Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework
Abstract
Tremendous progress has been made in basic neuroscience in recent decades. One area that has been especially successful is research on how the brain detects and responds to threats. Such studies have demonstrated comparable patterns of brain-behavior relationships underlying threat processing across a range of mammalian species, including humans. This would seem to be an ideal body of information for advancing our understanding of disorders in which altered threat processing is a key factor, namely, fear and anxiety disorders. But research on threat processing has not led to significant improvements in clinical practice. The authors propose that in order to take advantage of this progress for clinical gain, a conceptual reframing is needed. Key to this conceptual change is recognition of a distinction between circuits underlying two classes of responses elicited by threats: 1) behavioral responses and accompanying physiological changes in the brain and body and 2) conscious feeling states reflected in self-reports of fear and anxiety. This distinction leads to a “two systems” view of fear and anxiety. The authors argue that failure to recognize and consistently emphasize this distinction has impeded progress in understanding fear and anxiety disorders and hindered attempts to develop more effective pharmaceutical and psychological treatments. The two-system view suggests a new way forward.
Recent events in psychiatry highlight a growing chasm between basic science and the clinic (1). Progress in understanding the brain has not been mirrored in improved clinical outcomes. Most current therapies with some efficacy emerged decades ago. Promising new treatments either have not turned out to be useful when tested with patients or exhibit potential adverse effects that limit their applicability to severe, treatment-refractory disorders.
Research on brain mechanisms engaged during confrontations with threats—stimuli with the potential to inflict harm to the organism—has shown high degrees of conservation across mammals, likely reflecting evolutionary advantages of an efficiently functioning threat-processing circuitry. This work provides a unique opportunity for developing novel treatments for conditions involving alterations in threat processing, especially the so-called anxiety disorders. Nevertheless, research in this area has been disappointing as a source of novel treatments (2). We argue that this state of affairs reflects how fear and anxiety have been conceived, and we offer a new framework to address the problem.
It has long been assumed that an innate “fear system” exists in the mammalian brain and that this system, in the presence of a threat, generates both the conscious feeling of “fear” and the behavioral and physiological responses typical of such experiences (Figure 1A). We propose instead a “two systems” framework, with one set of circuits for generating conscious feelings and a second set for controlling behavioral and physiological responses to threats (Figure 1B). The first system primarily involves cortical areas, and the second mostly involves subcortical regions, such as the amygdala, although certain cortical areas interact with and regulate processing in these regions. While the first system generates conscious feelings, the second largely operates nonconsciously. Conflation of these circuits and their functions has hampered clinical extension of basic research. While the actual circuitry is considerably more complex than implied by the two-system label, the framework represents a useful heuristic around which to restructure translational efforts to achieve a deeper, more nuanced understanding than currently exists about how brain mechanisms give rise to normal and pathological feelings of fear and anxiety and the behavioral and physiological symptoms that accompany these subjective experiences.
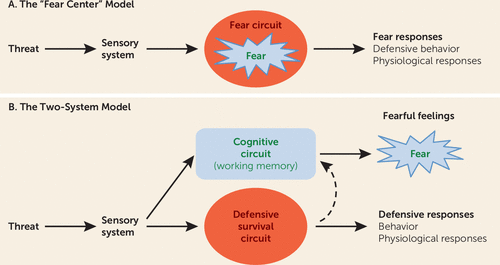
FIGURE 1. The Traditional “Fear Center” View Versus the “Two-System” View of “Fear”a
a In the traditional “fear center” model, the subjective experience of “fear” in the presence of a threat is innately programmed in subcortical circuits that also control defensive behaviors and physiological responses. The two-system framework views “fear” as a product of cortical circuits that underlie cognitive functions such as working memory; subcortical circuits control defensive behaviors and physiological responses and only indirectly contribute to conscious “fear.” The traditional view thus requires different mechanisms of consciousness in the brain for emotional and nonemotional states, whereas in the two-system framework, both emotional and nonemotional states of consciousness are treated as products of the same system. In the two-system framework, what distinguishes an emotional from a nonemotional state of consciousness, and what distinguishes different kinds of emotional states of consciousness, are the input processes by the cortical consciousness networks (see Figure 3).
A Note on Terminology
The terms “fear” and “anxiety” are used in many ways. Consider fear. Most often it refers to a subjective state, a feeling that one experiences when threatened. However, it also describes behaviors, such as facial expressions, freezing, flight, and avoidance, as well as physiological changes that accompany such behaviors. Using the same term for both subjective states and objective responses implies that they are entwined in a common neural circuit. We and others argue that this assumption is incorrect—that the different processes referred to by the term “fear” reflect different mechanisms rather than operations of one “fear center” or “fear circuit” (3–9).
Because precise terminology is essential for scientific progress, we propose that the use of mental state terms, like fear and anxiety, be limited to their primary, as opposed to their extended, meanings, that is, to mental states—subjective feelings of fear and anxiety. We further propose that these mental state terms be avoided when referring to behavioral and physiological responses that also may occur when one feels fearful or anxious. Accordingly, we refer to brain circuits that detect and respond to threats as defensive circuits, to behaviors that occur in response to threats as defensive behaviors, and to peripheral changes in physiology that support defensive behaviors as defensive physiological adjustments.
Confusion also results from interchangeable use of the terms fear and anxiety. To avoid such confusion, we propose using a common distinction consistently—that the mental state term fear be used to describe feelings that occur when the source of harm, the threat, is either immediate or imminent, and anxiety be used to describe feelings that occur when the source of harm is uncertain or is distal in space or time.
The Innate Fear Circuit View
Fear is often said to be an innate function of subcortical brain areas. This view stems from the idea that humans inherited from animals certain basic, universally expressed emotions (10, 11), often described as products of the brain’s so-called limbic system (12, 13). For example, fear is often said to be a product of the limbic area called the amygdala, which is frequently said to be a “fear center,” or, in more contemporary terminology, the hub of a “fear circuit” (13–22).
An immediately present threat activates the lateral nucleus of the amygdala, which by way of connections to the central nucleus of the amygdala initiates the expression of defensive behavioral reactions, such as freezing, and supports defensive physiological reactions (Figure 2A). And through connections from the lateral amygdala to the basal amygdala, and from there to the nucleus accumbens, defensive actions such as avoidance are controlled (5, 23). Although key components of this circuitry are subcortical, the ability of the circuits to control defense reactions and actions is modulated by certain cortical areas. For example, the extinction of defensive responses elicited by learned threats is regulated by connections from the ventral medial prefrontal cortex and hippocampus to the amygdala (24–27).
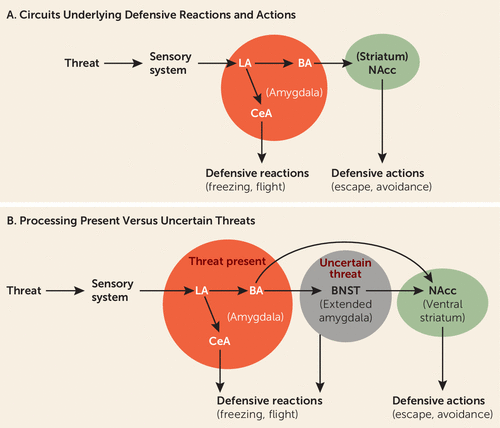
FIGURE 2. Neural Control of Reactions and Actions Elicited by Present Versus Uncertain Threatsa
a As shown in panel A, the amygdala is the central hub of circuits that control reactions and actions elicited by an immediately present threat. The lateral amygdala (LA) receives sensory inputs about the threat. Connections from LA to the central nucleus of the amygdala (CeA) control reactions, whereas connections from LA to the basal nucleus (BA), and from there to the ventral striatum (nucleus accumbens, NAcc), control the performance of actions, such as escape and avoidance. As shown in panel B, when the threat is uncertain, and thus only a possible outcome in the future, connections from the amygdala and hippocampus (not shown) to the extended amygdala (the bed nucleus of the stria terminalis, BNST) are engaged in the control of reactions, and actions using similar output pathways are used by the amygdala to control responses to present threats.
Findings in humans are consistent with the animal data. Thus, people with amygdala damage fail to exhibit bodily reactions to threats (28, 29). Furthermore, functional imaging studies show that threats activate the amygdala in healthy people (28, 30) and induce exaggerated amygdala activation in patients with anxiety disorders (31–34). In addition, the nucleus accumbens has been implicated in defensive actions such as avoidance in humans (35, 36). Medial cortical areas down-regulate the amygdala in healthy humans (25, 37), and this capacity is weakened in people with anxiety disorders (26, 27). Such findings are commonly viewed as support for the idea that the amygdala is a hub in an evolutionary conserved fear circuit that, in the presence of a threat, controls both feelings of fear and behavioral and physiological sequelae.
The assumption that in the presence of a threat the same circuit controls conscious feelings of fear and behavioral and physiological responses is expressed most forcefully by Panksepp (13, 16). He notes that “fear is an aversive state of mind” and that the major driving force for this “subjective component of fear seems to be … a subcortical FEAR system” (16). Since both the behavioral and physiological responses and the subjective feelings of fear elicited by a threat are viewed as products of the same circuit, it should be possible, according to Panksepp, to identify the “feeling circuit” in humans by studying behavioral and physiological responses in animals or humans. But if, as we have argued, different circuits underlie the two consequences of threat detection, studying behavioral or physiological responses will not reveal the circuits responsible for feelings.
Just as findings demonstrating that the amygdala detects and controls behavioral and physiological responses to immediate threats have supported views of the amygdala as fear-circuit hub, other findings, about responses to uncertain threats, have led to a view of the circuitry of anxiety. Thus, in recent years, animal research has suggested the bed nucleus of the stria terminalis (BNST) is engaged when threats are uncertain (38, 39), resulting in behavioral inhibition and risk assessment (40) (Figure 2B). Imaging studies in healthy humans (41) and in humans with anxiety disorders confirm the contribution of the BNST in processing uncertainty (42, 43). The BNST has thus come to be for anxiety what the amygdala is for fear—a circuit hub out of which anxious feelings emerge.
Proponents of subcortical explanations do not all mean the same thing when they use the terms fear and anxiety. For a number of contemporary neuroscientists, emotions are not subjective experiences but central physiological states (“central states” for short) that intervene between trigger stimuli and behavioral and physiological responses (14, 17–19, 40, 44, 45). A strong version of the central state view of fear is expressed by Fanselow and colleagues (45). They argue that a goal of science should be to “replace inaccurate subjective explanations … with more scientifically grounded explanations.” This approach ignores subjective states to avoid the scientific problems that result from attributing subjective experiences to animals. But it does so at the expense of making subjective experience off-limits as a scientific topic in humans. This is a serious shortcoming of any approach attempting to translate animal research to human clinical problems. Subjective experiences of fear or anxiety typically are the problems that lead people to seek help; moreover, therapies are judged as successful largely on the basis of their capacity to change these experiences. Softer versions of the central state view that allow the central state to contribute to both feelings of fear and bodily responses (19) are essentially “fear circuit” views and are subject to the criticisms mentioned above.
Problems With the Subcortical Fear and Anxiety Center/Circuit Views
That a brain area, like the amygdala, controls behavioral and physiological responses to threats does not mean that the experience of fear arises from this brain area. In other words, it is an assumption, not a fact, that both consequences of threat detection are products of a single circuit. And this assumption is contradicted by several sets of findings.
First, it has long been known that subjective experiences of fear and anxiety do not correlate well with measures of behavioral and physiological responses (46–48). This should not be the case if the same circuit controls all these consequences of threat detection. Second, patients with amygdala damage still can feel fear, panic, and pain (49–51). Earlier reports of diminished feelings following amygdala damage (52) may reflect the elimination of the indirect consequences of amygdala activity on feelings (see below). Third, threats presented subliminally increase amygdala activity and trigger peripheral physiological responses, even when one remains unaware of the stimulus and lacks feelings of fear (28, 30, 53–59). Fourth, “blindsight” patients, who, as a result of damage to the right visual cortex, lose the ability to consciously experience stimuli presented to their left visual hemifield (60), nevertheless exhibit threat-elicited amygdala activity and defensive behavioral and physiological responses, all in the absence of conscious awareness of the stimulus and without feeling fear (61–63). All four sets of findings dissociate the circuitry underlying feelings from circuitry underlying defensive behavior and physiologic responding. How can this be if the amygdala is a fear center?
We thus argue that the amygdala is not itself responsible for the experience of fear. Its job can be more appropriately viewed as detecting and responding to present or imminent threats. The amygdala contributes to fear indirectly but is not an innate fear center out of which fear percolates. And the BNST is not itself responsible for the experience of anxiety, but instead is a key part of an innate defense circuit that detects and processes uncertain threats. The BNST contributes to anxiety indirectly in the way that the amygdala contributes indirectly to fear—consequences of its activation generate signals that modulate circuits that are directly responsible for subjective feelings of fear and anxiety (5).
The Emergence of Conscious Experience From Cortical Circuits
Significant progress has been made in neuroscience research on the cognitive and neural underpinnings of subjective experiences (60, 64–72). This work assumes that conscious experience is cognitively derived from nonconscious processes that allow cortical regions to rerepresent lower-order information, and that this rerepresentation enables conscious awareness of nonconscious processing about external stimuli.
We propose that subjective feelings of fear or anxiety are not products of subcortical circuits underlying defensive responses, but instead depend on the same circuits that underlie any other form of conscious experience—namely, circuits in the so-called higher-order association cortex that are responsible for cognitive processes such as attention and working memory (Figure 3). Included are areas of the lateral and medial prefrontal cortex, as well as the parietal neocortex (73–77). The lateral prefrontal areas may be especially important since they have been most consistently implicated in conscious awareness (see below), are interconnected with the other key cortical areas, are particularly well developed in primates, and have unique attributes in humans (78–83). The insula, another frontal region, has been implicated in the conscious experience of somatic sensations (84–86) and may be particularly relevant to threats signaled by perturbations in the organism’s physiologic milieu and in forms of anxiety initiated by interoceptive stimuli (87, 88).
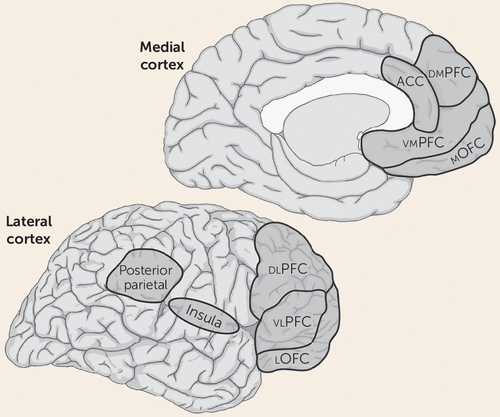
FIGURE 3. Cortical Consciousness Networksa
a Functional imaging studies have implicated circuits spread across frontal and parietal areas in conscious experiences in humans. ACC=anterior cingulate cortex; dlPFC=dorsolateral prefrontal cortex; dmPFC=dorsomedial prefrontal cortex; lOFC=lateral orbital frontal cortex; mOFC=medial orbital frontal cortex; vlPFC=ventrolateral prefrontal cortex; vmPFC=ventromedial prefrontal cortex.
When one is aware of a visual stimulus, prefrontal and parietal circuits are engaged, and when awareness degrades, the circuits are not engaged (64, 89–91). Two leading theories propose two different ways that this neural architecture gives rise to conscious experience. In global workspace theory, subjective experience emerges through widely distributed reentrant circuitry, with prefrontal areas playing an especially prominent role (64, 65, 92). In higher-order theory, subjective experience arises from a more delimited circuitry, especially involving a prefrontal hub, which supports thoughts about lower-order information (67, 93, 94).
While attention, working memory, and their underlying circuits support consciousness, working memory can be engaged without generating conscious content (71, 92, 95–97). An additional layer of cognitive representation, likely also involving the frontal cortex, is thus required beyond nonconscious representation in working memory (94). Differentiating neural features of cognitive processing that do and do not result in introspective awareness is a significant challenge and a major focus of current research.
Consistent with findings described so far, when participants in a brain imaging study are conscious of a visual threat, prefrontal and parietal areas are active, but when awareness is degraded, prefrontal and parietal areas are not (28, 30, 53–59). Importantly, as also noted above, in such studies, the amygdala is engaged even when people are not consciously aware of the threat and do not report feeling fear. This has two important implications. First, amygdala processing is dissociable from conscious awareness of threat. Second, conscious awareness of threats comes about in the same way as the conscious awareness of nonemotional stimuli.
We thus propose that emotional and nonemotional states of consciousness both emerge from cortical consciousness networks. The difference between an emotional and a nonemotional state of consciousness, in this view, reflects different kinds of inputs to the cortical consciousness network in the two kinds of states: the inputs required to feel an emotion elicited by a threat are more elaborate than those required to see a nonthreatening stimulus. While the feeling of fear thus does not arise directly from subcortical circuits that control behavioral and physiological responses to threats, these circuits indirectly contribute to the feeling of fear by generating responses, such as brain and body arousal, which can affect working memory function.
Although feelings of fear and anxiety are most often discussed in terms of circuits that evolved for predatory defense, this view is too narrow. We can also be afraid or anxious about activity related to other survival circuits (3–5), including circuits that signal low energy supplies (fear of starvation), fluid imbalance (fear of dehydration), or hypothermia (fear of freezing to death). In each example, fear/anxiety reflects awareness of a potential for harm, occurring when one cognitively monitors and interprets signals from the brain and/or body, and integrates these signals with information about the external situation. But there is more. Humans can also be fearful or anxious in relation to existential concerns, such as not leading a meaningful life and the eventuality of death. A scientific account of “fear” and “anxiety” has to accommodate all the various ways that fear and anxiety can manifest within and between individuals. The two-system view, which treats fear and anxiety as cognitively generated conscious experiences, accomplishes this.
Separating circuits underlying conscious feelings and response control also makes it easier to understand the role language and culture play in the shaping of experience (98–101). In English alone, there are more than three dozen words for gradations of fear and anxiety, reflecting the importance of these states to humans (102). Language enables symbolic representation of emotions like fear and anxiety without actual exposure to danger (103, 104). In certain circumstances, this can help keep us safe, but it can also become a vehicle for excessive rumination and worry.
The two-system view also may explain why infants react emotionally long before they report emotions (105). Interestingly, the defense circuitry in infants is not simply an immature version of the adult circuitry (106). Developmental changes in linguistic capacity allow categorization of experience and may affect the way fear is expressed at different points in early life: children report different fears than adolescents, who report different fears than adults (107, 108). Understanding these changes requires a deeper understanding of brain development (109). As discussed later, this also affects perspectives on treatment.
Whatever degree of self-awareness and conscious experience is possible without language, it is clear that language changes the self-and-consciousness game in the brain. We are not necessarily suggesting that animals lack conscious experiences, but rather that it is problematic to draw inferences about conscious experiences, and thus problematic to use words like fear and anxiety, when describing animal behavior and physiology. Moreover, because, as noted, defensive behaviors and physiological responding are not reliable indicators of subjective fear in humans, such responses in animals cannot be used to identify circuits responsible for feelings of fear or anxiety in animals or humans.
The two-system framework provides a way to understand what animal research can and cannot tell us about human fear and anxiety without making the impossible-to-evaluate assumption that in animals exposure to threat elicits states that are comparable to what humans call feelings of fear and anxiety. Another important impact of the two-system view is that it helps us understand why efforts to develop medications have not been more effective. If feelings of fear or anxiety are not products of circuits that control defensive behavior, studies of defensive behavior in animals will be of limited value in finding medications that can relieve feelings of fear and anxiety in people. Once this is realized, animal research can be viewed in a more realistic light as being relevant to a particular subset of symptoms in anxiety disorders (those arising from subcortical circuits). While it may never be possible to directly study conscious states in animals, animal research, especially nonhuman primate research, may nevertheless be able to make some indirect contributions by revealing neural underpinnings of cognitive processes, such as attention and working memory, that contribute to conscious experiences, including conscious feelings, in humans.
In sum, being clearer about when research is relevant to feelings of fear and anxiety, as opposed to defensive behavior and physiological responses, should enhance the translational impact of animal research. In particular, it will clarify when these two sets of phenomena show overlapping and distinct associations with clinical variables.
Implications for Stalled Progress in Therapeutics
The two-system framework offers a path forward for finding better medications and psychotherapies. We focus on medication development, given recent discussions about the poor outcome of this effort (1). In spite of major expenditures over several decades, the results have been disappointing (2). The pharmaceutical industry has concluded that the probability of discovering new treatments is low (2, 110), and it has curtailed investment in future psychotropic medication discovery.
To better understand the problem, consider how novel agents are tested for anxiolytic properties. The usual approach is to study effects on the behavioral responses of rodents in challenging situations. For example, rodents tend to avoid brightly lit, open areas where they can be easily seen, captured, and eaten. If a novel compound leads animals to spend more time in open areas or other threatening situations, it becomes a candidate for treating anxiety in people. While new medications have emerged using this strategy, they inconsistently reduce the feelings of fear or anxiety that cause people to seek help.
We argue that the disappointment in the results reflects two faulty assumptions: 1) that a common circuit underlies the expression of defensive responses and feelings of fear when threatened, and 2) that the circuits that contribute to defensive responses in animals can be used to determine how the human brain gives rise to feelings of fear and anxiety.
If, as we argue, feelings of fear and anxiety arise from a different system than do defensive behaviors, medications that make rats or mice less defensive, avoidant, and/or physiologically aroused in challenging situations will not necessarily make people feel less fearful or anxious. Incomplete response to benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), or other anxiolytics in humans, we propose, may reflect the actions of these medications in the two systems. While these compounds can act on both cortical and subcortical brain circuits (111–114), the key question is the extent to which the clinical efficacy of a given medication reflects changes in a particular circuit.
In general, clinicians judge whether therapy is succeeding on the basis of the degree to which the patient reports feeling better (115). The expectation that medications that decrease defense and avoidance in animals should also make people feel less anxiety needs reevaluation, as new medications are not meeting this expectation. This distinction can be characterized as one between the effects of medication on behavioral inhibition on the one hand (116) and anxious feelings on the other (5). While behavioral inhibition is often used as if it were synonymous with “anxiety,” the two-system framework treats behavioral inhibition as separable from anxious feelings.
Available research demonstrates the importance of this distinction. For example, efforts generally have failed to translate a replicable pattern of behavioral and physiological findings in rodents over 20 years with corticotropin-releasing hormone (CRH) receptor manipulations (117), even though CRH antagonists engage the CRH receptor in humans (118). CRH antagonists reduce defensive behaviors and associated physiological responses in animals, but they result in minimal clinical effect in humans, a finding that is consistent with predictions from the two-system perspective. Future research may clarify the extent to which different medications affect the two systems, as well as the degree to which partial response reflects an effect on behaviors, as opposed to conscious feelings, but this is not known at present.
Medications such as SSRIs and benzodiazepines can make some people feel less anxious. But it is important to ask whether this effect is due specifically to a change in anxiety itself. Might the positive effects on anxiety be part of a general blunting of emotion experience? And given that emotions, in our theory, are cognitively assembled states, to what extent are the effects of medications on anxiety due to changes in cognitive underpinnings, such as alterations in attention and memory (including working and long-term memory), rather than in anxious feelings themselves? If the anxiolytic effects of a medication are due to general emotional blunting or to impaired cognitive processing, the term anxiolytic would be a misnomer as a characterization of its treatment properties. While such indirect changes in anxious feelings are certainly useful clinically, progress in developing improved treatments would be facilitated by recognition of the exact ways in which the effect is achieved.
It is worth considering whether it is even possible to develop a pharmaceutical treatment that would specifically target anxious feelings. Because of the diverse effects of the medications, it may be difficult to do so without producing effects on other phenomena, such as feelings of sadness or worthlessness, or cognitive processes such as attention and memory. Existing medications are blunt tools with broad effects. But even with improved specificity, it may not be possible to alter specific feelings. If fear, anxiety, and other emotions are indeed cognitively assembled states that emerge from information integration, rather than being an innate experience prewired into subcortical neural circuits, there is no fear or anxiety locus to target, and the changes in fear and anxiety may always be at the expense of emotional or cognitive capacities.
Medications that fail to reduce feelings of fear or anxiety may nevertheless have beneficial effects by reining in defensive systems that are overly sensitive and are producing exaggerated defensive reactions, excessive avoidance, hyperarousal, hypervigilance, and so forth. The two-system approach suggests that a detailed understanding of neural circuits and their functions can help set realistic expectations about what medications might achieve.
Improving Treatment Outcomes
We suggest two paths for improving treatment outcomes. One involves adapting existing treatments to increase their impact, and the other, tailoring treatments to particular patients. The two paths are complementary, and each informs both medication and psychotherapy.
Adapting Treatments to Increase Their Effectiveness
The impact of current treatments might be increased through adaptations informed by basic science. For example, because exposure during cognitive-behavioral therapy (CBT) shares features with laboratory studies of extinction, findings about extinction in animals inform attempts to adapt CBT. This approach has been used to suggest adaptations of CBT through the use of partial reinforcement, exposure in multiple contexts, and particular timing parameters for exposures (5, 119–128). Similarly, medications that facilitate extinction in animals have been used to enhance initial changes in defensive responding that are achieved during a therapy session. This has been attempted using manipulations of glutamate, cortisol, and noradrenaline, with d-cycloserine being studied most extensively. While initial results were promising (129), later work has been inconsistent, with no overall benefits in aggregated results from the first 21 studies (130). A variable clinical effect could reflect influences of avoidance (131), levels of anxiety evoked during exposure (132), postexposure consolidation (120), or the use of particular outcome measures to draw conclusions on efficacy. Different conclusions might arise from measures that more completely dissociate effects on subjective feelings and behavioral or physiological responses to threats. Even if d-cycloserine turns out not to be as useful as initially hoped, the innovative conception underlying the work can be used to test other compounds that might improve CBT outcomes.
Other attempts to find more effective treatments might begin with differential targeting of one system while observing changes in the other. Some novel treatments may focus specifically on circuits that operate nonconsciously, using procedures such as subliminal exposures or manipulations of attention, which information technologies make increasingly possible (5). Indeed, subliminal extinction (133) may prove to be a way to expose patients to threats without inducing excessive feelings of fear. Attention bias modification treatment is another procedure with similar features in that it also acts on rapidly deployed processes and may remain independent of awareness (107). These and other treatments could weaken automatic deployment of nonconscious threat-processing systems, which in some patients could malfunction and inappropriately initiate defensive behavior and physiological arousal. Through indirect influences on cortical circuitry, this could have some partial effect on the conscious experience of fear or anxiety, much as a patient may partially respond to an SSRI. Other treatments, especially psychotherapeutic ones, then might target the conscious experience of fear and anxiety. Observing the way particular treatments targeting rapidly deployed circuits lead to other changes in the brain, in behavior, and in feelings could generate an iterative process whereby novel treatments are continually refined.
Alternatively, treatments might focus initially on circuits that are more closely related to conscious feeling states. For example, some patients may exhibit aberrant stimulus generalization and fail to recognize or appropriately label the boundaries separating different gradations of threat, leading them to inappropriately label ambiguous stimuli as threatening. In such patients, treatment might begin by slowly and carefully teaching them to improve their ability to recognize, describe, and label these nuanced boundaries (107, 119). With repeated experience, these new capacities might only later come to change activity in systems supporting rapid defensive responding. Thus, a novel treatment might target either of the two systems in an attempt to change the other.
Finally, the two-system perspective informs developmental perspectives. Longitudinal research shows that chronic anxiety in adults typically begins in childhood (107). However, many affected children mature to become healthy adults. The two-system perspective suggests that differential forms of brain maturation account for diverging developmental profiles, a suggestion with preliminary support from cross-sectional brain imaging studies (134). For example, cortical brain systems supporting cognitive processes related to feeling states mature later than subcortical circuitry. As a result, adolescents who overcome problems with anxiety may exhibit different patterns of interactions between the two sets of circuits, as compared with adolescents who mature to become adults with anxiety disorders. Adolescents who fail to overcome their problems could benefit from treatments that teach them how to deploy these same cortical systems in a mature fashion. Alternatively, differences in adolescent compared with adult circuitry may be identifiable in a subset of anxious individuals in adolescence. In some, one pattern of alterations in the two systems may predict chronicity, whereas in others a different pattern may predict remission. Thus, as studies map the ways in which particular treatments shape the two systems, it might be possible to tailor treatments in children according to the developmental status of the two systems and their subsystems.
Tailoring Treatments to Specific Patients
Basic science findings suggest individual differences in defensive reaction (freezing) and action (avoidance) (135–137) and underlying circuitry (138, 139). This suggests that it may be possible to tailor treatments to the specific needs of individual patients based on their symptoms.
Building on progress in using functional imaging to identify threat processing circuitry in humans (see earlier discussion), recent studies have helped illuminate how subcortical, amygdala-based circuits shape attention (56, 140–142). Forms of psychotherapy that emphasize reappraisal or other effortful, consciously deployed strategies in the service of emotion regulation may not be ideal for patients who exhibit hyperactivity in the subcortical circuitry at baseline. For these patients, their dysfunction may lie in the more rapidly deployed subcortical circuitry, and such patients could be guided toward other treatments, including medications or psychotherapies that target the subcortical defensive circuits.
On the other hand, anxious patients with standard activity profiles in subcortical circuitry but with altered activity profiles in cortical circuitry underlying working memory might be selected to receive therapies that emphasize reappraisal and similar top-down strategies as a first line of treatment. Indeed, imaging methods can quantify dysfunctions in cortical to more slowly evolving processes, involving appraisal of feeling states (134, 143–145). Here, perturbed activity in circuitry within the prefrontal cortex may identify patients who are particularly likely to benefit from techniques that teach patients how to regulate emotion through reappraisal and other effortful, consciously deployed cognitive strategies (146). In this way, the two-system perspective may allow clinicians to tailor particular treatments to perturbed functions in circuits that control immediate behavior as opposed to conscious feeling states. Reappraisal can in fact be used to change either the self-reported emotional experience (147) or autonomic consequences of amygdala activation (148), depending on the approach used.
Improvements in brain imaging methods are providing techniques that are increasingly capable of quantifying functions in distributed neural circuits (149, 150). Research using these approaches suggests that imaging-based biomarkers predict treatment outcomes in different ways than do clinical factors. As such, imaging-based biomarkers could allow a form of precision medicine in psychiatry in which treatments are delivered to particular patients based on the degree to which the treatment targets a particular form of circuitry dysfunction. The two-system perspective suggests how this advanced approach could be adapted to link particular functions to distinct aspects of threat responding. With this approach, some aspects of brain function could be linked to treatment-related changes in the immediate behavioral response, whereas other aspects of brain function could be linked to treatment-related changes in fearful and anxious feelings.
Conclusions
Progress has stalled in treatment development for mental disorders. Cross-species conservation of brain-behavior relationships occurring in the presence of threats provides unique opportunities and creates the potential for mutually informative basic and clinical research in the service of treatment development for anxiety disorders. However, current approaches fail to recognize adequately the distinctions between neural circuitry supporting subjective feeling states as opposed to defensive responding. Failure to do so prevents the field from leveraging cross-species conservation of threat-processing circuits. The two-system framework we propose distinguishes neural circuitry supporting defensive responding from circuitry supporting feeling states and provides a new heuristic for basic, clinical, and translational research on anxiety disorders.
1 : Psychiatric drug development: diagnosing a crisis. Cerebrum, Mar-Apr 2013 (http://www.dana.org/Cerebrum/2013/Psychiatric_Drug_Development__Diagnosing_a_Crisis/)Medline, Google Scholar
2 : 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 2013; 12:667–687Crossref, Medline, Google Scholar
3 : Rethinking the emotional brain. Neuron 2012; 73:653–676Crossref, Medline, Google Scholar
4 : Coming to terms with fear. Proc Natl Acad Sci USA 2014; 111:2871–2878Crossref, Medline, Google Scholar
5 : Anxious: Using the Brain to Understand and Treat Fear and Anxiety. New York, Viking, 2015Google Scholar
6 : On the concepts of fear. Harv Rev Psychiatry 1995; 3:231–234Crossref, Medline, Google Scholar
7 : Understanding the effects of temperament, anxiety, and guilt. Panel: The Affect of Emotions: Laying the Groundwork in Childhood. Washington, DC, Library of Congress/NIMH Decade of the Brain Project, Jan 3, 2003 (http://www.loc.gov/loc/brain/emotion/Kagan.html)Google Scholar
8 : Of mice and men: natural kinds of emotions in the mammalian brain? A response to Panksepp and Izard. Perspect Psychol Sci 2007; 2:297–312Crossref, Medline, Google Scholar
9 : The ecology of human fear: survival optimization and the nervous system. Front Neurosci 2015; 9:55Crossref, Medline, Google Scholar
10 : The Expression of the Emotions in Man and Animals. London, Fontana Press, 1872Crossref, Google Scholar
11 Ekman P: Universals and cultural differences in facial expressions of emotions, in Nebraska Symposium on Motivation. Edited by Cole J. Lincoln, University of Nebraska Press, 1972, pp 207–283Google Scholar
12 : The triune brain, emotion, and scientific bias, in The Neurosciences: Second Study Program. Edited by Schmitt FO. New York, Rockefeller University Press, 1970, pp 336–349Google Scholar
13 : Affective Neuroscience. New York, Oxford University Press, 1998Google Scholar
14 : The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992; 15:353–375Crossref, Medline, Google Scholar
15 : The Emotional Brain. New York, Simon and Schuster, 1996Google Scholar
16 : The basic neuroscience of emotional experiences in mammals: the case of subcortical FEAR circuitry and implications for clinical anxiety. Appl Anim Behav Sci 2011; 129:1–17Crossref, Google Scholar
17 : The neuroscience of mammalian associative learning. Annu Rev Psychol 2005; 56:207–234Crossref, Medline, Google Scholar
18 : Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res 2006; 156:3–29Crossref, Medline, Google Scholar
19 : The biology of fear. Curr Biol 2013; 23:R79–R93Crossref, Medline, Google Scholar
20 : What can ethobehavioral studies tell us about the brain’s fear system? Trends Neurosci 2016; 39:420–431Crossref, Medline, Google Scholar
21 : The many paths to fear. Nat Rev Neurosci 2012; 13:651–658Crossref, Medline, Google Scholar
22 : Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16:317–331Crossref, Medline, Google Scholar
23 : Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J Neurosci 2015; 35:3470–3477Crossref, Medline, Google Scholar
24 : Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 1993; 163:109–113Crossref, Medline, Google Scholar
25 : Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 2006; 16:723–727Crossref, Medline, Google Scholar
26 : The contextual brain: implications for fear conditioning, extinction, and psychopathology. Nat Rev Neurosci 2013; 14:417–428Crossref, Medline, Google Scholar
27 : Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63:129–151Crossref, Medline, Google Scholar
28 : Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 2006; 57:27–53Crossref, Medline, Google Scholar
29 : Fear, faces, and the human amygdala. Curr Opin Neurobiol 2008; 18:166–172Crossref, Medline, Google Scholar
30 : Amygdala automaticity in emotional processing. Ann N Y Acad Sci 2003; 985:348–355Crossref, Medline, Google Scholar
31 : Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65:568–576Crossref, Medline, Google Scholar
32 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
33 : Neuroimaging in social anxiety disorder: a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 2014; 47:260–280Crossref, Medline, Google Scholar
34 : Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress Anxiety 2015; 32:656–663Crossref, Medline, Google Scholar
35 : Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 2009; 3:33Crossref, Medline, Google Scholar
36 : Neuroimaging the temporal dynamics of human avoidance to sustained threat. Behav Brain Res 2013; 257:148–155Crossref, Medline, Google Scholar
37 : Overlapping neural systems mediating extinction, reversal, and regulation of fear. Trends Cogn Sci 2010; 14:268–276Crossref, Medline, Google Scholar
38 : Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 2008; 213:29–42Crossref, Medline, Google Scholar
39 : Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav Neurosci 2015; 129:673–678Crossref, Medline, Google Scholar
40 : The Neuropsychology of Anxiety, 2nd ed. Oxford, UK, Oxford University Press, 2000Google Scholar
41 : Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 2010; 68:416–424Crossref, Medline, Google Scholar
42 : Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 2013; 14:488–501Crossref, Medline, Google Scholar
43 : Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 2016; 21:450–463Crossref, Medline, Google Scholar
44 : From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350Crossref, Medline, Google Scholar
45 : Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem 2015; 22:417–425Crossref, Medline, Google Scholar
46 : Fear reduction and fear behavior: problems in treating a construct, in Research in Psychotherapy, vol 3. Edited by Schlien JM. Washington, DC, American Psychological Association, 1968, pp 90–103Crossref, Google Scholar
47 : Hypothetical constructs versus intervening variables: a reappraisal of the three-systems model of anxiety assessment. Behav Assess 1982; 4:347–358Google Scholar
48 : I. Synchrony and desynchrony in fear and avoidance. Behav Res Ther 1974; 12:311–318Crossref, Medline, Google Scholar
49 : Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci 2002; 14:709–720Crossref, Medline, Google Scholar
50 : Fear and panic in humans with bilateral amygdala damage. Nat Neurosci 2013; 16:270–272Crossref, Medline, Google Scholar
51 : Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct 2016; 221:1499–1511Crossref, Medline, Google Scholar
52 : The human amygdala and the induction and experience of fear. Curr Biol 2011; 21:34–38Crossref, Medline, Google Scholar
53 : Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry 2002; 52:927–937Crossref, Medline, Google Scholar
54 : Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 2007; 45:174–194Crossref, Medline, Google Scholar
55 : Human amygdala responsivity to masked fearful eye whites. Science 2004; 306:2061Crossref, Medline, Google Scholar
56 : Emotional automaticity is a matter of timing. J Neurosci 2010; 30:5825–5829Crossref, Medline, Google Scholar
57 : Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry 2008; 165:124–132Link, Google Scholar
58 : The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17:317–323Crossref, Medline, Google Scholar
59 : Awareness of emotional stimuli determines the behavioral consequences of amygdala activation and amygdala-prefrontal connectivity. Sci Rep 2016; 6:25826Crossref, Medline, Google Scholar
60 : Consciousness Lost and Found: A Neuropsychological Exploration. New York, Oxford University Press, 1997Google Scholar
61 : Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 2001; 124:1241–1252Crossref, Medline, Google Scholar
62 : Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci 2010; 11:697–709Crossref, Medline, Google Scholar
63 : I am blind, but I “see” fear. Cortex 2013; 49:985–993Crossref, Medline, Google Scholar
64 : Experimental and theoretical approaches to conscious processing. Neuron 2011; 70:200–227Crossref, Medline, Google Scholar
65 : Cognitive theories of consciousness, in The Cambridge Handbook of Consciousness. Edited by Zelazo PD, Moscovitch M, Thompson E. New York, Cambridge University Press, 2007, pp 177–205Crossref, Google Scholar
66 : Toward a cognitive neuropsychology of awareness: implicit knowledge and anosognosia. J Clin Exp Neuropsychol 1990; 12:155–178Crossref, Medline, Google Scholar
67 : Higher-order awareness, misrepresentation and function. Philos Trans R Soc Lond B Biol Sci 2012; 367:1424–1438Crossref, Medline, Google Scholar
68 : Introspective physicalism as an approach to the science of consciousness. Cognition 2001; 79:161–196Crossref, Medline, Google Scholar
69 : The social functions of consciousness, in Frontiers of Consciousness: Chichele Lectures. Edited by Weiskrantz L, Davies M. Oxford, UK, Oxford University Press, 2008, pp 225–244Crossref, Google Scholar
70 : The Conscious Brain: How Attention Engenders Experience. New York, Oxford University Press, 2012Crossref, Google Scholar
71 : Consciousness and the prefrontal parietal network: insights from attention, working memory, and chunking. Front Psychol 2012; 3:63Crossref, Medline, Google Scholar
72 : The attentional requirements of consciousness. Trends Cogn Sci 2012; 16:411–417Crossref, Medline, Google Scholar
73 : Working memory, neural basis, in MIT Encyclopedia of Cognitive Sciences. Edited by Wilson RA, Keil FC. Cambridge, Mass, MIT Press, 1999Google Scholar
74 : An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24:167–202Crossref, Medline, Google Scholar
75 : The cognitive neuroscience of working memory. Annu Rev Psychol 2015; 66:115–142Crossref, Medline, Google Scholar
76 : Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther 2007; 113:523–536Crossref, Medline, Google Scholar
77 : The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 2013; 14:143–152Crossref, Medline, Google Scholar
78 : Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex 2011; 21:1485–1497Crossref, Medline, Google Scholar
79 : Evidence for expansion of the precuneus in human evolution. Brain Struct Funct (Epub ahead of print, Jan 2, 2016)Google Scholar
80 : Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage 2015; 108:124–137Crossref, Medline, Google Scholar
81 : The human brain: rewired and running hot. Ann N Y Acad Sci 2011; 1225(suppl 1):E182–E191Crossref, Medline, Google Scholar
82 : Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain Behav Evol 2014; 84:156–166Crossref, Medline, Google Scholar
83 : An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain 2012; 135:1154–1164Crossref, Medline, Google Scholar
84 : How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70Crossref, Medline, Google Scholar
85 : An interoceptive neuroanatomical perspective on feelings, energy, and effort. Behav Brain Sci 2013; 36:685–686Crossref, Medline, Google Scholar
86 : Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Crossref, Medline, Google Scholar
87 : Panic, suffocation false alarms, separation anxiety, and endogenous opioids. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:603–612Crossref, Medline, Google Scholar
88 : Panic disorder: the psychobiology of external treat and introceptive distress. CNS Spectr 2008; 13:26–30Crossref, Medline, Google Scholar
89 : Methodologies for identifying the neural correlates of consciousness, in A Companion to Consciousness. Edited by Velmans M, Schneider S. Oxford, UK, Blackwell, 2007Crossref, Google Scholar
90 : The neural correlates of conscious experience: an experimental framework. Trends Cogn Sci 1999; 3:105–114Crossref, Medline, Google Scholar
91 : Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA 2006; 103:18763–18768Crossref, Medline, Google Scholar
92 : Causal role of prefrontal cortex in the threshold for access to consciousness. Brain 2009; 132:2531–2540Crossref, Medline, Google Scholar
93 : Empirical support for higher-order theories of conscious awareness. Trends Cogn Sci 2011; 15:365–373Crossref, Medline, Google Scholar
94 : The HOROR theory of phenomenal consciousness. Philos Stud 2015; 172:1783–1794Crossref, Google Scholar
95 : Unconscious activation of the cognitive control system in the human prefrontal cortex. J Neurosci 2007; 27:5805–5811Crossref, Medline, Google Scholar
96 : Working memory without consciousness. Curr Biol 2011; 21:R912–R913Crossref, Medline, Google Scholar
97 : Executive control over unconscious cognition: attentional sensitization of unconscious information processing. Front Hum Neurosci 2012; 6:61Crossref, Medline, Google Scholar
98 Bowerman M, Levinson SC (eds): Language Acquisition and Conceptual Development. Cambridge, UK, Cambridge University Press, 2001Crossref, Google Scholar
99 Kitayama S, Markus HR (eds): Emotion and Culture: Empirical Studies of Mutual Influence. Washington, DC, American Psychological Association, 1994Crossref, Google Scholar
100 : Emotion, language, and cultural scripts, in Emotion and Culture: Empirical Studies of Mutual Influence. Edited by Kitayama S, Markus HR. Washington, DC, American Psychological Association, 1994, pp 133–196Crossref, Google Scholar
101 : The construction of emotion in interactions, relationships, and cultures. Emot Rev 2012; 4:221–229Crossref, Google Scholar
102 : Fears, Phobias, and Rituals: Panic, Anxiety, and Their Disorders. New York, Oxford University Press, 1987Google Scholar
103 : The language of feeling and the feeling of anxiety: contributions of the behaviorisms toward understanding the function-altering effects of language. Psychol Rec 1996; 46:607–649Crossref, Google Scholar
104 : The paradigmatic behaviorism theory of emotions: basis for unification. Clin Psychol Rev 1990; 10:539–566Crossref, Google Scholar
105 : The Rise of Consciousness and the Development of Emotional Life. New York, Guilford, 2013Google Scholar
106 : Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne) 2014; 5:33Medline, Google Scholar
107 : Childhood antecedents and risk for adult mental disorders. Annu Rev Psychol 2015; 66:459–485Crossref, Medline, Google Scholar
108 : Developmental trends in the appraisal of anxiety-provoking situations. J Pers 1984; 52:46–57Crossref, Medline, Google Scholar
109 : Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J Child Psychol Psychiatry 2011; 52:1015–1025Crossref, Medline, Google Scholar
110 : Is pharma running out of brainy ideas? Science 2010; 329:502–504Crossref, Medline, Google Scholar
111 : Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 2016; 22:26–45Crossref, Medline, Google Scholar
112 : Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry 2006; 60:479–490Crossref, Medline, Google Scholar
113 : Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Hum Brain Mapp 2014; 35:646–658Crossref, Medline, Google Scholar
114 : Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neuroscience 1988; 25:771–795Crossref, Medline, Google Scholar
115 : Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety 2010; 27:417–425Crossref, Medline, Google Scholar
116 : The Neuropsychology of Anxiety. New York, Oxford University Press, 1982Google Scholar
117 : Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology 2012; 62:705–714Crossref, Medline, Google Scholar
118 : The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 2015; 40:1053–1063Crossref, Medline, Google Scholar
119 : Optimizing inhibitory learning during exposure therapy. Behav Res Ther 2008; 46:5–27Crossref, Medline, Google Scholar
120 : Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 2014; 58:10–23Crossref, Medline, Google Scholar
121 : A modern learning theory perspective on the etiology of panic disorder. Psychol Rev 2001; 108:4–32Crossref, Medline, Google Scholar
122 : Novelty-facilitated extinction: providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biol Psychiatry 2015; 78:203–209Crossref, Medline, Google Scholar
123 : Rethinking extinction. Neuron 2015; 88:47–63Crossref, Medline, Google Scholar
124 : Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 2009; 324:951–955Crossref, Medline, Google Scholar
125 : Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci USA 2013; 110:20040–20045Crossref, Medline, Google Scholar
126 : Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010; 463:49–53Crossref, Medline, Google Scholar
127 : Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010; 330:1108–1112Crossref, Medline, Google Scholar
128 : Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 2010; 35:136–146Crossref, Medline, Google Scholar
129 : Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 2006; 60:369–375Crossref, Medline, Google Scholar
130 : Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. Cochrane Database Syst Rev 2015; 5:CD007803Medline, Google Scholar
131 : Enhancement of psychosocial treatment with d-cycloserine: models, moderators, and future directions. Biol Psychiatry (Epub ahead of print, Sept 25, 2015)Google Scholar
132 : The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: the case of OCD and PTSD. Annu Rev Clin Psychol 2016; 12:1–28Crossref, Medline, Google Scholar
133 : What you cannot see can help you: the effect of exposure to unreportable stimuli on approach behavior. Conscious Cogn 2011; 20:173–180Crossref, Medline, Google Scholar
134 : Response to learned threat: an fMRI study in adolescent and adult anxiety. Am J Psychiatry 2013; 170:1195–1204Link, Google Scholar
135 : Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress 2007; 20:413–422Crossref, Medline, Google Scholar
136 : Heterogeneity in threat extinction learning: substantive and methodological considerations for identifying individual difference in response to stress. Front Behav Neurosci 2013; 7:55Crossref, Medline, Google Scholar
137 : Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues. Front Syst Neurosci 2014; 8:179Crossref, Medline, Google Scholar
138 : Active vs reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn Mem 2013; 20:446–452Crossref, Medline, Google Scholar
139 : Basal variability in CREB phosphorylation predicts trait-like differences in amygdala-dependent memory. Proc Natl Acad Sci USA 2013; 110:16645–16650Crossref, Medline, Google Scholar
140 : Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry 2013; 74:273–279Crossref, Medline, Google Scholar
141 : Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol 2013; 92:492–512Crossref, Medline, Google Scholar
142 : How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci 2005; 9:585–594Crossref, Medline, Google Scholar
143 : Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med 2013; 43:1475–1486Crossref, Medline, Google Scholar
144 : Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 2009; 66:275–285Crossref, Medline, Google Scholar
145 : Early childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evalution. Psychol Sci 2016; 27:821–835Crossref, Medline, Google Scholar
146 : Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry 2013; 70:1048–1056Crossref, Medline, Google Scholar
147 : The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Crossref, Medline, Google Scholar
148 : Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 2008; 59:829–838Crossref, Medline, Google Scholar
149 : Brain connectomics predict response to treatment in social anxiety disorder. Mol Psychiatry 2016; 21:680–685Crossref, Medline, Google Scholar
150 : Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 2013; 70:87–97Crossref, Medline, Google Scholar



