Prefrontal Cortex 5-HT2 Receptors in Depression: An [18F]Setoperone PET Imaging Study
Abstract
OBJECTIVE: Widespread disturbances of serotonin (5-HT) are implicated in the pathophysiology of depression. Of 5-HT receptor abnormalities reported, the most replicated finding is increased 5-HT2 receptor binding in the postmortem prefrontal cortex of depressed suicide victims. The extent to which these findings exist in depressed persons without recent suicide attempts is uncertain. The objective of this study was to evaluate 5-HT2 receptors in depressed patients who were medication-free and who had not made recent suicide attempts. METHOD: With the use of [18F]setoperone and positron emission tomography (PET), 5-HT2 receptor binding potential was assessed in 14 depressed and 19 healthy subjects. Exclusion criteria for depressed patients included use of antidepressant medication within the past 6 months, a history of suicide attempts within the past 5 years, other current axis I disorders including bipolar disorder, and the presence of psychotic symptoms. The 5-HT2 (setoperone) binding potential in the two groups of subjects was compared by analysis of covariance with age as the covariate. RESULTS: Age had a significant effect on 5-HT2 binding potential, but depression did not. The interaction of age and depression was not significant. CONCLUSIONS: The 5-HT2 binding potential is not increased in untreated depressed subjects who have not made recent suicide attempts. This negative finding does not rule out the possibility that there is a role for 5-HT2 receptors in treatment or that 5-HT2 receptors are increased in highly suicidal states.
Several indirect techniques provide evidence that serotonin (5-HT) physiology is disturbed in depression. Brain uptake of the 5-HT precursor 5-hydroxytryptophan is reduced, and the cerebrospinal fluid concentration of the 5-HT metabolite 5-hydroxyindoleacetic acid is usually low (1, 2). Prolactin response to fenfluramine, a 5-HT releasing agent, is decreased (3, 4). In treated depressed subjects, depletion of the 5-HT precursor tryptophan induces a relapse into sad mood (5). These findings represent generalized 5-HT disturbances but do not indicate the neurochemical level(s) at which they may occur. Such changes could reflect alterations in 5-HT synthesis, 5-HT metabolism, postsynaptic receptors, or linked second messenger systems.
Postmortem and platelet receptor binding studies suggest that 5-HT abnormalities exist at the postsynaptic receptor level in depressed subjects who are suicidal. Postmortem studies of antidepressant-free depressed suicide victims report either significant (6, 7) or nonsignificant (8) increases in prefrontal cortex 5-HT2 receptor binding. Cortex 5-HT2 receptor binding may be reflected by platelet receptors, and elevated platelet 5-HT2 receptor binding is commonly found in depression and suicidal states (9–18). These findings do not exclude the possibility that changes occur at other neurochemical levels; however, the presence of increased prefrontal 5-HT2 receptor binding in depressed suicide victims has considerable support.
Depressed suicide victims usually have increased prefrontal cortex 5-HT2 receptor binding (6–8). However, this finding may not be true for depressed subjects who have not made recent suicide attempts. Since increased 5-HT2 receptor binding is present in undiagnosed suicide victims, it could represent the presence of suicidality rather than depression (19–21). In postmortem studies, it is difficult to investigate the effects of depression independent of suicide. McKeith et al. (22) chose a group of subjects with major depressive disorder who had not been victims of suicide and showed a trend toward increased prefrontal cortex 5-HT2 receptor binding. However, this study group was heterogeneous and included patients with histories of medication use, psychotic symptoms, and euthymia at the time of death.
Use of medication is a major confounding factor with respect to postmortem studies of 5-HT2 receptor binding. In the one postmortem study that examined the effects of antidepressants, Yates et al. (7) found an increase in prefrontal cortex 5-HT2 receptor binding in unmedicated depressed subjects; however, those receiving chronic antidepressant treatment did not differ from the control group. In animal cortex, postmortem 5-HT2 receptor binding changes with several types of chronic antidepressant treatment (23–26). Additional variance in the results of postmortem studies may be attributable to cause of death, delay in tissue processing after death, and choice of ligand (27).
Two neuroimaging studies investigated 5-HT2 receptors in primary depression. Using single photon emission computed tomography (SPECT), D’haenen et al. (28) reported an increase in left-to-right prefrontal cortex 2-[123I]ketanserin uptake. Using positron emission tomography (PET), Biver et al. (29) found a significant decrease in [18F]altanserin uptake in the left inferofrontal cortex. Unfortunately, both findings were confounded by recent psychotropic drug use, and it is not clear whether the subjects participating in these studies had made recent suicide attempts. In both studies one-half of the subjects had used psychotropic medication as recently as 10 days before scanning.
The purpose of this study was to investigate the effects of depression on 5-HT2 receptors in patients without recent suicide attempts by means of [18F]setoperone PET. [18F]Setoperone has a number of properties that make it favorable for imaging. Setoperone is an antagonist with high affinity and specificity for 5-HT2 receptors. It has 100 times greater affinity for 5-HT2A than 5-HT2C receptors, thus making it relatively specific for the 5-HT2A receptor (30). Setoperone has only a 10- to 50-fold higher affinity for 5-HT2 as compared to D2 receptors, but D2 receptors have very low density in the cortex (31). 5-HT2 and D2 antagonists displace [18F]setoperone in the striatum. Only 5-HT2 antagonists displace [18F]setoperone in the cortex; hence, [18F]setoperone is still a suitable ligand for 5-HT2 receptor imaging in the cortex (32, 33). In fact, D2 antagonists have no effect on cortical [18F]setoperone signal (33). [18F]Setoperone has high brain uptake and a sufficiently high specific-to-nonspecific binding ratio (32–34). Metabolites of [18F]setoperone do not appear to have significant brain uptake (35). Since the cerebellum is practically devoid of 5-HT2 and D2 receptors, it may be used as a reference region to simplify image quantification (31–36). At our center, PET imaging with [18F]setoperone has been shown to be reliable (test-retest reliability within 7%) (37), and at another center, it has successfully confirmed an in vivo decrease in 5-HT2 receptors in patients with Alzheimer’s dementia (38).
On the basis of the foregoing evidence that unmedicated depressed suicide victims had increased 5-HT2 receptor binding (6, 7), and that in the only postmortem study of unmedicated depressed subjects who died of natural causes, a trend for increased 5-HT2 receptor binding was also found (22), it was hypothesized that increased frontal cortex 5-HT2 receptor binding would be demonstrated in unmedicated depressed subjects who do not have a recent history of suicide attempts.
METHOD
This study was approved by the University of Toronto Human Subjects Review Committee. Fourteen depressed subjects, aged 18–40 years, and 19 healthy subjects, aged 18–45 years, were recruited by advertisement. All subjects had been free of any psychotropic drug use for more than 6 months, and no subject had taken any nonpsychotropic medication in the past 6 weeks. All were physically healthy and right-handed. Written informed consent was obtained from each subject after the procedures had been fully explained.
The 19 healthy subjects, who have been previously reported (37, 39), were screened with the Structured Clinical Interview for DSM-III-R—Non-Patient Edition (40).
The patients’ diagnoses of a major depressive episode secondary to major depressive disorder were confirmed by the Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P) (41), which was administered by a trained research assistant. Each patient received a psychiatric consultation (with J.H.M. or S.H.K.) to verify the SCID-P diagnosis. A score higher than 17 on the Hamilton Depression Rating Scale (42) was required for entry into the study. Patients with psychotic symptoms, bipolar disorder (type I or type II), or comorbid axis I diagnoses were excluded from the study. Potential subjects with a history of alcohol or drug abuse or dependence were also excluded, and any and all past drug use was recorded. Those with a history of substance use that did not meet criteria for the SCID-P diagnosis of substance abuse received a urine drug screen and then were included in the study only if the result was negative. Four subjects had previously completed a 6-week trial of an antidepressant, but none had received any antidepressant or psychotropic medication/substance within the past 6 months. The exclusion criteria also included a history of a suicide attempt during the previous year. Three subjects had a history of past suicide attempts, but no attempt had occurred within the past 5 years. Each depressed subject had routine tests (thyroid function, electrolytes, CBC) to rule out common medical causes of depression.
[18F]Setoperone was prepared by [18F]fluoride displacement on the nitroderivative precursor of setoperone on the basis of the procedure described by Crouzel et al. (43). [18F]setoperone was of high radiochemical purity (>99%) and high specific activity (mean=46 GBq/µmol, SD=20, at the time of injection). Imaging was based on the approach described by Blin et al. (32, 33). An intravenous bolus of 185 MBq of [18F]setoperone was injected. PET images were obtained with the use of a GEMS 2048-15B camera (General Electric Medical Systems, Milwaukee, Wis.). Images were obtained in five 1-minute frames followed by 17 5-minute frames. The images were corrected for attenuation with a 68Ge transmission scan and reconstructed by filtered back projection (Hanning filter, 5 mm full width at half maximum).
To obtain a semiquantitative measure of 5-HT2 receptor binding potential, we used the pseudoequilibrium prefrontal cortex-to-cerebellum ratio (37). The cerebellum has no displaceable [18F]setoperone binding (32, 33). Between 65 and 90 minutes, there is no significant change in the prefrontal cortex-to-cerebellum ratio (37). If the cerebellar concentration of [18F]setoperone is used as an index for free and nonspecifically bound ligand in the cortex, then it can be shown that the cortex-to-cerebellum ratio between 65 and 90 minutes is linearly proportional to k3/k4 and f2 × Bmax/Kd(34) (k3 and k4 are rate constants for ligand transfer between free and specific binding compartments; f2 represents the free fraction of ligand; Bmax represents the total number of receptors available to a ligand; Kd is the dissociation constant for the ligand at the receptor; and pseudoequilibrium refers to a period of time in which the k3/k4 ratio is stable). Petite-Taboue et al. (34) empirically demonstrated that the pseudoequilibrium cortex-to-cerebellum ratio is highly correlated with the k3/k4 ratio (r=0.91–0.97). Use of the cortex-to-cerebellum ratio avoids the need for an arterial puncture, making this approach less invasive (34).
The 5-HT2 binding potential does not estimate Bmax or Kd separately. However, Kd is almost always unchanged in postmortem studies of depression and/or suicide, whereas changes are observed in Bmax(6–8, 19–22).
For image analysis, each subject had a magnetic resonance imaging (MRI) scan (GE Sigma 1.5-T scanner; spin-echo sequence T2-weighted image; x, y, z voxel dimensions=0.78, 0.78, and 3 mm, respectively). MRI scans were coregistered to each PET image by using the surface matching function in ANALYZE (CNS Software, Rochester, Minn.). For region-of-interest analysis, the regions of interest were drawn on the [18F]setoperone scans with reference to the coregistered MRI by a rater blind to the identity of the subjects of the scans. The prefrontal cortex region of interest was drawn on five adjacent slices for each hemisphere, as described previously (37). This region included Brodmann’s area 9 and parts of areas 8, 10, 46, and 32. The cerebellum region of interest was drawn bilaterally on two adjacent slices. Decay-corrected time-activity curves were obtained for each of these regions of interest.
The technique as described above is well standardized in our laboratory (37, 39, 44). Pseudoequilibrium occurs between 65 and 90 minutes, since no significant change was found in the cortex-to-cerebellum ratio during this time in 20 healthy subjects (37). To test the reliability of this measure, 11 normal subjects were scanned twice, and their data showed a test-retest standard deviation of 6%–9% and a mean scan-rescan difference of 5%–7% (37). The intraclass correlation coefficient was greater than 0.98. Furthermore, this method of region-of-interest analysis has been applied to the same data with high interrater and intrarater reliability (37). We have already demonstrated that this technique is sensitive to age-related and medication-induced changes in 5-HT2 receptors (39, 44).
RESULTS
Fourteen patients (12 male and two female; mean age=32.3 years, SD=6.4) were compared with 19 healthy subjects (eight male and 11 female; mean age=31.8 years, SD=6.9). There was no significant difference in age between the patients and the healthy subjects (t=0.26, df=31, p=0.80). There were significantly more women in the comparison group (p=0.01, Fisher’s exact test). The mean baseline score of the patients on the Hamilton depression scale was 22.5 (SD=3.7). On the suicide subscale, five subjects scored 0, one subject scored 1, seven subjects scored 2, and two subjects scored 3. The mean prefrontal cortex binding potential was 1.6 (SD=0.6) in the depressed patients and 1.8 (SD=0.7) in the healthy subjects.
The effects of age and gender on binding potential were assessed. Age was associated with a significant decrease in 5-HT2 binding potential for all subjects (analysis of covariance [ANCOVA] with age as a covariant, F=42.3, df=1, 28, p<0.001), but gender was not (F=0.1, df=1, 28, p=0.70). The effects of age were also significant within the healthy group (F=31.9, df=1, 17, p<0.001) and the depressed group (F=11.2, df=1, 12, p=0.006); therefore, age was entered as a covariate in all further analyses. Gender was not significant within the healthy group (F=1.9, df=1, 16, p=0.20) or the depressed group (F=1.2, df=1, 11, p=0.30); therefore, gender was not considered a necessary covariate.
There were no discernible effects of diagnosis on 5-HT2 binding potential (ANCOVA, F=1.6, df=1, 30, p=0.22) (Figure 1). The interaction between age and diagnosis was not significant (F=0.1, df=1, 29, p=0.70).
The three subjects with a history of a suicide attempt did not have a 5-HT2 binding potential which was significantly different from that of the healthy subjects (ANCOVA, age as covariant, F=0.6, df=1, 19, p=0.46); however, this subgroup was small.
Given the previous report of an increased right-to-left ratio of prefrontal cortex 2-[123I]ketanserin uptake in depression (28), the right-to-left ratio of prefrontal cortex 5-HT2 binding potential was calculated for each scan. The mean ratio for the depressed subjects was 1.02 (SD=0.03). For the healthy subjects, the ratio was 1.01 (SD=0.02). Depression was not associated with right-to-left prefrontal cortex asymmetry (analysis of variance, F=0.2, df=1, 30, p=0.69).
DISCUSSION
To our knowledge, this is the first investigation of 5-HT2 receptors in drug-free depressed subjects who have not recently attempted suicide, and we found no evidence for increased 5-HT2 receptor binding in the prefrontal cortex. We had hypothesized an increase in 5-HT2 binding potential, which would have been consistent with postmortem studies of depressed suicide victims (6–8), but our data do not support this theory.
Unlike postmortem studies of depressed subjects, most of whom ended their lives by suicide, this was a study of depressed patients who had not made recent suicide attempts (6–8). Given that increased 5-HT2 receptor binding in undiagnosed suicide victims has been reported, it appears that increased 5-HT2 receptor binding is linked to suicide attempts but not depression without suicide attempts (19–21).
Our findings replicate in part those reported by D’haenen et al. (28). They imaged 5-HT2 receptors in 19 depressed and 10 healthy subjects by means of 2-[123I]ketanserin SPECT. All of their depressed subjects had been free of psychotropic drugs for 7 days, and 10 of their depressed subjects had been free of antidepressants for at least 3 weeks. As in our study, D’haenen and colleagues found that age was a significant covariate and that no changes in bilateral 5-HT2 binding were present. In contrast to our results, D’haenen et al. also reported an increase in left-to-right prefrontal cortex 2-[123I]ketanserin uptake. Their unexpected finding was not hypothesized a priori and is not supported by postmortem data. Neither the current study nor another recent study (29) confirmed their finding. It is possible that their unreplicated finding represents a subgroup with treatment-refractory illness or the effects of medication withdrawal (23–26).
Biver et al. (29) measured 5-HT2 binding potential in 22 healthy and eight depressed subjects by means of [18F]altanserin PET. Using statistical parametric mapping (45, 46), they detected two marginally significant regions of approximately 4-cm3 total size in bilateral inferofrontal cortex. These regions reflected decreased 5-HT2 binding potential in the depressed group. Spatial normalization of statistical parametric mapping has not been validated for [18F]altanserin (47). Such localized findings may be diluted in our region of interest. Our results may also differ because of sampling differences: we selected untreated patients, whereas Biver et al. chose patients with depression who were withdrawn from treatment (and may have had treatment-refractory illness). Also, the effect of medication use in their study is not clear; of the eight depressed subjects studied, one took prednisone, four had taken benzodiazepines until 10 days before scanning, and four had taken antidepressants until 3 weeks prior to scanning. Steroids and antidepressants are known to affect 5-HT2 receptor regulation (7, 23–26, 48), and γ-aminobutyric acid receptors influence serotonin release (49). The mean age of their patient group was 48 years, 16 years older than the mean age of our patients. Six of their eight patients had recurrent major depression, as compared with three of 14 in our study. Their finding may reflect a vulnerability to recurrent major depression rather than the state of depression itself.
Mayberg and colleagues (50), using PET with [11C]N-methylspiperone, also found no increases in 5-HT2 binding potential in a group of subjects with poststroke depression or dysthymia, as compared with age-matched healthy volunteers. Unlike our study, the theoretical basis for investigating up-regulation of 5-HT2 receptors was that localized decreases of 5-HT and norepinephrine near an infarct location could cause changes in 5-HT2 binding potential.
Whenever negative results are found, the sensitivity of the approach to detecting a difference and the expected magnitude of such a difference have to be considered. Our approach is reasonably sensitive to changes in 5-HT2 receptor binding because it has detected the effects of age (37, 39, 44), atypical neuroleptic occupancy (44), and Alzheimer’s disease (38). Even in the present study group, robust age effects were observed. Postmortem studies of depressed suicide victims report increases in 5-HT2 receptor binding of approximately 70% within the prefrontal cortex (6, 7). Thus, if differences in vivo were similar in magnitude to those reported in postmortem studies, we should have had 99% power to detect such change (51). In fact, our study had 80% power to detect a difference as small as 20%. The present study cannot rule out a change of less than 10%, since it had only limited power (27%) to detect such a small change. A study group of 70 depressed and 70 healthy subjects would be required to detect a 10% change.
The second limitation of our approach is that we measured 5-HT2 binding potential in a large volume of the prefrontal cortex that included Brodmann’s area 9 and parts of areas 8, 10, 46, and 32. It is conceivable that highly localized regional changes within the prefrontal cortex may have been undetected. Voxel-by-voxel image analysis is available for regional cerebral blood flow data (45–47); however, we are not aware of any spatially validated voxel analyses for 5-HT2 binding potential images.
Other limitations also need to be considered. Rather than determining Bmax and Kd individually, we measured the 5-HT2 binding potential, which is linearly proportional to Bmax/Kd. If our patients had an increase in receptor number (i.e., increased Bmax) and a decrease in affinity (i.e., increased Kd), then these two changes could have cancelled each other out in 5-HT2 binding potential. Such an event is highly unlikely because Bmax and Kd would have to change in the same direction and by the same quantity. Furthermore, this possibility is not supported by previous reports of elevated 5-HT2 Bmax in drug-free depressed suicide victims (6, 7), in whom changes in Kd were not found.
Our findings do not rule out a role for prefrontal 5-HT2 receptors in the treatment of depression. Using [18F]setoperone PET, Massou et al. (52) found increased 5-HT2 binding potential in six depressed subjects who were treated with selective serotonin reuptake inhibitors (SSRIs), as compared with eight untreated depressed subjects. Many tricyclic antidepressants bind to 5-HT2 receptors, and both tricyclic antidepressants and SSRIs are known to affect 5-HT2 receptor binding in animal models (23–26, 53). It is proposed that 5-HT2 receptors may mediate antidepressant action through postreceptor cellular targets such as the cAMP response element-binding protein via the stimulation of Ca2+-dependent protein kinases (54).
We made a special effort to exclude subjects with a recent history of suicide attempts because it might have confounded the effects of depression. On the other hand, it may be of scientific interest and clinical importance to study patients who have a prominent suicidal history or who have made recent suicide attempts. Elevated 5-HT2 receptor binding has been well replicated in suicide victims; hence, it is likely that suicidal depressed subjects are different from depressed subjects with regard to 5-HT2 receptor binding (6, 7, 19–21).
In conclusion, we found no increase in 5-HT2 receptor binding in the prefrontal cortex in unmedicated patients with major depression. This result is in contrast to postmortem studies that showed an increase of 65%–75% (6, 7). It is still possible that vulnerability to suicide attempts is associated with increased 5-HT2 receptor binding and that 5-HT2 receptors may have an important role in the therapeutics of depression, even though a primary role for increased 5-HT2 receptor density in depression seems unlikely.
Received April 13, 1998; revision received Nov. 21, 1998; accepted Dec. 10, 1998. From the Clarke Institute of Psychiatry, Department of Psychiatry, University of Toronto; and the Rotman Research Institute, University of Toronto. Address reprint requests to Dr. Meyer, Clarke Institute of Psychiatry, Department of Psychiatry, University of Toronto, 250 College St., Toronto, Ont., Canada M5T 1R8; [email protected] (e-mail). Supported by the Medical Research Council of Canada (grant MT-14337), the Canadian Psychiatric Research Foundation (grant 8764), the National Alliance for Research on Schizophrenia and Depression (grant 8817), and salary support awards from the Medical Research Council of Canada to Dr. Meyer and Dr. Kapur. The authors thank research assistants Erin Toole and Corey Jones and technicians Doug Hussey, Kevin Cheung, Stephen Dobbin, Armando Garcia, and Li Jin for their help.
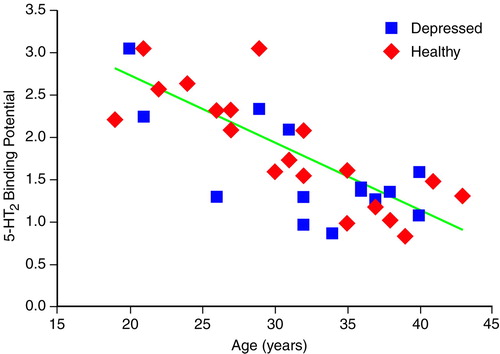
FIGURE 1. Relation Between 5-HT2 Binding Potential and Age in 14 Depressed Patients and 19 Healthy Subjects
1. Agren H, Reibring L: PET studies of presynaptic monoamine metabolism in depressed patients and healthy volunteers. Pharmacopsychiatry 1994; 27:2–6Crossref, Medline, Google Scholar
2. Asberg M, Traskman L, Thoren P: 5-HIAA in the cerebrospinal fluid: a biochemical suicide predictor? Arch Gen Psychiatry 1976; 33:1193–1197Google Scholar
3. Heninger GR, Charney DS, Sternberg DE: Serotonergic function in depression. Arch Gen Psychiatry 1984; 41:398–402Crossref, Medline, Google Scholar
4. Siever LJ, Murphy DL, Slater S, de la Vega E, Lipper S: Plasma prolactin changes following fenfluramine in depressed patients compared to controls: an evaluation of central serotonergic responsivity in depression. Life Sci 1984; 34:1029–1039Google Scholar
5. Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR: Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 1990; 47:411–418Crossref, Medline, Google Scholar
6. Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M:5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res 1993; 614:37–44Google Scholar
7. Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN:5-HT2 receptor changes in major depression. Biol Psychiatry 1990; 27:489–496Google Scholar
8. Cheetham SC, Crompton MR, Katona CLE, Horton RW: Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res 1988; 443:272–280Crossref, Medline, Google Scholar
9. Biegon A, Weizman A, Karp L, Ram A, Tiano S, Wolff M: Serotonin 5-HT2 receptor binding on blood platelets—a peripheral marker for depression? Life Sci 1987; 41:2485–2492Google Scholar
10. Pandey GN, Pandey SC, Janicak PG, Marks RC, Davis JM: Platelet serotonin-2 receptor binding sites in depression and suicide. Biol Psychiatry 1990; 28:215–222Crossref, Medline, Google Scholar
11. Arora RC, Meltzer HY: Increased serotonin-2 (5-HT2) receptor binding as measured by [3H] lysergic acid diethylamide ([3H]LSD) in the blood platelets of depressed patients. Life Sci 1989; 44:725–734Crossref, Medline, Google Scholar
12. Mikuni M, Kusumi I, Kagaya A, Kuroda Y, Mori H, Takahashi K: Increased 5-HT2 receptor function as measured by serotonin-stimulated phosphoinositide hydrolysis in platelets of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 1991; 15:49–61Crossref, Medline, Google Scholar
13. Hrdina PD, Bakish D, Chudzik J, Ravindran A, Lapierre YD: Serotonergic markers in platelets of patients with major depression: upregulation of 5-HT2 receptors. J Psychiatry Neurosci 1995; 20:11–19Medline, Google Scholar
14. Cowen PJ, Charig EM, Fraser S, Elliott JM: Platelet 5-HT receptor binding during depressive illness and tricyclic antidepressant treatment. J Affect Disord 1987; 13:45–50Crossref, Medline, Google Scholar
15. McBride PA, Brown RP, DeMeo M, Keilp J, Mieczkowski T, Mann JJ: The relationship of platelet 5-HT2 receptor indices to major depressive disorder, personality traits and suicidal behavior. Biol Psychiatry 1994; 35:295–308Crossref, Medline, Google Scholar
16. Bakish D, Cavazzoni P, Chudzik J, Ravindran A, Hrdina PD: Effects of selective serotonin reuptake inhibitors on platelet serotonin parameters in major depressive disorder. Biol Psychiatry 1997; 41:184–190Crossref, Medline, Google Scholar
17. Biegon A, Grinspoon A, Blumenfeld B, Bleich A, Apter A, Mester R: Increased serotonin 5-HT2 receptor binding on blood platelets of suicidal men. Psychopharmacology (Berl) 1990; 100:165–167Crossref, Medline, Google Scholar
18. Pandey GN, Pandey SC, Dwivedi Y, Sharma RP, Janicak PG, Davis JM: Platelet serotonin-2A receptors: a potential biological marker for suicidal behavior. Am J Psychiatry 1995; 6:850–855Google Scholar
19. Stanley M, Mann JJ: Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983; 1:214–216Crossref, Medline, Google Scholar
20. Mann JJ, Stanley M, McBride PA, McEwen BS: Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry 1986; 43:954–959Crossref, Medline, Google Scholar
21. Arango V, Ernsberger P, Marzuk PM, Chen J-S, Tierney H, Stanley M, Reis DJ, Mann JJ: Autoradiographic demonstration of increased serotonin 5-HT2 and β-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 1990; 47:1038–1047Google Scholar
22. McKeith IG, Marshall EF, Ferrier IN, Armstrong MM, Kennedy WN, Perry RH, Perry EK, Eccleston D:5-HT receptor binding in post-mortem brain from patients with affective disorder. J Affect Disord 1987; 13:67–74Google Scholar
23. Peroutka SJ, Snyder SH: Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science 1980; 210:88–90Crossref, Medline, Google Scholar
24. Peroutka SJ, Snyder SH: Regulation of serotonin (5-HT2) receptors labelled with (3H) spiroperidol by chronic treatment with the antidepressant amitriptyline. J Pharmacol Exp Ther 1980; 215:582–587Medline, Google Scholar
25. Dumbrille-Ross A, Tang SW: Manipulations of synaptic serotonin: discrepancy of effects on serotonin S1 and S2 sites. Life Sci 1983; 32:2677–2684Google Scholar
26. Hrdina PD, Vu TB: Chronic fluoxetine treatment upregulates 5-HT uptake sites and 5-HT2 receptors in rat brain: an autoradiographic study. Synapse 1993; 14:324–331Crossref, Medline, Google Scholar
27. Mann JJ, Underwood MD, Arango V: Postmortem studies of suicide victims, in Biology of Schizophrenia and Affective Disease. Edited by Watson SJ. Washington, DC, American Psychiatric Press, 1996, pp 197–221Google Scholar
28. D’haenen H, Bossuyt A, Mertens J, Bossuyt-Piron C, Gijsemans M, Kaufman L: SPECT imaging of serotonin2 receptors in depression. Psychiatry Res Neuroimaging 1992; 45:227–237Crossref, Medline, Google Scholar
29. Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Mendlewicz J: Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. Br J Psychiatry 1997; 171:444–448Crossref, Medline, Google Scholar
30. Seeman P: Receptor Tables, vol 2: Drug Dissociation Constants for Neuroreceptors and Transporters. Toronto, SZ Research, 1993Google Scholar
31. De Keyser J, Clayes A, De Backer J-P, Ebinger G, Roels F, Vauquelin G: Autoradiographic localization of D1 and D2 dopamine receptors in the human brain. Neurosci Lett 1988; 91:142–147Crossref, Medline, Google Scholar
32. Blin J, Pappata S, Kiyosawa M, Crouzel C, Baron J-C: [18F]setoperone: a new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur J Pharmacol 1988; 147:73–82Crossref, Medline, Google Scholar
33. Blin J, Sette G, Fiorelli M, Bletry O, Elghozi JL, Crouzel C, Baron JC: A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem 1990; 54:1744–1754Google Scholar
34. Petit-Taboue M-C, Landau B, Osmont A, Tillet I, Barre L, Baron J-C: Estimation of neocortical serotonin-2 binding potential by single dose fluorine-18-setoperone kinetic PET data analysis. J Nucl Med 1996; 37:95–104Medline, Google Scholar
35. Blin J, Crouzel C: Blood-cerebrospinal fluid and blood-brain barriers imaged by 18F-labeled metabolites of 18F-setoperone studied in humans using positron emission tomography. J Neurochem 1992; 58:2303–2310Google Scholar
36. Schotte A, Maloteaux JM, Laduron PM: Characterization and regional distribution of serotonin S2-receptors in human brain. Brain Res 1983; 276:231–235Crossref, Medline, Google Scholar
37. Kapur S, Jones C, DaSilva J, Wilson AA, Houle S: Reliability of [18F]setoperone in humans. Nucl Med Comm 1997; 18:395–399Crossref, Medline, Google Scholar
38. Blin J, Baron JC, Dubois B, Crouzel C, Fiorelli M, Attar-Levy D, Pillon B, Fournier D, Vidailhet M, Agid Y: Loss of brain 5-HT2 receptors in Alzheimer’s disease: in vivo assessment with positron emission tomography and [18F]setoperone. Brain 1993:497–510Google Scholar
39. Lewis R, Kapur S, Jones C, DaSilva J, Roy P, Brown G, Wilson A, Houle S, Zipursky R: PET Study of 5-HT2 receptor density in schizophrenia (abstract). Biol Psychiatry 1997; 41(suppl):63SGoogle Scholar
40. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
41. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P). Washington, DC, American Psychiatric Press, 1995Google Scholar
42. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
43. Crouzel C, Guillaume M, Barre L, Lemaire C, Pike VW: Ligands and tracers for PET studies of the 5-HT system—current status. Int J Rad Appl Instrum B 1992; 19:857–870Crossref, Medline, Google Scholar
44. Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S: PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: implications for the treatment of schizophrenia. Am J Psychiatry 1997; 154:1525–1529Google Scholar
45. Friston KJ, Ashburner J, Frith CD, Poline J-P, Heather JD, Frackowiak RSJ: Spatial registration and normalization of images. Hum Brain Mapp 1995; 2:165–189Crossref, Google Scholar
46. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
47. Friston KJ, Frith CD, Liddle PF, Frackowiak RS: Plastic transformation of PET images. J Comput Assist Tomogr 1991; 15:634–639Crossref, Medline, Google Scholar
48. Kurodo Y, Mikuni M, Ogawa T, Takahashi K: Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor mediated wet-dog shake behaviours. Psychopharmacology (Berl) 1992; 108:27–32Crossref, Medline, Google Scholar
49. Tao R, Ma Z, Auerbach SB: Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br J Pharmacol 1996; 119:1375–1384Google Scholar
50. Mayberg HS, Robinson RG, Wong DF, Parikh R, Bolduc P, Starkstein SE, Price T, Dannals RF, Links JM, Wilson AA, Ravert HT, Wagner HN Jr: PET Imaging of cortical S2 serotonin receptors after stroke: lateralized changes and relationship to depression. Am J Psychiatry 1988; 145:937–943Link, Google Scholar
51. Rosner B: Fundamentals of Biostatistics. Toronto, Duxbury Press, 1995, pp 283–285Google Scholar
52. Massou JM, Trichard C, Attar-Levy D, Feline A, Corruble E, Beaufils B, Martinot JL: Frontal 5-HT2A receptors in depressive patients during chronic treatment by selective serotonin reuptake inhibitors. Psychopharmacology (Berl) 1997; 133:99–101Crossref, Medline, Google Scholar
53. Richelson E: Antidepressants and brain neurochemistry. Mayo Clin Proc 1990; 65:1227–1236Google Scholar
54. Duman RS, Henninger GR, Nestler EJ: A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54:597–608Crossref, Medline, Google Scholar



