Cognitive Impairment in Adolescents With Schizophrenia
Abstract
OBJECTIVE: The purpose of this study was to determine whether adolescent schizophrenia is characterized by neuropsychological deficits. METHOD: The performance on a battery of neuropsychological tests of 17 adolescents with schizophrenia (mean age=15.71 years) was compared with that of 17 normal adolescents (mean age=15.12 years). RESULTS: Compared with the normal subjects, the patients were impaired on 10 of the 13 measures; larger effect sizes were shown for measures involving working memory and attention than for those involving secondary memory, generative naming, and executive functions. CONCLUSIONS: Adolescents with schizophrenia have generalized cognitive dysfunction, which is most apparent on tests of attention and working memory. (Am J Psychiatry 1997; 154:1613–1615)
There are few studies of adolescent schizophrenia (1, 2). During adolescence there are neurodevelopmental events including changes in synaptic density and morphology in the frontal lobe (3), a decline in the ratio of gray matter to white matter (4), marked sleep EEG changes, and a decline in cerebral metabolism (3). These changes are believed to involve maturational reorganization consisting of selective synaptic enhancement and elimination of redundant axons (i.e., pruning). These maturational events have been summoned in support of what Keshaven et al. (5) have described as a “late” neurodevelopmental model of the etiology of schizophrenia (3, 6) in contrast to the “early” developmental model (7), which posits a fixed lesion from early life that interacts with normal neurodevelopmental events occurring at a later point. A neurodevelopmental perspective suggests that substantial neurocognitive deficits should be evident very early in the course of schizophrenia.
We report the first comprehensive neuropsychological assessment of adolescent schizophrenic patients.
METHOD
Seventeen adolescent patients (12 male and five female) were included in this study. They were aged 13–18 years (mean=15.71 years, SD=1.65) and were diagnosed with schizophrenia (N=12), schizophreniform disorder (N=1), or schizoaffective disorder, mainly schizophrenic (N=4). The patients' mean age at onset of illness was 13.79 years (SD=2.42). The patients were stabilized on antipsychotic medication regimens at testing. Seventeen normal adolescent subjects (nine male and eight female) aged 13–18 years were recruited through advertisements. Their mean age was 15.12 years (SD=1.27). Written informed consent was obtained from all subjects and their parents or legal guardians.
All subjects were medically healthy. No subject had had a neurological illness or major head trauma with loss of consciousness for more than 20 minutes. The subjects did not have histories of substance abuse, as reported by the parents, and all subjects had an estimated IQ of at least 70.
Clinical diagnostic interviews were conducted by physician-investigators (S.C.S., R.L.F.). Subjects were also interviewed by a psychologist (T.P.S.) using the Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version (K-SADS-E) (8). Consensus diagnoses were based on DSM-III-R criteria. Patients were studied after being clinically stabilized either early in the course of outpatient treatment or at the end of inpatient care. All of the normal subjects were also assessed with the K-SADS-E and were free of clinically significant psychopathology.
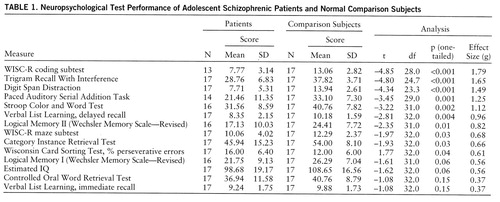
The test battery description, along with the rationale supporting the cognitive constructs purported to be measured by the individual tests, has been presented elsewhere (9, 10). The neuropsychological tests included measures of attention (Stroop Color and Word Test [11], Digit Span Distraction [12], and Paced Auditory Serial Addition Task [13]), working memory (Trigram Recall With Interference Test [14, 15]), a measure sensitive to both attention and working memory (the coding subtest of the Wechsler Intelligence Scale for Children—Revised [WISC-R] [16]), verbal learning and memory (Selective Reminding, Categorical Clustering, and Verbal List Learning, immediate recall and delayed recall [17] and Logical Memory I and Logical Memory II, Wechsler Memory Scale—Revised [18]), generative naming (Controlled Oral Word Retrieval Test [19] and Category Instance Retrieval Test [20]), and executive functions (Wisconsin Card Sorting Test [21] and the maze subtest of the WISC-R). An estimated IQ was created on the basis of three IQ subtests (information, similarities, and block design [WISC-R]). Thus, there were 11 neuropsychological tests with 13 total measures and a single IQ estimate.
In the statistical analysis, patients and normal subjects were compared on cognitive measures by one-tailed tests, because the patients were expected to perform more poorly than the comparison group. Effect sizes were scaled as “g” (22, 23) and indicate mean group differences in standard deviation units. To assess the number of adolescent patients with clinically significant impairment, a threshold of 2 SD from the mean of the normal subjects was used.
RESULTS
The results of the primary analysis are presented in table 1. The measures are ordered by effect size. The patients were impaired with respect to the normal subjects (p<0.05, one-tailed) on 10 of the 13 neuropsychological measures (excluding IQ). The overall average effect size (g=0.92) was quite large (24).
The measures showing the greatest impairment (i.e., the largest effect sizes) were those with a strong component of working memory or attention. Also, the percentage of patients who performed more than 2 SD below the mean of the normal subjects was greater for WISC-R coding (47%), Trigram Recall With Interference (65%), and Digit Span Distraction (71%) than for the measures of other functions (range=12%–35%).
DISCUSSION
The main finding was that adolescents with schizophrenia showed generalized neuropsychological impairment on measures of attention, working memory, secondary memory, generative naming, and executive functions. The effect sizes were moderate to very large (24). To our knowledge, this study is the first to identify neuropsychological dysfunction in adolescents with schizophrenia with the use of a comprehensive test battery and with nonpsychiatric comparison subjects.
The largest effect sizes were on measures of focused and divided attention as well as working memory. Also, a large percentage of the patients performed at a clinically significant level of impairment. Taken together, these findings attest to the relatively greater role of attention and working memory deficits in adolescent schizophrenia.
The precise neurodevelopmental etiology of the impaired cognitive performance of adolescent schizophrenic patients is unclear. It is unknown whether the neurodevelopmental and disease processes are independent or, alternatively, whether the neurodevelopmental process itself is diseased.
 |
Presented at the International Congress on Schizophrenia Research, Warm Springs, Va., April 6–12, 1995. Received Dec. 30, 1996; revision received June 6, 1997; accepted June 19, 1997. From the Department of Psychiatry, Case Western Reserve University School of Medicine, Cleveland, and the Psychology Service, VA Medical Center, Brecksville, Ohio. Address reprint requests to Dr. Kenny, Psychology Service-116 B (B), VA Medical Center, 10000 Brecksville Road, Brecksville, OH 44141. Supported by the Stanley Foundation.
1. Findling RL, Friedman L, Kenny JT, Swales TP, Cola DM, Schulz SC: Adolescent schizophrenia: a methodologic review of the current neuroimaging and neuropsychologic literature. J Autism Dev Disord 1995; 25:627–639Crossref, Medline, Google Scholar
2. Goldberg TE, Hyde TM, Kleinman JE, Weinberger DR: Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull 1993; 19:797–804Crossref, Medline, Google Scholar
3. Feinberg I, Thode HC, Chugani HT, March JD: Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol 1990; 142:149–161Crossref, Medline, Google Scholar
4. Jernigan TL, Tallal P: Late changes in brain morphology observable with MRI. Dev Med Child Neurol 1990; 32:379–385Crossref, Medline, Google Scholar
5. Keshaven MS, Anderson S, Pettegrew JW: Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? the Feinberg hypothesis revisited. J Psychiatr Res 1994; 28:239–265Crossref, Medline, Google Scholar
6. Feinberg I: Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1983; 17:319–334Google Scholar
7. Weinberger D: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
8. Orvaschel H, Puig-Antich J: Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version (K-SADS-E), 5th revision. Fort Lauderdale, Fla, Nova University, 1987Google Scholar
9. Lezak MD: Neuropsychological Assessment, 2nd ed. New York, Oxford University Press, 1983Google Scholar
10. Kenny JT, Meltzer HY: Attention and higher cortical functions in schizophrenia. J Neuropsychiatry Clin Neurosci 1991; 3:269–275Crossref, Medline, Google Scholar
11. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Stoelting, 1978Google Scholar
12. Oltmanns TF: Selective attention in schizophrenic and manic psychoses: the effect of distraction on information processing. J Abnorm Psychol 1978; 87:212–225Crossref, Medline, Google Scholar
13. Gronwall D: Paced Auditory Serial Addition Task: a measure of recovery from concussion. Percept Mot Skills 1977; 44:367–373Crossref, Medline, Google Scholar
14. Baddeley A: Working Memory. New York, Oxford University Press, 1986Google Scholar
15. Petersen LR, Peterson MJ: Short-term retention of individual items. J Exp Psychol 1959; 58:193–198Crossref, Medline, Google Scholar
16. Wechsler D: WISC-R Manual: Wechsler Intelligence Scale for Children—Revised. New York, Psychological Corp/Harcourt Brace Jovanovich, 1974Google Scholar
17. Buschke H, Fuld PA: Evaluating storage, retention and retrieval in disordered memory and learning. Neurology 1974; 11:1019–1025Google Scholar
18. Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp/Harcourt Brace Jovanovich, 1987Google Scholar
19. Benton AL: Differential behavioural effects in frontal lobe disease. Neuropsychologia 1968; 6:53–60Crossref, Google Scholar
20. Perret E: The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia 1974; 12:323–330Crossref, Medline, Google Scholar
21. Robinson AL, Heaton RK, Lehman RA, Stilson DW: The utility of the Wisconsin Card Sorting Test in detecting localized frontal lobe lesions. J Consult Clin Psychol 1980; 48:605–614Crossref, Medline, Google Scholar
22. Rosenthal R: Parametric measures of effect size, in The Handbook of Research Synthesis. Edited by Cooper H, Hedges LV. New York, Russell Sage Foundation, 1994Google Scholar
23. Johnson BT: Software for the Meta-Analytic Review of Research Literatures. Hillsdale, NJ, Lawrence Erlbaum Associates, 1989Google Scholar
24. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar



