Effect of a Novel NMDA Receptor Modulator, Rapastinel (Formerly GLYX-13), in OCD: Proof of Concept
To the Editor: A single intravenous dose of ketamine, an N-methyl-d-aspartate receptor (NMDAR) full antagonist, produces robust and rapid antiobsessional effects in obsessive-compulsive disorder (OCD) (1, 2), but ketamine’s side effects, including dissociation and nausea, may limit clinical use (1, 3–5). Rapastinel (formerly GLYX-13), a putative NMDAR functional glycine site partial agonist, has shown rapid antidepressant activity without ketamine-like side effects (6) and may be a new therapeutic strategy for OCD. We conducted the first test of the tolerability and potential efficacy of rapastinel administration in OCD. Specifically, we explored the drug’s acute effects on obsessive-compulsive symptoms, depression, and anxiety at 90 minutes and at 230 minutes postinfusion and at 1 week postinfusion.
Method
We received approval from an institutional review board and recruited seven unmedicated outpatients with OCD (ages 18–55) between March 2014 and March 2015, and they provided written informed consent. Patients met criteria for OCD (as defined in both DSM-IV and DSM-5) with at least moderate symptoms (score ≥16 on the Yale-Brown Obsessive Compulsive Scale [YBOCS]) (7, 8). Exclusion criteria included severe depression (score >25 on the 17-item version of the Hamilton Depression Rating Scale [HAM-D]) (9), current cognitive-behavioral therapy (CBT), and other comorbid psychiatric or general medical conditions that made participation unsafe.
Patients (N=7) received a single 3- to 5-minute intravenous push of rapastinel (10 mg/kg). At baseline, 90 minutes postinfusion, and 230 minutes postinfusion, patients self-rated the severity of their obsessions and compulsions using the YBOC Challenge Scale (10), a 10-item self-report form that assesses OCD symptoms (i.e., time spent, degree of control, severity) (total score range=0–40) over the previous 60 minutes, facilitating symptom evaluation over shorter time intervals. At the same time intervals, patients also self-rated the severity of their anxiety using the Beck Anxiety Inventory (BAI) (11) and the severity of their depression using the Beck Depression Inventory (BDI) (12). Side effects of dissociation (13), mania (14), and psychosis (15) were assessed at baseline, 90 minutes postinfusion, and 230 minutes postinfusion. At baseline and at 1 week postinfusion, an independent evaluator who was blind to study design evaluated patients using the YBOCS, which appraises obsessive and compulsive symptoms over the prior week, and patients self-rated anxiety and depression symptoms using the BAI and the BDI, respectively. Treatment response was defined a priori as a reduction of ≥35% in YBOCS scores (16). Outcomes were analyzed using a nonparametric Wilcoxon matched-pairs signed ranks test (alpha=0.05, two-tailed) without adjustment for multiple comparisons, given the exploratory nature of this study.
Results
All seven patients who received rapastinel completed the infusion. Patients had severe OCD symptoms: the mean YBOCS score at baseline was 28.9 (SD=4.4), with a mean duration of illness of 24.9 years (SD=10.6). The mean number of prior adequate serotonin reuptake inhibitor (SRI) trials was 3.4 (SD=2.8, median=3, range=0–7). In our sample, 86% received at least one adequate trial of an SRI, 29% failed at least one prior adequate trial of antipsychotic augmentation, and 57% failed at least one prior adequate trial of CBT with exposure and response prevention. Three of the seven OCD subjects (43%) had no other psychiatric comorbidity. Two subjects (29%) met criteria for comorbid generalized anxiety disorder. Two (29%) met criteria for comorbid major depression, with baseline HAM-D scores of 11 (mild) and 14 (moderate). Compared with baseline, scores on the YBOC Challenge Scale, the BAI, and the BDI were significantly lower at 90 minutes and 230 minutes postinfusion (all p<0.05; Figure 1; see also Figure S1 in the data supplement that accompanies the online edition of this letter); the percentage decrease in YBOC Challenge Scale scores from baseline to 230 minutes postinfusion was 46.4%. OCD severity, as measured by the YBOCS, was not significantly decreased from baseline to 1 week postinfusion, and neither were scores on the BDI, although scores on the BAI were significantly decreased (p=0.02). No patient met the treatment response criterion (reduction of ≥35% in the YBOCS score) at 1 week postinfusion (the mean YBOCS score at baseline was 28.9 [SD=4.4] and was 26.0 [SD=5.4] at 1 week postinfusion). One individual with mild comorbid depression had a further reduction in HAM-D scores, from 11 (at baseline) to 1 (at 230 minutes), with a slight increase to 7 (by 1 week after infusion of rapastinel).
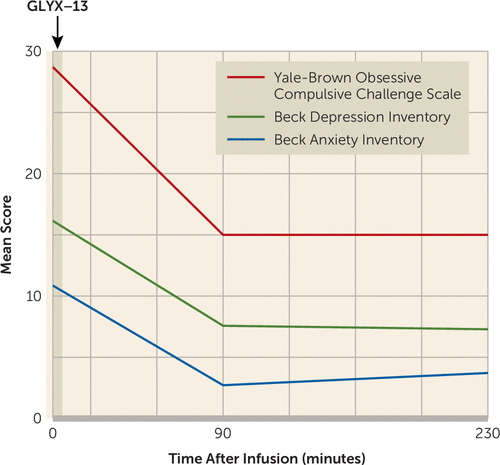
FIGURE 1. Obsessive-Compulsive, Depression, and Anxiety Severity Mean Scores at Baseline, 90 Minutes, and 230 Minutes After a Single Infusion of Rapastinel (N=7)a
a At baseline, 90 minutes postinfusion, and 230 minutes postinfusion (rapastinel at 10 mg/kg), patients self-rated the severity of their obsessions and compulsions using the Yale-Brown Obsessive Compulsive Challenge Scale (range=0–40), the severity of their anxiety using the Beck Anxiety Inventory (range=0–63), and the severity of their depression using the Beck Depression Inventory (range=0–63). For the 90-minute and 230-minute assessments, patients were instructed to rate their symptoms over the past 60 minutes for all three measures. Mean scores are plotted for each assessment measure.
Rapastinel was well tolerated. Of note, in contrast to participant reports in a prior study of intravenous ketamine in OCD (1), participants did not report adverse events (e.g., dizziness, nausea, vomiting, or headache). Assessments of dissociation, mania, and psychosis were not significantly changed from baseline.
Discussion
The findings suggest that rapastinel is well tolerated in unmedicated OCD patients, as it is in patients with depression (6). Specifically, rapastinel did not increase psychotomimetic effects following dosing in this sample of OCD patients, unlike ketamine in prior studies (1, 3–5). In this small open-label sample, rapastinel had acute effects on obsessions and compulsions and on symptoms of anxiety and depression. However, rapastinel did not have significant effects on OCD symptoms 1 week postinfusion. To have clinical utility, glutamate modulators should refine molecular targets for rapid and sustained action while minimizing side effects. Mechanistic preclinical data suggest drugs like rapastinel (17) and ketamine’s metabolite hydroxynorketamine (1, 18) that act on AMPA receptor modulation pathways may be promising therapeutic strategies.
1 : Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 2013; 38:2475–2483Crossref, Medline, Google Scholar
2 : Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? An open-label trial. J Clin Psychiatry 2016; 77:408–409Crossref, Medline, Google Scholar
3 : Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2011; 72:567–569Crossref, Medline, Google Scholar
4 : Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry 2012; 72:964–970Crossref, Medline, Google Scholar
5 : Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol 2013; 27:651–654Crossref, Medline, Google Scholar
6 : GLYX-13, an NMDA receptor glycine site functional partial agonist, enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 2014; 23:243–254Crossref, Medline, Google Scholar
7 : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
8 : The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
9 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
10 : Pharmacological challenges in obsessive compulsive disorder, in The Psychobiology of Obsessive Compulsive Disorder. Edited by Zohar J, Insel T, Rasmussen S. New York, Springer, 1991, p 183Google Scholar
11 : An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Crossref, Medline, Google Scholar
12 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
13 : Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11:125–136Crossref, Medline, Google Scholar
14 : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
15 : The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
16 : Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown obsessive compulsive scale. J Clin Psychiatry 2005; 66:1549–1557Crossref, Medline, Google Scholar
17 : GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38:729–742Crossref, Medline, Google Scholar
18 : NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533:481–486Crossref, Medline, Google Scholar



