Identification of Two Heritable Cross-Disorder Endophenotypes for Tourette Syndrome
Abstract
Objective:
Phenotypic heterogeneity in Tourette syndrome is partly due to complex genetic relationships among Tourette syndrome, obsessive-compulsive disorder (OCD), and attention deficit hyperactivity disorder (ADHD). Identifying symptom-based endophenotypes across diagnoses may aid gene-finding efforts.
Method:
Assessments for Tourette syndrome, OCD, and ADHD symptoms were conducted in a discovery sample of 3,494 individuals recruited for genetic studies. Symptom-level factor and latent class analyses were conducted in Tourette syndrome families and replicated in an independent sample of 882 individuals. Classes were characterized by comorbidity rates and proportion of parents included. Heritability and polygenic load associated with Tourette syndrome, OCD, and ADHD were estimated.
Results:
The authors identified two cross-disorder symptom-based phenotypes across analyses: symmetry (symmetry, evening up, checking obsessions; ordering, arranging, counting, writing-rewriting compulsions, repetitive writing tics) and disinhibition (uttering syllables/words, echolalia/palilalia, coprolalia/copropraxia, and obsessive urges to offend/mutilate/be destructive). Heritability estimates for both endophenotypes were high and statistically significant (disinhibition factor=0.35, SE=0.03; symmetry factor=0.39, SE=0.03; symmetry class=0.38, SE=0.10). Mothers of Tourette syndrome probands had high rates of symmetry (49%) but not disinhibition (5%). Polygenic risk scores derived from a Tourette syndrome genome-wide association study (GWAS) were significantly associated with symmetry, while risk scores derived from an OCD GWAS were not. OCD polygenic risk scores were significantly associated with disinhibition, while Tourette syndrome and ADHD risk scores were not.
Conclusions:
The analyses identified two heritable endophenotypes related to Tourette syndrome that cross traditional diagnostic boundaries. The symmetry phenotype correlated with Tourette syndrome polygenic load and was present in otherwise Tourette-unaffected mothers, suggesting that this phenotype may reflect additional Tourette syndrome (rather than OCD) genetic liability that is not captured by traditional DSM-based diagnoses.
Tourette syndrome is highly heritable (1), yet etiological and phenotypic heterogeneity, including high rates of co-occurrence with attention deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) (2–4), has hampered gene-finding efforts (5–7). Decreasing the observed phenotypic heterogeneity, for example using latent variable modeling, may improve efforts to clarify Tourette syndrome pathophysiology and genetic architecture by identifying more homogeneous Tourette-related endophenotypes (8). In particular, the simultaneous use of distinct and complementary latent modeling approaches, such as exploratory factor analysis, to identify subsets of symptoms that group together, and latent class analysis, to identify subgroups of individuals based on patterns of symptom expression, can provide convergent evidence in support of a novel phenotypic subtype (endophenotype) that might better capture the underlying genetic liability and/or neural circuitry of complex disorders such as Tourette syndrome (9).
Only a few studies have previously used these multivariate methods to explore Tourette-associated symptom patterns (10–15). These studies identified three to five symptom groups, including simple tics, complex tics, compulsive behaviors (or OCD), aggressive behaviors (or ADHD), and self-injurious behaviors (summarized in reference 16). However, prior studies have been small and have not used the full range of potentially relevant symptoms (16). In addition, few examined familial patterns of the identified factors, and no information exists about the heritability of the derived symptom subtypes or their genetic relationships to categorical Tourette syndrome and OCD diagnoses (16). Thus, the aims of this study were to 1) identify and characterize unique Tourette-related endophenotypes with tic, OCD, and ADHD symptom data using exploratory factor and latent class analyses in a large sample of Tourette-affected families, 2) replicate the identified factors and latent classes in an independent sample, and 3) estimate their prevalence in parents of Tourette-affected probands, heritability, as well as their Tourette- and OCD-associated polygenic burden to determine their utility for genetic studies of Tourette syndrome and/or OCD.
Method
Samples
The discovery sample included 3,850 individuals from 1,365 families collected by the Tourette Syndrome Association International Consortium for Genetics for genetic studies between 1992 and 2011. Participants were referred from Tourette syndrome specialty clinics in the United States, Canada, Great Britain, and the Netherlands and from the U.S. Tourette Association of America (formerly the Tourette Syndrome Association). Probands were defined as the first identified Tourette-affected individual in the family. Additional family members were subsequently recruited and assessed. Inclusion criteria for probands were as follows: age ≥6 years, established Tourette syndrome diagnosis, and availability of living parents for family-based genetic analyses. Exclusion criteria included intellectual disability and tics caused by neurologic disorders other than Tourette syndrome. For children, the parents and children were interviewed jointly or separately, depending on the family’s preference. For adults, the parents were interviewed whenever possible to corroborate data. Families were ascertained as affected sib-pairs (two or more Tourette-affected siblings plus parents; N=283) or trio families (Tourette-affected individuals plus both parents; N=1,082). The sib-pair families analyzed in our previous diagnosis-based latent class analysis (11) are included in the current study. Due to the design of the original genetic study, sib-pair families were excluded at the time of enrollment if both parents had chronic tics or OCD. No such exclusions were made for trio families. Probands were excluded from analysis if they were missing all responses for any diagnostic subset of items (e.g., all responses to tic, OCD, or ADHD symptom items). Family members were excluded if they were missing all responses regarding tics. Additionally, cases were excluded if family role data was missing (e.g., it was unknown whether an individual was a proband or parent) or symptom data were not verified by clinicians (the final N for each analysis is shown in Table ST1 in the data supplement accompanying the online version of this article). Missing data patterns did not differ by site.
A replication sample derived from the same sources under the same protocol was used for independent replication of the findings. This sample included 882 individuals from 565 families collected between 2003 and 2013.
All participants provided written informed consent (parental consent and written assent were obtained for individuals under 18). This study was approved by the institutional review boards of all participating sites.
Procedure
Research staff administered clinical assessments using a standardized protocol. Demographic data and tic, OCD, and ADHD symptoms were assessed using a comprehensive, validated semistructured interview, the Tic and Comorbid Symptom Inventory (6, 17) (items used in this study are provided in Table ST2 in the online data supplement). Psychiatric diagnoses were validated using a best-estimate process (shown in Appendix SA1 in the online data supplement) (18).
Statistical Analyses
Descriptive statistical analyses were calculated using SPSS version 19 (IBM, Armonk, N.Y.). MPlus version 7.1 was used for latent variable modeling (19). PLINK was used to generate polygenic risk scores (20). Statistical analyses were conducted with R (v2.1). The R package “stats” (lm) was used to calculate R2 reported for polygenic risk score analyses. Given the fact that the majority of tests were highly correlated with one another, a strict Bonferroni correction would be overly conservative. Thus, significance for the analyses to characterize the latent classes and heritabilities was conservatively set at p<0.005 to account for multiple testing. Because the polygenic burden analyses were exploratory in nature, they were not corrected for multiple testing.
Exploratory factor analyses.
Exploratory factor analyses were conducted on symptom data using robust weighted least squares estimation, as recommended for dichotomous variables (21), and oblique rotation (geomin). Data were limited to the probands to examine independent cases. The factor solution was chosen based on the following criteria: “elbow” of the scree plot, eigenvalue >1, clinical interpretability, the presence of minimal cross-loading (i.e., a single item loading on ≥1 factor at ≥0.40), and fit statistics (see Appendix SA2 in the online data supplement) (22–24). Within each factor model, items were retained if factor loadings were ≥0.40; items that loaded ≥0.40 on two factors were retained on both. Items with loadings <0.40 were excluded from the final model. Items that failed to load on any factor were excluded. Cronbach’s alpha was calculated for each factor.
Latent class analyses.
Models with 2–6 classes were fit in the probands and replicated with probands plus family members for heritability analyses. The lowest Bayesian information criterion and results of the Lo, Mendel, and Rubin likelihood ratio test were used to determine the number of classes to retain (24). Specifically, the lowest Bayesian information criterion and a significant likelihood ratio test (p<0.05) were used to indicate good fit. If these criteria left the model choice unclear, the clinical interpretability of the solutions was examined (i.e., if clinically relevant patterns distinguished the classes in one solution but not another). Classes were labeled according to the group of symptoms that individuals in the class endorsed with a high frequency. We compared the rates of psychiatric comorbidity and rates of parents in each class using the auxiliary variable function of MPlus.
Replication sample analyses.
Confirmatory factor analysis employing a robust weighted least squares estimator was used to examine whether the best factor model fit the replication data among probands. Goodness of fit was evaluated using the comparative fit index, the nonnormed fit index, and the root mean square error of approximation (25). To replicate the latent class results, we followed the same procedures used with the discovery sample.
Heritability estimates.
Heritability estimates were calculated for factor sum scores and class membership within the discovery sample using the Sequential Oligogenic Linkage Analysis Routine (SOLAR) statistical package (26). SOLAR employs a variance component approach and calculates kinship coefficients using information from all available family members across generations. Families were included only if a proband was present. Although the majority of the families consisted of parent-child trios, some with additional affected siblings, there were also 91 unaffected siblings, and 26 families had extended family members (including grandparents, uncles or aunts, and cousins). However, we note that, because most were nuclear families, we cannot with confidence separate shared genetic from shared environmental effects in these analyses. SOLAR automatically corrects for proband status to minimize potential ascertainment bias. Age, sex, and the sex-by-age interaction were used as covariates. To allow for generalization of the data and for the heritability analyses, mean factor sum scores were calculated for each participant by dividing the number of items the individual endorsed by the total number of items answered in the factor (27). We inverse-normalized all mean sum scores because of the skewed distribution of the raw data. Because the probability distributions from the latent class analyses (i.e., probabilities that an individual will belong to each class from 0 [no probability] to 1 [100% probability]) approximated a binary distribution, we assigned each individual to his or her most likely class. Class membership was categorical and mutually exclusive.
Polygenic burden analyses.
Polygenic burden analyses were conducted in the sample of probands who had both detailed phenotype data and genotype data to test for associations between multiple genes of small effect implicated in Tourette syndrome, OCD, or ADHD pathogenesis from GWAS data (28–31) and phenotypes of interest identified from the latent variable modeling (see Appendix SA3 in the online data supplement). The polygenic risk score was calculated as the sum of the number of risk alleles at each locus weighted by the allele effect size estimated from the GWAS of the discovery sample. The single-nucleotide polymorphisms (SNPs) used in polygenic risk analyses were linkage disequilibrium pruned (r2<0.2), and their GWAS p values passed predetermined significance thresholds (p<0.01, 0.1, 0.2, 0.3, 0.4, and 0.5). A cross-validation approach was used in calculating the Tourette syndrome polygenic risk score to avoid overfitting. The factor sum scores for the phenotype of interest in the target sample were regressed on the polygenic risk scores and potential confounders (principal component factors that capture the population stratification and genotyping/imputation platforms) to assess the association between the novel phenotypes identified by the factor and/or latent class analyses and genomic variants implicated in Tourette syndrome, OCD, or ADHD risk (in aggregate) from GWAS.
Results
Sample Characteristics
The final discovery sample included 1,191 probands (254 from sib-pair families, 937 from trios) and 3,494 total participants (1,147 from sib-pair families and 2,347 from trios) (Table 1).
| Original Sample | Validation Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Probands Only (N=1,191) | Probands and Family Members (N=3,494) | Probands Only (N=527) | Probands and Family Members (N=882) | ||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age (years) | 1,191 | 15.3 | 10.0 | 3,494 | 30.6 | 17.2 | 522 | 21.4 | 15.6 | 864 | 30.0 | 17.5 |
| Tourette syndrome | ||||||||||||
| Age at onset (years) | 1,131 | 5.8 | 2.5 | 1,692 | 6.1 | 2.7 | 521 | 6.2 | 2.8 | 569 | 6.3 | 2.8 |
| Severitya | 1,181 | 11.4 | 2.6 | 3,490 | 4.1 | 4.6 | 478 | 11.7 | 2.5 | 764 | 7.9 | 5.6 |
| OCD | ||||||||||||
| Age at onset (years) | 442 | 7.1 | 4.0 | 712 | 8.2 | 5.3 | 249 | 7.4 | 3.8 | 299 | 8.0 | 4.4 |
| Severitya | 694 | 4.3 | 3.4 | 2,442 | 3.0 | 3.3 | 450 | 5.0 | 3.9 | 681 | 3.9 | 3.8 |
| Total N | N | % | Total N | N | % | Total N | N | % | Total N | N | % | |
| Male | 1,191 | 944 | 79.3 | 3,494 | 2,140 | 61.2 | 527 | 408 | 77.4 | 882 | 581 | 65.9 |
| Parental history of Tourette syndrome or chronic motor or vocal tic disorder | 864 | 385 | 44.6 | — | — | — | — | — | — | — | — | — |
| Personal history | ||||||||||||
| OCD | 1,135 | 570 | 50.2 | 3,286 | 1,125 | 34.2 | 504 | 219 | 43.5 | 847 | 336 | 39.7 |
| ADHD | 1,116 | 628 | 56.3 | 3,220 | 1,013 | 31.5 | 494 | 181 | 36.6 | 830 | 293 | 35.3 |
| Mood disorders | 498 | 132 | 26.5 | 1,603 | 487 | 30.4 | — | — | — | — | — | — |
| Anxiety disorders | 507 | 176 | 34.7 | 1,620 | 515 | 31.8 | — | — | — | — | — | — |
| Disruptive behavior disorders | 390 | 121 | 31.0 | 662 | 192 | 29.0 | — | — | — | — | — | — |
TABLE 1. Characteristics of Tourette-Affected Probands and Their Family Members
Exploratory Factor Analyses
We fit exploratory factor models with up to 10 factors using all 126 tic, OCD, and ADHD items simultaneously (see Figure SF1 in online data supplement). The 8-factor model demonstrated the best fit (see Table ST2 and Figure SF1 in online data supplement), but substantial cross-loading on this, as well as other higher-factor models, cast doubt on the stability of this model. Since the ADHD items consistently cross-loaded on all models tested, we subsequently fit factor models using the 108 tic and OCD items only. The 4-factor model demonstrated the best fit (Table 2; see also Tables ST3 and ST4 and Figure SF1 in online data supplement), with tics as factor 1, obsessive-compulsive symptoms as factor 2, disinhibited symptoms as factor 3, and symmetry symptoms as factor 4. The first factor includes most simple and complex tics as well as the OCD item “needs to touch, tap, or rub things.” The disinhibited factor (factor 3) includes rude or obscene gestures and words, echolalia, palilalia, and animal/bird noises, plus hoarding and OCD items regarding urges to harm or offend. The symmetry factor (factor 4) includes tic items on repeatedly writing things and slower movements and OCD items on repeating routines, ordering, evening up, and symmetry. The obsessive-compulsive symptoms factor (factor 2) contains the remainder of the OCD items. Fifteen items (7 OCD, 8 tics) failed to load on any of the factors. The internal consistency was good for each factor (Cronbach’s alpha was 0.77 to 0.92).
| Factor Loading | |||||
|---|---|---|---|---|---|
| Category | Item | Factor 1: Tics (Cronbach’s alpha=0.88) | Factor 2: Obsessive-Compulsive Symptoms (Cronbach’s alpha=0.92) | Factor 3: Disinhibition (Cronbach’s alpha=0.77) | Factor 4: Symmetry Symptoms (Cronbach’s alpha=0.87) |
| Tourette | Lift chin | 0.67 | 0.02 | –0.09 | –0.06 |
| Tourette | Knee-bending | 0.66 | 0.02 | 0.07 | 0.14 |
| Tourette | Teeth bearing | 0.63 | 0.10 | 0.07 | –0.17 |
| Tourette | Touch chin to shoulder | 0.60 | 0.04 | –0.08 | –0.08 |
| Tourette | Tensing buttocks | 0.58 | 0.02 | –0.16 | 0.18 |
| Tourette | Deep knee bending | 0.57 | –0.14 | 0.19 | 0.28 |
| Tourette | Quick eye turn | 0.56 | 0.13 | –0.07 | –0.04 |
| Tourette | Tensing abdomen | 0.56 | –0.03 | –0.15 | 0.12 |
| Tourette | Lip pouting | 0.56 | 0.13 | –0.04 | –0.11 |
| Tourette | Rolling eyes to one side | 0.55 | 0.02 | –0.10 | –0.01 |
| Tourette | Broadening nostrils | 0.55 | 0.20 | –0.22 | –0.08 |
| Tourette | Smiling | 0.54 | 0.07 | 0.03 | –0.04 |
| Tourette | Opening eyes wide | 0.52 | 0.10 | –0.10 | 0.01 |
| Tourette | Flexing or extending ankle | 0.52 | 0.12 | –0.13 | 0.07 |
| Tourette | Kicking | 0.52 | –0.01 | 0.32 | 0.03 |
| Tourette | Sticking tongue out | 0.51 | 0.06 | 0.24 | –0.13 |
| Tourette | Shrugging shoulders | 0.51 | 0.00 | 0.00 | –0.07 |
| Tourette | Nose twitching | 0.50 | 0.06 | –0.18 | –0.06 |
| Tourette | Squatting | 0.49 | –0.17 | 0.19 | 0.29 |
| Tourette | Biting tongue | 0.48 | 0.13 | –0.03 | 0.02 |
| Tourette | Touching | 0.48 | –0.14 | 0.22 | 0.35 |
| Tourette | Squinting | 0.48 | 0.13 | –0.15 | –0.01 |
| Tourette | Tapping | 0.48 | –0.07 | 0.12 | 0.26 |
| Tourette | Jerking shoulder | 0.47 | 0.00 | –0.05 | 0.02 |
| Tourette | Counting with fingers | 0.46 | 0.02 | 0.03 | 0.20 |
| Tourette | Bending or gyrating | 0.45 | –0.03 | 0.31 | 0.07 |
| Tourette | Pulling back pencil | 0.43 | –0.02 | 0.13 | 0.17 |
| Tourette | Throwing head back | 0.43 | 0.00 | –0.09 | 0.04 |
| Tourette | Skipping | 0.42 | –0.08 | 0.28 | 0.05 |
| Tourette | Chewing on lip | 0.42 | 0.15 | –0.04 | –0.11 |
| Tourette | Unusual postures | 0.42 | –0.03 | 0.28 | 0.09 |
| Tourette | Slower movements | 0.41 | –0.02 | 0.20 | 0.41 |
| OCD | Needs to touch, tap, or rub things | 0.41 | 0.13 | 0.14 | 0.39 |
| Tourette | Eye blinking | 0.41 | 0.15 | –0.27 | –0.04 |
| Tourette | Syllables | 0.40 | –0.16 | 0.40 | 0.05 |
| OCD | Contamination concern | 0.00 | 0.77 | –0.23 | –0.05 |
| OCD | Hoarding obsessions | –0.24 | 0.76 | 0.43 | –0.01 |
| OCD | Illness concern | 0.04 | 0.73 | –0.13 | 0.02 |
| OCD | Hoarding compulsions | –0.21 | 0.70 | 0.42 | –0.03 |
| OCD | Dirt or germ obsessions | –0.04 | 0.70 | –0.16 | 0.08 |
| OCD | Fears harming others if not careful | 0.03 | 0.66 | 0.14 | 0.07 |
| OCD | Fears responsible for something terrible | 0.03 | 0.65 | 0.05 | 0.19 |
| OCD | Thoughts may influence the outcome of some events if does certain things | –0.02 | 0.63 | –0.09 | 0.32 |
| OCD | Checks no self-harm | 0.03 | 0.62 | 0.08 | –0.08 |
| OCD | Fears harming others | 0.08 | 0.62 | 0.01 | 0.09 |
| OCD | Fears losing things | –0.02 | 0.62 | 0.10 | 0.18 |
| OCD | Contamination compulsions | 0.08 | 0.61 | –0.12 | 0.04 |
| OCD | Contamination obsessions | 0.22 | 0.61 | –0.08 | 0.03 |
| OCD | Checks nothing terrible | 0.05 | 0.60 | 0.11 | –0.02 |
| OCD | Fears will steal things | 0.06 | 0.60 | 0.22 | –0.16 |
| OCD | Religious obsessions | 0.06 | 0.60 | 0.03 | 0.06 |
| OCD | Concerned with bodily waste | 0.05 | 0.59 | –0.03 | 0.09 |
| OCD | Morality obsessions | 0.03 | 0.58 | –0.08 | 0.20 |
| OCD | Compulsions to prevent harm | 0.25 | 0.57 | –0.03 | 0.10 |
| OCD | Urges to offend | –0.05 | 0.57 | 0.56 | –0.10 |
| OCD | Checks no harm | 0.07 | 0.57 | 0.08 | 0.12 |
| OCD | Violent obsessions | 0.02 | 0.56 | 0.13 | 0.08 |
| OCD | Fears self-harm | 0.19 | 0.56 | –0.03 | 0.04 |
| OCD | Thoughts can influence the outcome of some events if does certain things | –0.02 | 0.54 | –0.10 | 0.33 |
| OCD | Mental rituals | –0.04 | 0.53 | 0.00 | 0.34 |
| OCD | Fears acting on an unwanted impulse | 0.09 | 0.53 | 0.29 | 0.12 |
| OCD | Self-harm urges | 0.11 | 0.52 | 0.35 | 0.01 |
| OCD | Superstitious fears | 0.02 | 0.50 | –0.14 | 0.35 |
| OCD | Sexual obsessions | 0.13 | 0.49 | 0.18 | 0.14 |
| OCD | Need to confess | 0.00 | 0.49 | 0.12 | 0.18 |
| OCD | Animal obsessions | –0.08 | 0.48 | 0.00 | 0.12 |
| OCD | Need to explore surroundings | –0.14 | 0.46 | 0.42 | 0.03 |
| OCD | Concerned with a part of body or appearance | 0.02 | 0.45 | 0.05 | 0.20 |
| OCD | Superstitious behaviors | 0.03 | 0.42 | –0.08 | 0.34 |
| OCD | Urges to do sudden and reckless things | 0.11 | 0.40 | 0.30 | 0.13 |
| Tourette | Coprolalia | 0.25 | 0.05 | 0.71 | 0.00 |
| Tourette | Copropraxia | 0.27 | 0.06 | 0.66 | –0.08 |
| Tourette | Echolalia | 0.25 | –0.10 | 0.56 | 0.23 |
| OCD | Urges to be destructive | 0.02 | 0.51 | 0.55 | –0.02 |
| OCD | Urges to injure or mutilate others | –0.10 | 0.52 | 0.55 | –0.17 |
| Tourette | Words | 0.31 | 0.04 | 0.54 | 0.05 |
| Tourette | Palilalia | 0.27 | –0.02 | 0.50 | 0.25 |
| Tourette | Animal or bird noises | 0.26 | 0.06 | 0.41 | –0.11 |
| OCD | Symmetry obsessions | –0.07 | 0.09 | –0.04 | 0.82 |
| OCD | Needs certain things to be symmetrical | –0.02 | 0.06 | –0.01 | 0.76 |
| OCD | Ordering or arranging compulsions | –0.10 | 0.11 | 0.07 | 0.73 |
| OCD | Needs to have certain things evened up | 0.08 | 0.06 | –0.05 | 0.73 |
| OCD | Thoughts about evening things up | 0.07 | 0.08 | –0.01 | 0.73 |
| OCD | Thoughts about lining things up | –0.01 | 0.09 | –0.01 | 0.71 |
| OCD | Obsessions about exactness | –0.03 | 0.20 | –0.05 | 0.70 |
| OCD | Rereads or rewrites things | 0.06 | 0.20 | 0.05 | 0.59 |
| OCD | Needs to repeat routine activities | 0.03 | 0.14 | 0.11 | 0.58 |
| OCD | Counting compulsions | 0.11 | 0.14 | 0.05 | 0.54 |
| OCD | Has to do things the same way every time | –0.11 | 0.22 | 0.21 | 0.47 |
| OCD | Needs to know or remember certain things | 0.08 | 0.36 | 0.05 | 0.44 |
| OCD | Checks that did not make mistakes | 0.05 | 0.40 | –0.09 | 0.43 |
| Tourette | Writing the same letter or word over and over | 0.20 | 0.04 | 0.03 | 0.42 |
| OCD | Checks things | 0.11 | 0.35 | –0.11 | 0.42 |
TABLE 2. Cronbach’s Alpha and Factor Loadings for the 4-Factor Modela
Latent Class Analyses
In the latent class analyses, a 4-class solution was the best fit for the Tourette syndrome probands (see Tables ST5 and Figure SF2 in online data supplement). Probands in class 1 were likely to endorse symptoms from all three disorders (Tourette syndrome, OCD, ADHD). Probands in class 2 tended to endorse primarily OCD symmetry symptoms (about 20% of this class also endorsed a few simple tics, e.g., eye blinking). Class 3 probands endorsed high rates of tic and ADHD symptoms but minimal OCD symptoms. Finally, class 4 probands endorsed only tics. When the latent class analyses were repeated including family members, a 5-class solution was the best fit (see Figure 1 and Table ST5 and Figure SF2 in online data supplement) and replicated the classes in the probands-only analysis, with the addition of an unaffected relative class.
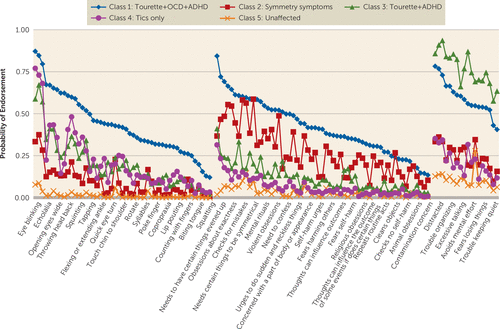
FIGURE 1. Probabilities of Endorsing Symptoms in Original Latent Class Analyses With Tourette-Affected Probands and Family Members
Significant differences in comorbidity rates were observed between classes (detailed in Figure 2) for OCD (χ2=1709.34, df=4, p≤0.001), ADHD (χ2=2796.01, df=4, p≤0.001), mood disorders (χ2=71.17, df=4, p≤0.001), anxiety disorders (χ2= 62.42, df=4, p≤0.001), and disruptive behavior disorders (χ2=60.31, df=4, p≤0.001). The proportion of mothers (χ2=833.81, df=4, p≤0.001) and fathers (χ2=426.12, df=4, p≤0.001) also differed between the classes; most mothers and fathers were in the unaffected (47% and 45%) and symmetry (49% and 29%) classes (Figure 2).
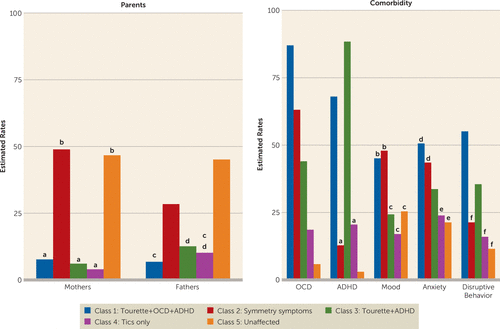
FIGURE 2. Parents’ Symptoms and Comorbidity Rates for Classes of Tourette-Affected Probands and Family Membersa
a Letters above bars indicate pairwise comparisons that are not significantly different at p<0.05. Significant differences were found for all diagnoses: OCD (χ2=1709.34, df=4, p≤0.001), ADHD (χ2=2796.01, df=4, p≤0.001), mood disorders (χ2=71.17, df=4, p≤0.001), anxiety disorders (χ2=62.42, df=4, p≤0.001), and disruptive behavior disorders (χ2=60.31, df=4, p≤0.001).
Replication Analyses
Characteristics of the 882 participants (527 probands and 355 family members) included in the replication analyses were similar to those of the discovery sample (Table 1). The confirmatory factor analysis of the 4-factor model with probands demonstrated a good fit (root mean square error of approximation=0.02; comparative fit index=0.93, nonnormed fit index=0.93). The results of the latent class analyses in the replication sample paralleled (confirmed) the original findings in the discovery sample using probands and parents: a 5-class solution was the best fit and identified a distinct “symmetry” class in addition to Tourette+OCD+ADHD, Tourette+ADHD, tics only, and unaffected classes (see Table ST5 and Figures SF2 and SF3 in online data supplement).
Heritability Analyses
Heritability estimates were calculated for the 4-factor and the 5-class latent class solutions (excluding the “unaffected” classes; Table 3). Heritabilities for the factors ranged from 0.25 to 0.46 (all p values ≤2.6×10−13). Heritabilities for the classes ranged from 0.28 (class 4, tics only, p=0.001) to 0.63 (class 5, Tourette+OCD+ADHD, p=2.0×10−13).
| Type of Analysis | H2r | SE | p |
|---|---|---|---|
| Latent class analyses | |||
| Class 1: Tourette+OCD+ADHD | 0.63 | 0.09 | 2.0×10–13 |
| Class 2: Symmetry symptoms | 0.38 | 0.10 | 0.001 |
| Class 3: Tourette+ADHD | 0.47 | 0.08 | 3.3×10–29 |
| Class 4: Tics only | 0.28 | 0.08 | 0.001 |
| Class 5: Unaffected | — | — | — |
| Exploratory factor analyses | |||
| Factor 1: Tics | 0.25 | 0.04 | 2.6×10–13 |
| Factor 2: Obsessive-compulsive symptoms | 0.46 | 0.03 | 8.6×10–41 |
| Factor 3: Disinhibition | 0.35 | 0.03 | 4.2×10–34 |
| Factor 4: Symmetry symptoms | 0.39 | 0.03 | 7.2×10–31 |
TABLE 3. Heritability Estimates for Classes of Tourette-Affected Probands and Family Membersa
As we identified two nontraditional patterns of symptom endorsement that might be useful for future studies of Tourette syndrome pathophysiology, symmetry in both the factor analyses and the latent class analyses, and disinhibited symptoms in the factor analyses, we conducted additional post hoc heritability and polygenic burden analyses. For symmetry, we combined the classes endorsing these symptoms at high rates (i.e., class 1 and class 2). Symmetry symptoms were endorsed at high rates by 997 individuals (29%); the heritability for symmetry was 0.53 (SE=0.09, p=6.3×10−10). Only the Tourette+OCD+ADHD endophenotype had a higher heritability estimate (h2r=0.63, Table 3). As noted above, the heritability for disinhibited symptoms, which was seen in the factor analyses but not the latent class analyses, was 0.35 (Table 3).
Polygenic Burden
We examined the association between Tourette syndrome, OCD, and ADHD polygenic burden and the two novel phenotypes, symmetry and disinhibition, in 947 Tourette syndrome probands with available genotype data (no genotype data are yet available on relatives). Both phenotypes were defined as continuous factor sum scores from the exploratory factor analysis. For exploratory purposes, the disinhibition phenotype was defined in two ways: 1) including the presence of hoarding symptoms in the factor sum score and 2) excluding hoarding symptoms, as these symptoms consistently factored out from the other disinhibition symptoms in higher factor solutions. The symmetry phenotype was positively associated with Tourette syndrome polygenic risk score (R2=0.57%, p=0.02) but not with OCD or ADHD polygenic risk scores (R2=0.19% [negative correlation], p=0.18, and R2=0.13% [negative correlation], p=0.26, respectively) (see Table ST6 and Figure SF4 in the online data supplement). In contrast, the disinhibition phenotype was significantly associated with OCD polygenic risk score (R2=0.52%, p=0.026) but was not significantly associated with Tourette syndrome or ADHD polygenic risk scores (R2=0.18%, p=0.19 and R2=0.23%, p=0.14, respectively). The disinhibition phenotype excluding hoarding symptoms was also significantly associated with OCD polygenic risk score (R2=0.56%, p=0.021) and had a positive but not statistically significant correlation with Tourette syndrome and ADHD polygenic risk scores (R2=0.27%, p=0.11 and R2=0.30%, p=0.10, respectively) (see Table ST6 and Figure SF4 in the online data supplement).
Discussion
To our knowledge, this is the first study to use multiple modeling approaches on symptom-level data in a large sample of individuals with Tourette syndrome and family members to identify heritable Tourette-associated endophenotypes. The use of factor and latent class analyses in the same data set provides an opportunity to thoroughly examine these complex phenotypes; the complementary findings across the approaches are notable. These analyses extend previous work and highlight two Tourette-related endophenotypes (disinhibition and symmetry symptoms) of potential use in future research. The heritability and polygenic burden analyses, which are also complementary, provide insight into the possible biological underpinnings of these cross-disorder phenotypes, and their likely utility in future genetic studies aimed at identifying Tourette-related susceptibility variants.
The symmetry phenotype was seen in both the factor and latent class analyses and parallels and refines the chronic tics+OCD class identified in our previous work using categorical diagnoses (11). We note that OCD with tics is now a specifier for OCD in DSM-5, acknowledging the growing awareness that the co-occurrence of tic symptoms represents a specific subtype of this disorder (32). The identification of this endophenotype is also consistent with previous literature suggesting that individuals with Tourette syndrome are more likely to have symmetry symptoms, which are associated with specific neural correlates in motor and limbic circuits (33), rather than other OCD symptoms. Of note, symmetry scores were positively associated with Tourette syndrome aggregated polygenic risk scores but not with OCD polygenic risk scores, even though these symmetry symptoms are derived from the Yale-Brown Obsessive Compulsive Scale and traditionally considered to be OCD-related. This finding is also consistent with previous GWAS analyses demonstrating that OCD with and without tics have some degree of nonoverlapping genetic risk (30), as well as with the clinical observations that symmetry symptoms seem to be driven by the need for things to feel “just right” (similar to premonitory urges for tics) rather than classic anxiety symptoms (34).
Additionally, in the latent class analysis, the symmetry class stood in contrast to all other classes, where categorical diagnoses appeared to be a bigger driver than symptom-level variation. Of note, the higher rate of mothers (including otherwise unaffected mothers of Tourette syndrome probands) in the symmetry class compared with the other affected classes (Figure 2) fits with prior observations that females in Tourette syndrome families are more likely to have OCD than tics, and it suggests that symmetry may represent an alternative Tourette-susceptibility phenotype that may be influenced by sex (35). The high heritability of this phenotype, and the increased Tourette syndrome, but not OCD, polygenic risk burden in symmetry-positive individuals, provides further support for this symptom-based cross-disorder phenotype as an appropriate substrate for further genetic analyses aimed at identifying Tourette-associated genetic variation.
The disinhibition phenotype was identified only in the factor models; however, it was identified in a “tic-only” item level latent class analysis in the same sample (36). Interestingly, the disinhibition factor is similar to an aggressive tic factor found in a previous study of Tourette syndrome, as well as an aggressive symptom cluster identified previously in OCD-affected individuals, combined with religious and sexual symptoms to form a “taboo” factor (12, 27). The heritability of this phenotype was among the highest identified, and although the polygenic risk score patterns were less clear for the disinhibition phenotype than for symmetry, there was some evidence for association between Tourette syndrome, OCD, and ADHD polygenic risk scores and the disinhibition phenotype, particularly when hoarding symptoms were excluded. Although not meeting criteria for statistical significance, and clearly requiring replication in larger samples, this pattern of association between disinhibition and increased polygenic burden for Tourette syndrome, OCD, and ADHD suggests that this endophenotype, rather than being specific for Tourette syndrome, may reflect deficits in top-down cognitive control that are seen across all three of these disorders (37–40).
Additional studies, including larger-scale GWAS, are needed to confirm and further parse the genetic and neurobiological relationships of the disinhibition phenotype to Tourette syndrome, OCD, and ADHD. This phenotype may also be of particular interest for neuroimaging studies of Tourette syndrome and OCD, again potentially correlating with impairment in top-down cortical control/response inhibition and with associated patterns of dysfunctional cortical-striatal-thalamic-cortical circuitry in these disorders (37–39).
As noted, both the symmetry and disinhibition phenotypes are relevant for future genetic studies, as both had high heritabilities and capture specific elements of the Tourette syndrome phenotype that might have different risk genes and/or pathophysiology than Tourette syndrome (or OCD) as defined using standard diagnostic criteria. Future research might also explore whether individuals endorsing these symptoms differ clinically (e.g., in terms of tic persistence or prognosis), pathophysiologically (e.g., severity of frontostriatal circuit disruption), or in treatment response.
Limitations
The primary limitation in these analyses reflects the fact that data were collected over an extended time period at many different sites. This limitation may also represent an advantage: the sample’s heterogeneity is likely to increase the generalizability of the findings. Also, statistical power was limited for some analyses, particularly for the polygenic burden analyses. Additionally, the small number of unaffected family members other than parents may limit the interpretation of the heritability estimates, as we cannot confidently separate shared genetic from shared environmental effects in the current sample. Nevertheless, the consistency of our findings across analytic approaches is striking and provides a novel and likely fruitful avenue of investigation for future genetic and neurobiological studies of Tourette syndrome and its co-occurring disorders.
1 : The inheritance of Tourette disorder: a review. J Obsessive Compuls Relat Disord 2014; 3:380–385Crossref, Medline, Google Scholar
2 : Anxiety disorders and tic severity in juveniles with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:562–568Crossref, Medline, Google Scholar
3 : Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry 2010; 197:36–44Crossref, Medline, Google Scholar
4 : Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015; 72:325–333Crossref, Medline, Google Scholar
5 : L-Histidine decarboxylase and Tourette’s syndrome. N Engl J Med 2010; 362:1901–1908Crossref, Medline, Google Scholar
6 : Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet 2007; 80:265–272Crossref, Medline, Google Scholar
7 : Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005; 310:317–320Crossref, Medline, Google Scholar
8 : Repetitive behaviours in patients with Gilles de la Tourette syndrome: tics, compulsions, or both? PLoS One 2010; 5:e12959Crossref, Medline, Google Scholar
9 : Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry 2014; 19:1017–1024Crossref, Medline, Google Scholar
10 : Tic symptom profiles in subjects with Tourette Syndrome from two genetically isolated populations. Biol Psychiatry 2007; 61:292–300Crossref, Medline, Google Scholar
11 : Latent class analysis of Gilles de la Tourette syndrome using comorbidities: clinical and genetic implications. Biol Psychiatry 2008; 64:219–225Crossref, Medline, Google Scholar
12 : Principal components analysis of a large cohort with Tourette syndrome. Br J Psychiatry 2008; 193:31–36Crossref, Medline, Google Scholar
13 : The Tourette’s Disorder Scale (TODS): development, reliability, and validity. Assessment 2003; 10:273–287Crossref, Medline, Google Scholar
14 : Factor-analytic study of the Yale Global Tic Severity Scale. Psychiatry Res 2007; 149:231–237Crossref, Medline, Google Scholar
15 : The Gilles de la Tourette syndrome: a principal component factor analytic study of a large pedigree. Psychiatr Genet 2007; 17:143–152Crossref, Medline, Google Scholar
16 : Clinical phenomenology and phenotype variability in Tourette syndrome. J Psychosom Res 2009; 67:491–496Crossref, Medline, Google Scholar
17 : A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. Am J Hum Genet 1999; 65:1428–1436Crossref, Medline, Google Scholar
18 : Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry 1982; 39:879–883Crossref, Medline, Google Scholar
19 : Mplus User’s Guide, 7th ed. Los Angeles, Muthén & Muthén, 1998Google Scholar
20 : PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575Crossref, Medline, Google Scholar
21 : Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. https://www.statmodel.com/download/Article_075.pdfGoogle Scholar
22 : Choosing the optimal number of factors in exploratory factor analysis: a model selection perspective. Multivariate Behav Res 2013; 48:28–56Crossref, Medline, Google Scholar
23 : Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess 1995; 7:286–299Crossref, Google Scholar
24 : Goodness of Fit Indices: Latent Variable Models. Mahwah, NJ, Lawrence Erlbaum Associates, 2004Crossref, Google Scholar
25 : Confirmatory Factor Analysis for Applied Research, 2nd ed. New York, Guilford Press, 2015Google Scholar
26 : Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62:1198–1211Crossref, Medline, Google Scholar
27 : Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav Genet 2010; 40:505–517Crossref, Medline, Google Scholar
28 : Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet 2013; 9:e1003864Crossref, Medline, Google Scholar
29 : Genome-wide association study of Tourette’s syndrome. Mol Psychiatry 2013; 18:721–728Crossref, Medline, Google Scholar
30 : Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette’s syndrome and OCD. Am J Psychiatry 2015; 172:82–93Link, Google Scholar
31 : Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:884–897Crossref, Medline, Google Scholar
32 : Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety 2010; 27:507–527Crossref, Medline, Google Scholar
33 : Limbic and motor circuits involved in symmetry behavior in Tourette’s syndrome. CNS Spectr 2013; 18:34–42Crossref, Medline, Google Scholar
34 : [Obsessive compulsive disorder with tics: a new subtype?]. Tijdschr Psychiatr 2011; 53:275–285Medline, Google Scholar
35 : Obsessive-compulsive disorder in Tourette’s syndrome. Adv Neurol 2005; 96:249–261Medline, Google Scholar
36 : Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology 2016; 87:497–504Crossref, Medline, Google Scholar
37 . The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010; 35:4–26Crossref, Medline, Google Scholar
38 : Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry 2012; 169:1100–1108Link, Google Scholar
39 : The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci 2015; 35:786–794Crossref, Medline, Google Scholar
40 : The effects of co-occurring ADHD symptoms on electrophysiological correlates of cognitive control in young people with Tourette syndrome. J Neuropsychol 2016; 10:223–238Crossref, Medline, Google Scholar



