article reviews the literature on the age-associated decline of testosterone in healthy men, starting with an overview of the endocrinology of the normal aging process and the effects of such changes on reproductive, psychosexual, and cognitive functions and muscle and bone mass and continuing with studies on the impact of testosterone replacement on these parameters and the potential adverse short- and long-term effects of such replacement. The clinical issues raised for the field of psychiatry by the literature on the decline of testosterone levels in men are also discussed.
THE HYPOTHALAMIC-PITUITARY-GONADAL AXIS AND AGING IN MEN
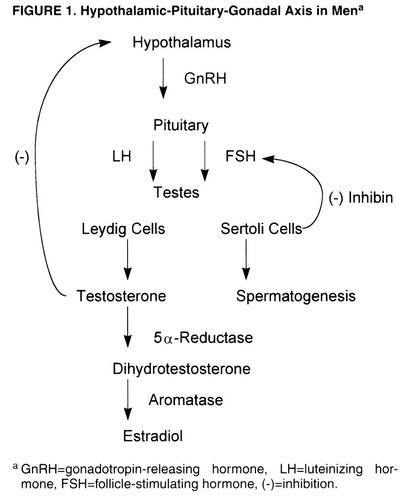
Normal male reproductive function depends on the intermittent secretion of LH and FSH by the pituitary gland under the influence of gonadotropin-releasing hormone (GnRH), which is itself secreted by the hypothalamic GnRH “pulse generator” (19) (figure 1). LH stimulates the testicular Leydig cells to secrete testosterone, in a pulsatile manner and in a diurnal rhythm of peak levels in the morning and nadir in the evening (20), with a negative feedback loop to the hypothalamus to modulate LH secretion. In normal men, approximately 2% of testosterone is in the “free” (i.e., unbound) form, while the remainder is bound to sex hormone-binding globulin (SHBG) and, to a lesser extent, albumin- and cortisol-binding globulin (17). The term “bioavailabletestosterone” refers to the testosterone that is not bound to SHBG (5), that is, the free and albumin-bound portion. Testosterone is then metabolized by 5α-reductase, in target organs, to the more potent androgen dihydrotestosterone, which itself is metabolized by aromatase to estradiol (17). FSH stimulates the testicular Sertoli cells/seminiferous tubules and promotes spermatogenesis; a nonsteroidal Sertoli cell compound, inhibin, in turn regulates FSH level by an inhibitory feedback loop to the pituitary gland and possibly the hypothalamus (20).
Measurement of serum testosterone is subject to several methodological problems. Rabkin et al. (21) discussed the variability in the ranges of normal values among laboratories, a problem recently examined by Boots et al. (22), who reported that the measurement of testosterone by commercially available kits may have limited clinical utility because of the high degree of variability among the kits. Rabkin et al. also noted the wide range of normal values (270 ng/dl to 1100 ng/dl), which indicates that a substantial percent decline in testosterone level could still fall within the normal range, and the variability they found between levels in the same patient on separate occasions even when the time of day for sampling was kept constant.
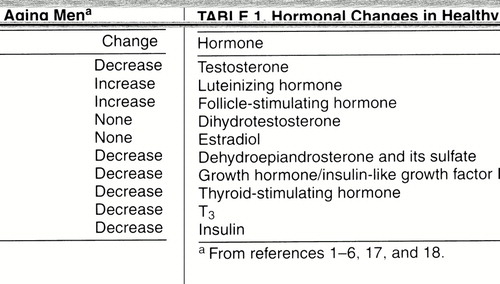
Aging has been associated with a number of changes in the levels of the above-mentioned hormones (table 1) and, consequently, in the target organs to which these hormones attach. As noted in several reports (1-6), testosterone levels in healthy, aging groups of men decline with age on the order of 100 ng/dl per decade (23), accompanied by increases in SHBG and therefore leading to a decline in free and bioavailable testosterone levels. In addition, LH levels increase (more markedly after age 70), while FSH levels increase and inhibin levels decrease, with a loss of the diurnal rhythm of testosterone secretion (2-6). These hormonal changes begin in the 40s and are well established by the age of 50 years. However, there is great interindividual variability in testosterone levels among healthy older men, and therefore, unlike women, not all older men will become hypogonadal as they age (2). As noted by Tenover (2), the definition of an older male as hypogonadal has not been established, and the percentage of men described as testosterone deficient depends on what measures are used as cutoff values. Tenover cited, as an example, a 20% rate of hypogonadism if total testosterone levels in men 55 years old with values below the range of normal for young adult men were used, while the rate would reach as high as 50% if bioavailable testosterone levels were used. Levels of dihydrotestosterone do not show a decrease with age, possibly reflecting an increased production by the prostate (4, 6), and levels of estrogen are also not changed in elderly men (6).
Testicular failure, due to a decline in Leydig cell mass, is thought not to be the only reason for the hormonal changes described above (4-6). The increase in LH levels, for example, is relatively small, except for men older than 70 years, and should be higher in middle-aged and “young old” men if a primary testosterone deficiency were the problem (4, 5). It is believed that the relatively small increase in LH levels, along with a decrease in relative responsiveness of LH and FSH to GnRH, in elderly men (4, 6) is more consistent with a failure of the hypothalamic-pituitary system and that the decline in testosterone levels, therefore, represents secondary hypogonadism (4-6). Also, since LH secretion is inhibited by endogenous opioid peptides (24), it has been hypothesized (5) that increased opioid tone in middle-aged men may keep LH levels from rising, a theory supported by the increase in LH and testosterone levels in hypogondal men treated with the opiate antagonist nalmefene (25). Finally, a potential change in androgen receptor site sensitivity with aging has been suggested by Bancroft (26), and aging is associated with a down-regulation of androgen receptors in the rat prostate (27) and hepatocytes (28), leading to androgen resistance during senescence.
OTHER HORMONAL CHANGES WITH AGING
In addition to the changes in the hypothalamic-pituitary-gonadal axis, other important changes in endocrine activity take place. As reviewed by Lamberts et al. (1), two clinically important changes occur in the pancreas and thyroid. Impaired glucose tolerance or diabetes mellitus affects approximately 40% of people between the ages of 65 and 74 years, and in nearly one-half of these it is undiagnosed (1, 29). This deterioration in glucose tolerance is attributed to several factors, including decreased beta cell secretion of insulin, peripheral insulin resistance, poor diet, increased abdominal fat mass, and physical inactivity (1, 30). Decrease in pituitary release of thyrotropin (TSH) and decreased peripheral conversion of T4 to T3 result in a slight decrease in T3 levels, which still remain within the range of normal limits. Although diet, oral hypoglycemics, insulin, and exercise are all aspects of managing impaired glucose tolerance, it is not known whether healthy aging individuals would benefit from T3 supplementation (1, 31).
Additional endocrine changes occur in the growth hormone (GH)/insulin-like growth factor I (IGF-I) system (1, 32), with a gradual decline in the pulse amplitude, duration, and fraction of GH secreted, in parallel with a drop in IGF-I levels. These declines, which have been called “somatopause,” are thought to be secondary to a decline in a hypothalamic triggering pacemaker (1). In addition, age-related declines in the adrenal hormones dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), termed “adrenopause,” appear related to a reduction in adrenal cortical zona reticularis cells (33, 34). DHEA is a precursor for the formation of estrogenic and androgenic steroids in peripheral tissues (1).
PHYSICAL AND NEUROPSYCHIATRIC CORRELATES OF AGING: RELATIONSHIP TO HORMONAL CHANGES
Reproductive Function
Although fertility may persist in elderly men, changes in spermatogenesis are reported, including a decrease in sperm motility, ejaculate volume, and total sperm production and an increase in numbers of nonviable, abnormal spermatozoa (35). Sperm production is reduced because of a decline in the number and functioning of Sertoli cells with age (35), although results of a test of sperm fertilizing capacity in the zona-free hamster egg preparation appear the same with the sperm of elderly men compared with those of younger men (36). Other age-associated histologic changes in the testes include an overall decrease in the testicular parenchyma, seminiferous tubular fibrosis, and sclerosis (see reference 35 for a more detailed review of these changes). However, notwithstanding the reported declines in testosterone, as discussed by Meacham and Murray (35), it is not possible to make definitive statements about the effect of male age on pregnancy and birth rates without properly designed studies that control for male and female fertility factors as well as timing and frequency of intercourse.
Sexual Function
Age-associated changes in male sexual response and function have been reviewed by Schiavi and Rehman (37) and others (4, 6, 35) and include 1) decline in libido; 2) decrease in the number and frequency of morning erections; 3) reduced penile sensitivity; 4) reduced sexual arousal, both mentally (visual, psychological) and physically with respect to erectile capacity (delayed, less rigid, more difficult to sustain), reduced scrotal vasocongestion, impaired testicular elevation, and poorly defined sense of impending orgasm; 5) prolonged duration of the plateau phase with decreased or absent pre-ejaculatory secretions and emission; 6) reduced duration and strength of orgasm with a shorter period of ejaculatory inevitability; 7) a prolonged refractory period; and 8) reduced swelling and erection of the nipples, absence of maculopapular rash, and extragenital muscle spasm at climax.
As noted by several authors (2, 6, 17, 37), it would be erroneous to attribute impotence (i.e., erectile dysfunction) to declining testosterone levels when there are other variables that must be considered in the etiology of complaints related to diminished libido, erectile and ejaculatory dysfunction, and diminished sexual activity. Such variables include availability of a partner, fear of performance failure, impaired penile perfusion, chronic illness, depression, medications, neuropathy, smoking, and alcohol and drug use.
In their review of the literature on male aging and sexuality, Schiavi and Rehman (37) noted that in addition to a decline in sexual interest, there is a decreased frequency of sexual activity and an increased prevalence of sexual dysfunction, although substantial individual differences exist, with the differences in the frequency and satisfaction of sexual activity in younger men being relatively stable characteristics that contribute to behavioral differences in older men.
The relationship between libido, erectile dysfunction, and testosterone level has been examined in a number of studies. Davidson et al. (38), studying hypogonadal men given testosterone replacement or placebo, found significant dose-related increases in the frequency of erections, including nocturnal erections, and intercourse among the men given replacement therapy. Bancroft and Wu (39) studied erections in response to erotic films and fantasy in hypogonadal men with and without testosterone replacement and in age-matched control subjects and found that there was no difference between the groups in erectile response to the films, although erections in response to fantasy were significantly smaller and slower to develop in the hypogonadal men and showed significant improvement during replacement treatment. These investigators did not confirm this finding in a different study (40). In a study of eugonadal men with complaints of either loss of libido or erectile failure, O’Carroll and Bancroft (41) found that testosterone significantly increased sexual interest but had no effect on erectile function in either group.
A number of studies have examined age, testosterone levels, sleep, and nocturnal penile tumescence. Spontaneous erections during sleep (i.e., nocturnal penile tumescence) are impaired in hypogonadism and improve with androgen treatment (39, 42-44). Carani et al. (45) found that testosterone administration to eugonadal men enhanced nocturnal penile tumescence in terms of rigidity but not circumference. Schiavi et al. (46) studied levels of testosterone, LH, and prolactin every 20 minutes during sleep in a group of healthy men aged 45–75 years and found that decreases in sleep efficiency, number of rapid eye movement (REM) episodes, and latency to onset of REM were associated with lower bioavailable testosterone levels, a finding that was independent of age. Further, their study did not find any changes in the pulsation of hormone release or any evidence that age-associated increases in sleep-disordered breathing contribute to the decline in androgen levels in healthy men. In an extension of their study of the same healthy men, Schiavi et al. (47) examined the relationship between nocturnal penile tumescence and hormone levels, noting that overall, age itself accounted for the apparent direct relationship between nocturnal penile tumescence and bioavailable testosterone, although there was an age-independent association between testosterone level and nocturnal penile tumescence in the men who were 55–64 years old, which the investigators believed confirmed Bancroft’s hypothesis (26) that the threshold required for the effects of testosterone increases with age.
Overall, the importance of testosterone for erections is minor (37), although in hypogonadal states testosterone administration appears to improve measures of libido and nocturnal penile tumescence. As Schiavi and Rehman (37) noted, however, age itself is the important variable when one is assessing sexual dysfunction, given the above-mentioned findings in healthy aging men.
Cognitive Function
The relationships among hormones, aging, and cognitive function have received attention as a result of studies of the effect of estrogen replacement therapy on cognition in women, a topic recently reviewed in a meta-analytic study (48). Studies of the influence of testosterone on cognitive function have focused primarily on the relationship between spatial cognition and testosterone. Spatial cognition—defined by tasks that include spatial attention, visual perception, object identification, and visual memory (49)—has been assessed in studies that examined correlations between gender and performance (50-53) and testosterone administration and performance (49, 54-56). Interpretation of the results of these studies of the influence of testosterone on cognitive function is hampered by the differences between studies in the tests administered, the populations tested (e.g., the elderly, young adults), the time of day of testing, subjects’ handedness, and the influence of other hormones (e.g., estrogen) (52, 57). Nevertheless, it appears that there exists a curvilinear relationship between testosterone level and performance on tests of spatial cognition such that low and high levels are associated with poorer performance (52). Janowsky et al. (49) found that in a group of older, hypogonadal men who received testosterone supplementation for 3 months, there was significant enhancement of spatial cognition as measured by the block design subtest of the WAIS-R, but not of other measures tested (verbal and visual memory, mood, and fine motor speed). These authors believed that the effects on spatial cognition that they found were also influenced by lower estradiol levels secondary to testosterone supplementation, and they noted that the effects on spatial cognition that they did find were not “strong,” which they interpreted as also indicating that other factors were influencing their results. In addition, there has been discussion in the literature about whether the influence of testosterone on cognitive function is the result of the activational effects of circulating testosterone or the result of a more permanent organizational effect during development (57).
Mood
The influence of aging-associated declines in testosterone on mood in men is not well studied. While reviews of the male climacteric mention depression, mood swings, anxiety, and irritability (2–5, 17), references documenting such changes in controlled studies are lacking. In studies that have examined the relation between levels of depression and testosterone in male depressive subjects (58-63), results have been inconsistent. The discrepancies in these studies can be accounted for by differences in patient groups (e.g., mean age), method of measuring testosterone (e.g., total versus free, serum versus salivary), cortisol levels (usually not measured but known to be elevated in some depressed persons, which could in turn lower testosterone levels), degree of weight loss (known to lower testosterone), and use of medication (63). In the one study (63) that examined depressed men whose mean age was 52.4 years and a control group whose mean age was 52.2 years, there were no differences between the depressed patients and the control subjects in salivary testosterone levels, although levels were significantly and negatively correlated with depression ratings.
While reviews (49,64) have noted the positive effects of testosterone supplementation in “involutional” depression, these conclusions are based on many early reports from the 1930s and 1940s of open-label treatment of patients variously diagnosed with “male climacteric,” “involutional melancholia,” “involutional psychosis,” “agitated depression,” and “depression,” that is, nonstandardized diagnoses. Also, not all such reports showed positive results (15, 16), and the preparations of testosterone administered were variable (e.g., testosterone propionate, androsterone, methyltestosterone). Rubinow and Schmidt (65) noted that many of these reports represented uncontrolled studies, and while they indicated that it is difficult to dismiss the approximately 4:1 ratio of positive to negative reports on the effect of testosterone treatment on middle-aged and elderly men, these reports did not address the important question of whether mood changes occur in a clinically significant and consistent fashion with declining testosterone levels associated with aging.
Several studies have examined the effects of testosterone administration on mood, although results have been inconsistent. Testosterone replacement in hypogonadal men was found to improve mood in some studies (43, 66) but not in others (67, 68); mood was often measured by self-rating diaries or scales (e.g., the Profile of Mood States). Rabkin et al. (69) found improvement in mood in hypogonadal (on the basis of total testosterone values) HIV-positive men who were treated in an open design with testosterone and rated on the 21-item Hamilton Depression Rating Scale. Anderson et al. (70) found no change in mood in a single-blind, placebo-controlled crossover study of testosterone in eugonadal men without baseline sexual complaints; similarly, double-blind studies (41, 71) of testosterone administered to eugonadal sexually dysfunctional men found no change in mood.
Muscle and Bone
Normal male aging has been associated with a decline in muscle strength and mass as well as an increase in upper and central body fat (1, 2, 5, 6). In their review of testosterone and frailty, Morley et al. (5) noted that the suggested ability of testosterone to protect against loss of muscle mass (i.e., its anabolic effect) may be mediated by its effects on increasing IGF-I and its binding protein. A number of studies (5, 72, 73) have reported benefit of testosterone supplementation in increasing muscle strength and lean body mass while decreasing fat mass. In addition, Swerdloff and Wang (6) pointed out that physical activity itself increases testosterone levels and that physical inactivity could lead to a cycle of further reduction in testosterone secretion and increased physical frailty.
The incidence of hip fractures among men over the age of 65 is 4–5/1,000 (5), with hypogonadism occurring in up to 20% of men who have vertebral crush fractures (74). However, as noted by several authors (4-6), it is not clear whether the decline in bone mass found in aging men is the result of declining testosterone levels. Premature osteoporosis is seen in hypogonadal men in association with anorexia nervosa, Klinefelter’s syndrome, castration, hyperprolactinemia, and hypothalamic dysfunction (6), although studies that have examined the correlation between testosterone levels, age, and bone mineral density have shown mixed results (2, 4-6). Proposed mechanisms for the effect of diminished testosterone on bone include reduction in calcitonin—an inhibitor of bone resorption (75), reduction in osteoblast function (6), and reduction in estrogen levels (76).
As noted by Morley et al. (5), studies of the effect of testosterone replacement therapy on bone replacement and markers of bone turnover (e.g., hydroxyproline, osteocalcin) have had mixed results, although these authors and Tenover (2) believe that testosterone therapy may have beneficial effects on bone density in older men.
TREATMENT OF TESTOSTERONE DEFICIENCY
Lamberts et al. (1) pointed out that there are very few long-term, placebo-controlled studies of testosterone replacement in elderly men. While there does not seem to be disagreement about testosterone replacement in men with low levels and sexual dysfunction, the problems in diagnosing the hypogonadal state (2), the resultant uncertainty about treatment for men with complaints of sexual dysfunction and low-normal levels of testosterone, and the potential adverse effects of treatment make definitive recommendations problematic. Bhasin and Bremner (77) suggested testosterone therapy for men with bioavailable levels less than 70 ng/dl, especially if associated with sexual dysfunction. Blood levels are best sampled in the morning and, if low, warrant further evaluation with measurement of LH, FSH, and prolactin levels (3). If normal free and/or bioavailable testosteronelevels are found, evaluation should be directed toward detecting other causes for the complaints.
Several forms of testosterone replacement are available: oral, injectable, transdermal patches (genital and nongenital), and implantable pellets. Testosterone is well absorbed after oral administration, but rapid hepatic degradation makes it difficult to achieve sustained plasma levels (15, 77). Also, while the oral 17-alpha-alkylated derivatives of testosterone are relatively resistant to hepatic degradation, their potential hepatotoxicity leaves them unsuitable for clinical use (77). Parenteral formulations of testosterone are the byproduct of esterification of the testosterone molecule at the 17-beta-hydroxy position. This esterification leads to an increase in hydrophobicity and extends the duration of action (77), with the longer side chains of testosterone enanthate and cypionate conferring a longer duration of action than the propionate form. The usual dosing of these parenteral formulations is 200–400 mg every 2–4 weeks, but because there is a rapid rise of serum testosterone levels into the high-normal or supraphysiologic range within 24 hours and then a decline into the hypogonadal range over the next 2 weeks, fluctuations in mood, libido, sexual activity, and energy level may occur accordingly. Transdermal preparations, available in scrotal and nonscrotal delivery systems, produce more physiologic levels than the parenteral forms and can mimic the diurnal rhythm of testosterone production (3, 77). A potential disadvantage of the scrotal system is production of supraphysiologic levels of dihydrotestosterone due to high scrotal 5α-reductase activity; the long-term clinical significance of elevated dihydrotestosterone levels is unknown (77). The nonscrotal patch, however, is associated with more skin irritation at the application site. Testosterone pellets, which have been available for more than 40 years (77), are implanted under the skin and release testosterone slowly over 3 months. Because of the need for a skin incision and occasional spontaneous extrusion, this formulation is rarely used.
Reported side effects due to testosterone therapy include increase in hematocrit, gynecomastia, water retention, irritability, acne, hair loss, testicular atrophy, decreased ejaculate, and worsening of sleep apnea (2–6, 17, 21, 77). The increase in hematocrit may occur as a result of an effect on erythropoietin or a direct effect on bone marrow stem cells; it was found in up to 25% of patients in one study and is potentially more significant in men with a smoking history, chronic obstructive lung disease, or sleep apnea (5, 6, 77). Platelet counts and aggregation have also been reported to increase (3), such that these changes, along with increases in hematocrit, raise concerns regarding adverse cerebrovascular and cardiovascular events.
Gynecomastia, which is believed to result from testosterone conversion to estradiol, leading to growth of breast tissue, is usually transient. Of great concern is the potential effect of testosterone therapy on the prostate and lipid metabolism.
Prostate cancer, which can occur in preclinical (microscopic) or clinical forms, is one of the most commonly diagnosed cancers in the United States; 50% of men have preclinical cancer by their seventh decade (6). There are no clear data indicating that testosterone therapy will enhance the progression from the preclinical to the clinical form, although it is known that androgens stimulate the growth of clinically diagnosed prostate cancer (78). Benign prostatic hypertrophy is known to benefit from a reduction in testosterone and dihydrotestosterone, but there is no evidence that replacement therapy will lead to the development of prostatic hyperplasia (6). The effect of testosterone on lipids is variable. When testosterone esters are given to hypogonadal or eugonadal men, small decreases in HDL cholesterol are seen, while small decreases or no changes are seen in LDL cholesterol, and this differs from the effects of 17-alpha-alkylated androgens, which increase LDL and lower HDL cholesterol and apolipoprotein A-I and A-II levels (2, 3, 6, 77).
Rabkin et al. (21) noted that in their studies of testosterone administration to HIV-positive men, an increase in self-reported irritability was the most common side effect, although for the majority of their patients, the effect was transient and “seldom a cause for concern to the patient.” Further, Rubinow and Schmidt (65) reviewed the relationships between androgens, the brain, and behavior and found that studies of testosterone administration to hypogonadal and eugonadal men did not report increased aggression, although most studies preferentially sampled mood rather than aggression per se. One study (79) found a decrease in anger in hypogonadal men who had been significantly more angry at baseline compared with control subjects.
Hair loss, secondary to the conversion of testosterone to dihydrotestosterone, was found in 6% of the HIV-positive men treated by Rabkin et al. (21). They also found acne in 8% of their subjects and decreased testicular size with diminished ejaculate in up to 20%, a side effect they reported treating successfully with human chorionic gonadotropin.
Given the potential for the adverse effects of testosterone described above, a number of reports (2, 3, 6, 17, 77) noted that the routine use of testosterone therapy should not be recommended unless hypogonadism exists; that is, testosterone therapy is not a remedy for aging in men in the absence of diminished levels of free or bioavailable testosterone.
Lamberts et al. (1) discussed the sparse literature on the use of DHEA or GH to treat adrenopause and somatopause, respectively, noting that it is not known whether the increase in sex steroid levels induced by DHEA is safe with respect to the development of prostate, ovarian, or other cancers, while the safety of administering GH and thereby elevating GH and IGF-I levels with respect to tumor formation is also unknown, since most human solid tumors express IGF-I receptors. (See reference 80 for a report on the role of DHEA and DHEAS in breast cancer and reference 81 regarding the positive association between IGF-I levels and prostate cancer.)
CLINICAL ISSUES FOR PSYCHIATRY
The concept of a testosterone deficiency syndrome introduces into psychiatry an additional consideration in the evaluation of middle-aged and older men for mood, anxiety, and cognitive disorders. A checklist for the “low-testosterone syndrome” (appendix 1) (5), with 10 screening questions, reveals considerable overlap with symptoms of primary psychiatric disorders, leaving the psychiatrist with the dilemma of how to distinguish hypogonadism requiring testosterone replacement from primary and other secondary mood, anxiety, and cognitive disorders without measurement of serum testosterone levels. Should all middle-aged and elderly men with mood, anxiety, and cognitive complaints now have routine measurement of serum free or bioavailable testosterone levels when their complaints include sexual dysfunction and/or anergia? Such an undertaking would be both prohibitively expensive and impractical in the context of managed care medicine. Further,as reviewed by Morley et al. (5), sexual desire was associated with both poor sensitivity and poor positive predictive value regarding low testosterone levels.
Given the complexity of the aging process and the lack of specificity of complaints such as low libido, depression, and anergia, psychiatrists must use their best clinical judgment when assessing middle-aged and elderly men who present with such symptoms. This must include a comprehensive psychosocial, sexual, medical, substance use, and medication history to narrow down the etiology of the patient’s complaints. Serum testosterone measurements should be considered when patients’ complaints cannot be attributed to other causes and in instances where symptoms are only partially responsive or are refractory to conventional psychiatric treatments. Referral to an endocrinologist or urologist should be made when one determines that the patient has a low serum free or bioavailable testosterone level. Education about the normal aging process as well as psychiatric interventions aimed at dealing with associated life transitions and concerns should be part of the treatment plan. In instances in which a patient who comes for psychiatric consultation is already receiving testosterone replacement therapy, it is important to review the circumstances that led to this intervention, including baseline testosterone level, response to treatment, and for those using parenteral preparations, any variations in symptoms that may coincide with fluctuations in serum levels. Finally, given the availability of DHEA as an over-the-counter preparation, patients should be queried about the use of this agent and dissuaded from self-medicating with it, given the unknown long-term risks.
CONCLUSIONS
The male climacteric is a controversial construct that has been suggested to represent a state analogous to female menopause and that exhibits significant interindividual variability in its presentation. The terms “male climacteric,” “male menopause,” “andropause,” and “viropause” appear to be misnomers, however, since most men do not seem to experience cessation of testosterone production and the complete loss of reproductive capacity that occurs in the female menopause. “Low-testosterone syndrome” might be a more appropriate term, but the existence of this syndrome requires further research and validation, especially with regard to the prevalence of mood, anxiety, and cognitive disorders that are directly attributable to the decline in testosterone levels as part of normal aging, which are separate from the other hormonal changes (e.g., thyroid, estrogen) that may be contributory. At this time, testosterone replacement therapy should be reserved for men with clear deficiency of free or bioavailable testosterone levels. Use of testosterone in men with low-normal levels, with the possible exception of HIV-positive men (21), has not been shown to be safe or beneficial and should, for now, be discouraged, as should self-medication with DHEA.
APPENDIX 1. St. Louis University Screening Checklist for Low-Testosterone Syndromea
| 1. | Do you have a decrease in libido (sex drive)? | ||||
| 2. | Do you have a lack of energy? | ||||
| 3. | Do you have a decrease in strength or endurance? | ||||
| 4. | Have you lost height? | ||||
| 5. | Have you noticed a decreased “enjoyment of life”? | ||||
| 6. | Are you sad or grumpy? | ||||
| 7. | Are your erections less strong? | ||||
| 8. | Are you falling asleep after dinner? | ||||
| 9. | Have you noted a recent deterioration in your ability to play sports? | ||||
| 10. | Has there been a recent deterioration in your work performance? | ||||
| 11. | aSee reference 5. | ||||
Received Jan. 9, 1998; revision received April 13, 1998; accepted May 15, 1998. From the Department of Psychiatry, UCLA-Neuropsychiatric Institute, Los Angeles.. Address reprint requests to Dr. Sternbach, Suite 325, 2730 Wilshire Blvd., Santa Monica, CA 90403. The author thanks Barry Sobel, M.D., for assistance in the preparation of the figure.
 |
1. Lamberts SW, van den Beld AW, van der Lely AJ: The endocrinology of aging. Science 1997; 278:419–424Crossref, Medline, Google Scholar
2. Tenover JL: Testosterone and the aging male. J Androl 1997; 18:103–106Medline, Google Scholar
3. Schow DA, Redmon B, Pryor JL: Male menopause: how to define it, how to treat it. Postgrad Med 1997; 101:62–79Crossref, Medline, Google Scholar
4. Vermeulen A: The male climacterium. Ann Med 1993; 25:531–534Medline, Google Scholar
5. Morley JE, Kaiser FE, Sih R, Hajjar R, Perry HM III: Testosterone and frailty. Clin Geriatr Med 1997; 13:685–695Crossref, Medline, Google Scholar
6. Swerdloff RS, Wang C: Androgen deficiency and aging in men. West J Med 1993; 159:579–585Medline, Google Scholar
7. Hamilton D: The Monkey Gland Affair. London, Chatto & Windus, 1986Google Scholar
8. Brown-Sequard CE: The effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet 1889; 2:105–107Crossref, Google Scholar
9. Bharke MS, Yesalis CE III, Wright JE: Psychological and behavioral effects of endogenous testosterone levels and anabolic-androgenic steroids among males: a review. Sports Med 1990; 10:303–337Crossref, Medline, Google Scholar
10. Schmitz G: Erfahrungen mit dem neuen synthetischen testes-hormon-preparat “Perandren.” Deutsche Medizinische Wochenschrift 1937; 63:230–231Crossref, Google Scholar
11. Werner AA: The male climacteric. JAMA 1939; 112:1441–1443Crossref, Google Scholar
12. Davidoff E, Goodstone GL: Use of testosterone propionate in treatment of involutional psychosis in the male. Arch Neurol Psychiatry 1942; 48:811–817Crossref, Google Scholar
13. Altschule MD, Tillotson KJ: The use of testosterone in the treatment of depression. N Engl J Med 1948; 239:1036–1038Crossref, Medline, Google Scholar
14. Lamar CP: Clinical endrocrinology of the male: with special reference to the male climacteric. J Fla Med Assoc 1940; 26:398–404Google Scholar
15. Kerman EF: Testosterone therapy of involutional psychosis. Arch Neurol Psychiatry 1943; 49:306–307Google Scholar
16. Pardoll DH, Belinson L: Androgen therapy in psychosis: effect of testosterone propionate in male involutional psychotics. J. Clin Endocrinol 1941; 1:138–141Crossref, Google Scholar
17. Burns-Cox N, Gingell C: The andropause: fact or fiction? Postgrad Med J 1997; 73:553–556Google Scholar
18. Vermeulen A, Kaufman JM: Ageing of the hypothalamic-pituitary-testicular axis in men. Horm Res 1995; 43:25–28Crossref, Medline, Google Scholar
19. Vermeulen A: Enviornment, human reproduction, menopause, and andropause. Enviorn Health Perspect 1993; 101 (suppl 2):91–100Google Scholar
20. McClure RD, Marshall L: Endocrinologic sexual dysfunction, in Sexual Dysfunction: A Neuro-Medical Approach. Edited by Singer C, Wiener WJ. Armonk, NY, Futura, 1994, pp 245–248Google Scholar
21. Rabkin JG, Rabkin R, Wagner GJ: Testosterone treatment of clinical hypogonadism in patients with HIV/AIDS. Int J STD AIDS 1997; 8:537–545Crossref, Medline, Google Scholar
22. Boots LR, Potter S, Potter D, Azziz R: Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril 1998; 69:286–292Crossref, Medline, Google Scholar
23. Morley JE, Kaiser FE, Perry HM III, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ: Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997; 46:410–413Crossref, Medline, Google Scholar
24. Morley JE, Baranetsky NG, Wingert TD, Carlson HE, Hershman JM, Melmed S, Levin SR, Jamison KR, Weitzman R, Chan RJ, Varner AA: Endocrine effects of naloxone-induced opiate receptor blockade. J Clin Endocrinol Metab 1980; 50:251–257Crossref, Medline, Google Scholar
25. Billington CJ, Shafer RB, Morley JE: Effects of opioid blockade with nalmefene in older impotent men. Life Sci 1990; 47:799–805Crossref, Medline, Google Scholar
26. Bancroft J: Androgens, sexuality and the ageing male, in Endocrinology. Edited by Labrie F, Proulx L. Amsterdam, Elsevier, 1984, pp 913–916Google Scholar
27. Shain SA, Boesel RW: Age-associated diminishing rat prostrate and receptor concentration is concurrrent with decreased androgen dependence. Mech Ageing Dev 1997; 6:219–282Crossref, Google Scholar
28. Chatterjee B, Roy AK: Changes in hepatic androgen sensitivity and gene expression during aging. J Steroid Biochem Mol Biol 1990; 37:437–445Crossref, Medline, Google Scholar
29. Harris MI: Epidemiology of diabetes mellitus among the elderly in the United States. Clin Geriatr Med 1990; 6:703–719Crossref, Medline, Google Scholar
30. Davidson MB: The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 1979; 28:688–705Crossref, Medline, Google Scholar
31. Mariotti S, Franceschi C, Cossarinzza A, Pinchera A: The aging thyroid. Endocr Rev 1995; 16:686–715Crossref, Medline, Google Scholar
32. Corpas E, Harman SM, Blackman MR: Human growth hormone and human aging. Endocr Rev 1993; 14:20–39Crossref, Medline, Google Scholar
33. Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzoferrato A, Gasbarrini G: The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest old: results from an Italian study of healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab 1996; 81:1173–1178Medline, Google Scholar
34. Hornsby PJ: Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann NY Acad Sci 1995; 774:29–46Crossref, Medline, Google Scholar
35. Meacham RB, Murray MJ: Reproductive function in the aging male. Urol Clin North Am 1994; 21:549–556Medline, Google Scholar
36. Nieschlag E, Lammers U, Freischem CW, Langer K, Wickings EJ: Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab 1982; 55:676–681Crossref, Medline, Google Scholar
37. Schiavi RC, Rehman J: Sexuality and aging. Urol Clin North Am 1995; 22:711–726Medline, Google Scholar
38. Davidson JM, Camargo CA, Smith ER: Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab 1979; 48:955–958Crossref, Medline, Google Scholar
39. Bancroft J, Wu FCW: Changes in erectile responsiveness during androgen replacement therapy. Arch Sex Behav 1983; 12:59–66Crossref, Medline, Google Scholar
40. Carani C, Granata ARM, Bancroft J, Marrama P: The effects of testosterone replacement on nocturnal penile tumesence and rigidity and erectile response to visual erotic stimuli in hypogonadal men. Psychoneuroendocrinology 1995; 20:743–753Crossref, Medline, Google Scholar
41. O’Carroll R, Bancroft J: Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry 1984; 145:146–151Crossref, Medline, Google Scholar
42. Kwan M, Greenleaf WJ, Mann J, Davidson JM: The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab 1983; 57:552–562Crossref, Google Scholar
43. O’Carroll R, Shapiro C, Bancroft J: Androgens, behaviour and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985; 23:527–538Crossref, Medline, Google Scholar
44. Cunningham GR, Hirshkowitz M, Korenman SG, Karacan I: Testosterone replacement therapy and sleep-related erections in hypogonadal men. J Clin Endocrinol Metab 1990; 70:792–797Crossref, Medline, Google Scholar
45. Carani C, Scuteri A, Marrama P, Bancroft J: The effects of testosterone administration and visual erotic stimuli on nocturnal penile tumesence in normal men. Horm Behav 1990; 24:435–441Crossref, Medline, Google Scholar
46. Schiavi RC, White D, Mandeli J: Pituitary-gonadal function during sleep in healthy aging men. Psychoneuroendocrinology 1992; 17:599–609Crossref, Medline, Google Scholar
47. Schiavi RC, White D, Mandeli J, Schreiner-Engel P: Hormones and nocturnal penile tumesence in healthy aging men. Arch Sex Behav 1993; 22:207–215Crossref, Medline, Google Scholar
48. Yaffe K, Sawaya G, Lieberburg I, Grady D: Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 1998; 279:688–695Crossref, Medline, Google Scholar
49. Janowsky JS, Oviatt SK, Orwoll ES: Testosterone influences spatial cognition in older men. Behav Neurosci 1994; 108:325–332Crossref, Medline, Google Scholar
50. Christiansen K, Knussman R: Sex hormones and cognitive functioning in men. Neuropsychobiology 1987; 18:27–36Crossref, Medline, Google Scholar
51. Gouchie C, Kimura D: The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology 1991; 16:323–334Crossref, Medline, Google Scholar
52. Moffat SD, Hampson E: A curvilinear relationship between testosterone and spatial cognition in humans: possible influences of hand preference. Psychoneuroendocrinology 1996; 21:323–337Crossref, Medline, Google Scholar
53. Gordon HW, Lee PA: A relationship between gonadotropins and visuopatial function. Neuropsychologia 1986; 24:563–576Crossref, Medline, Google Scholar
54. Vogel W, Broverman DM, Klaiber EL, Abraham G, Cone FL: Effects of testosterone infusions upon EEGs of normal male adults. Electroencephalogr Clin Neurophysiol 1971; 31:400–403Crossref, Medline, Google Scholar
55. Kertzman C, Robinson DL, Sherins RJ, Schwankhaus JD, Mc Clurkin JW: Abnormalities in visual spatial attention in men with mirror movements associated with hypogonadotropic hypogonadism. Neurology 1990; 40:1057–1063Crossref, Medline, Google Scholar
56. Cappa SF, Guariglia C, Papagno C. Pizzamiglio L, Vallar G, Zoccolotti P, Ambrosi B, Santiemma V: Patterns of lateralization and performance levels for verbal and spatial tasks in congenital androgen deficiency. Behav Brain Res 1988; 31:177–183Crossref, Medline, Google Scholar
57. Hampson E, Moffat SD: Is testosterone related to spatial cognition and hand preference in humans? Brain Cogn 1994; 26:255–266Google Scholar
58. Sachar EJ, Halpern F, Rosenfeld RS, Gallagher TF, Hellman L: Plasma and urinary testosterone levels in depressed men. Arch Gen Psychiatry 1973; 28:15–18Crossref, Medline, Google Scholar
59. Vogel W, Klaiber EL, Broverman DL: Roles of gonadal steroid hormones in psychiatric depression in men and women. Prog Neuropsychopharmacol 1978; 2:487–503Crossref, Google Scholar
60. Yesavage JA, Davidson J, Widrow L, Berger PA: Plasma testosterone levels, depression, sexuality, and age. Biol Psychiatry 1985; 20:222–225Crossref, Medline, Google Scholar
61. Levitt AJ, Joffe RT: Total and free testosterone in depressed men. Acta Psychiatr Scand 1988; 77:346–348Crossref, Medline, Google Scholar
62. Rubin RT, Poland RE, Lesser IM: Neuroendocrine aspects of primary endogenous depression, VIII: pituitary-gonaldal axis activity in male patients and matched control subjects. Psychoneuroendocrinology 1989; 14:217–229Crossref, Medline, Google Scholar
63. Davies RH, Harris B, Thomas DR, Cook N, Read G, Riad-Fahmy D: Salivary testosterone levels and major depressive illness in men. Br J Psychiatry 1992; 161:629–632Crossref, Medline, Google Scholar
64. Danziger L, Schroeder HT, Unger AA: Androgen therapy for involutional melancholia. Arch Neurol Psychiatry 1944; 51:457–461Crossref, Google Scholar
65. Rubinow DR, Schmidt PJ: Androgens, brain, and behavior. Am J Psychiatry 1996; 153:974–984Link, Google Scholar
66. Skakkebaek NE, Bancroft J, Davidson DW, Warner P: Androgen replacement with oral testosterone undeconoate in hypogonadal men: a double-blind controlled study. Clin Endocrinol 1981; 14:49–61Crossref, Medline, Google Scholar
67. Salmimies P, Kockott G, Pirke KM, Vogt HJ, Schill WB: Effects of testosterone replacement on sexual behavior in hypogonadal men. Arch Sex Behav 1982; 11:345–353Crossref, Medline, Google Scholar
68. Pirke KM, Kockott G: Endocrinology of sexual function. Clin Endocrinol Metab 1982; 11:625–637Crossref, Medline, Google Scholar
69. Rabkin JG, Rabkin R, Wagner G: Testosterone replacement therapy in HIV illness. Gen Hosp Psychiatry 1995; 17:37–42Crossref, Medline, Google Scholar
70. Anderson RA, Bancroft J, Wu FCW: The effects of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab 1992; 75:1503–1507Medline, Google Scholar
71. Schiavi RC, White D, Mandeli J, Levine AC: Effect of testosterone administration on sexual behavior and mood in men with erectile dysfunction. Arch Sex Behav 1997; 26:231–241Crossref, Medline, Google Scholar
72. Tenover JS: Effects of testosterone supplementation in the aging male. J. Clin Endocrinol Metab 1992; 75:1092–1098Google Scholar
73. Sih R, Morley JE, Kaiser FE, Perry HM III, Patrick P, Ross C: Tesotsterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997; 82:1661–1667Crossref, Medline, Google Scholar
74. Scane AC, Sutcliffe AM, Francis RM: Osteoporosis in men (review). Baillieres Clin Rheumatol 1993; 7:589–601Crossref, Medline, Google Scholar
75. Jackson JA, Kleerekoper M, Parfitt AM, Rao DS, Villanueva AR, Frame B: Bone histomorphometry in hypogonadal and eugonadal men with spinal osteoporosis. J Clin Endocrinol Metab 1987; 65:53–58Crossref, Medline, Google Scholar
76. Crilly RG, Francis RM, Nordin BE: Steroid hormones, ageing and bone. Clin Endocrinol Metab 1981; 10:115–139Crossref, Medline, Google Scholar
77. Bhasin S, Bremner WJ: Emerging issues in androgen replacement theray. J Clin Endocrinol Metab 1997; 82:3–8Medline, Google Scholar
78. Schroder FH: Androgens and carcinoma of the prostate, in Testosterone Action, Deficiency, Substitution. Edited by Nieschlag E, Behre HM. Berlin, Springer Verlag, 1990, pp 245–260Google Scholar
79. Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ: A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl 1992; 13:297–304Medline, Google Scholar
80. Dorgan JF, Stanczyk FZ, Longcope C, Stephenson HE Jr, Chang L, Miller R, Franz C, Falk RT, Kahle L: Relationship of serum dehydroepiandrosterone (DHEA), DHEA sulfate and 5-androstene-3 beta, 17 beta-diol to risk of breast cancer in postmenopausal woman. Cancer Epidemiol Biomarkers Prev 1997; 6:177–181Medline, Google Scholar
81. Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M: Plasma insulin-like growth factor-1 and prostate cancer risk: a prospective study. Science 1998; 279:563–566Crossref, Medline, Google Scholar



