Dopaminergic Enhancement of Striatal Response to Reward in Major Depression
Abstract
Objective:
Major depressive disorder is characterized by reduced reward-related striatal activation and dysfunctional reward learning, putatively reflecting decreased dopaminergic signaling. The goal of this study was to test whether a pharmacological challenge designed to facilitate dopaminergic transmission can enhance striatal responses to reward and improve reward learning in depressed individuals.
Method:
In a double-blind placebo-controlled design, 46 unmedicated depressed participants and 43 healthy control participants were randomly assigned to receive either placebo or a single low dose (50 mg) of the D2/D3 receptor antagonist amisulpride, which is believed to increase dopamine signaling through presynaptic autoreceptor blockade. To investigate the effects of increased dopaminergic transmission on reward-related striatal function and behavior, a monetary incentive delay task (in conjunction with functional MRI) and a probabilistic reward learning task were administered at absorption peaks of amisulpride.
Results:
Depressed participants selected previously rewarded stimuli less frequently than did control participants, indicating reduced reward learning, but this effect was not modulated by amisulpride. Relative to depressed participants receiving placebo (and control participants receiving amisulpride), depressed participants receiving amisulpride exhibited increased striatal activation and potentiated corticostriatal functional connectivity between the nucleus accumbens and the midcingulate cortex in response to monetary rewards. Stronger corticostriatal connectivity in response to rewards predicted better reward learning among depressed individuals receiving amisulpride as well as among control participants receiving placebo.
Conclusions:
Acute enhancement of dopaminergic transmission potentiated reward-related striatal activation and corticostriatal functional connectivity in depressed individuals but had no behavioral effects. Taken together, the results suggest that targeted pharmacological treatments may normalize neural correlates of reward processing in depression; despite such acute effects on neural function, behavioral modification may require more chronic exposure. This is consistent with previous reports that antidepressant effects of amisulpride in depression emerged after sustained administration.
Major depressive disorder is a highly prevalent psychiatric condition characterized by blunted reward processing and diminished positive affect (1). Preclinical research has shown that phasic dopamine signaling, particularly in the striatum, constitutes an important neural mediator of reward-related behaviors, including reinforcement learning (2, 3) and incentive motivation (4). Functional MRI (fMRI) studies in humans have corroborated the central role of striatal function in reinforcement learning (5) and reward processing (6) and demonstrated that these striatal functions are disrupted in depression (7, 8). Accordingly, reduced striatal dopamine functioning is believed to play a key role in the pathophysiology of depression, particularly in the context of impaired reward processing and reward learning (9–11). fMRI studies have further suggested that reward dysfunction in depression is related to disrupted corticostriatal functional connectivity (12, 13), consistent with the notion that altered communication among dopamine-rich striatal regions and cortical regulatory systems is an important substrate of depression (14).
Despite theories implicating striatal dopamine dysfunction in depression, it is unknown whether an acute manipulation thought to transiently increase dopamine signaling might normalize reward processing in depression. In healthy individuals, studies combining fMRI with acute pharmacologically induced dopaminergic enhancements have shown increased reward-related striatal responses and improved reward learning relative to placebo (15–17). For instance, acute administration of amisulpride (200 mg) improved healthy participants’ ability to select the better of two rewarding options, purportedly by enhancing reinforcement learning signals in the striatum and ventromedial prefrontal cortex (15). However, no study to date has tested whether pharmacologically induced enhancement of dopaminergic transmission can improve reward learning or striatal activity and corticostriatal connectivity in response to reward in depression.
To address these important gaps in the literature, we conducted a double-blind randomized placebo-controlled study integrating neural and behavioral measures of reward processing in conjunction with a dopamine pharmacological challenge. To this end, 46 unmedicated depressed individuals and 43 healthy control subjects were randomly assigned to receive either placebo or a single low dose (50 mg) of the D2/D3 receptor antagonist amisulpride, which has a particularly high affinity for mesolimbic pathways and is believed to increase dopaminergic transmission by means of presynaptic D2/D3 autoreceptor blockade (18, 19) (see also the Supplementary Methods section in the data supplement that accompanies the online edition of this article). After administration of amisulpride or placebo, participants underwent fMRI scanning during a monetary incentive delay task involving anticipation and receipt of monetary rewards and penalties (7). After the scan, participants completed a probabilistic selection task that separately measured the ability to learn from rewards or penalties (20). We selected a 50-mg dose in light of previous reports that a (sustained) 50-mg dosage of amisulpride has antidepressant and antianhedonic effects in depressive disorders (21, 22) and in order to avoid postsynaptic blockade (23), with the goal of maximizing the likelihood of autoreceptor effects. We hypothesized that this pharmacological manipulation would be associated with increased striatal response to reward and improved reward learning, and that such effects would be largest among depressed individuals.
Method
Participants
Participants were recruited from the Boston metropolitan community. The depressed and control groups were matched for age, gender, ethnicity, and years of education (Table 1). Inclusion criteria restricted recruitment to right-handed individuals 18–45 years of age with no contraindications to MRI, no lifetime substance dependence, no past-year substance abuse, and no serious medical conditions. For the depression group, participants had to have a diagnosis of major depressive disorder according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (24). Exclusion criteria for the depressed group included use of any psychotropic medication in the past 2 weeks (6 weeks for fluoxetine, 6 months for dopaminergic drugs or antipsychotics) and a psychiatric history of other major axis I disorders. For the control group, inclusion criteria included medication-free status for at least 3 weeks, absence of current or past psychiatric illnesses (based on the SCID interview), and absence of first-degree familial psychiatric illness. Participants received $15/hour in compensation plus earnings in the fMRI task. All participants provided written informed consent to a protocol approved by Partners Human Research Committee.
| Depression Group | Healthy Control Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Amisulpride (N=23) | Placebo (N=23) | Amisulpride (N=23) | Placebo (N=20) | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 27.7 | 8.2 | 26.3 | 5.2 | 26.5 | 6.8 | 25.3 | 5.6 |
| Education (years) | 10.9 | 5.1 | 11.4 | 6.1 | 13.0 | 5.0 | 12.9 | 5.2 |
| Beck Depression Inventory–IIb | 26.6 | 8.1 | 28.0 | 9.4 | 1.3 | 2.1 | 1.7 | 2.6 |
| Mood and Anxiety Symptom Questionnaire | ||||||||
| Total score | 170.3 | 15.0 | 176.6 | 25.0 | 91.0 | 13.5 | 91.3 | 14.2 |
| General distress depression subscale scoreb | 39.3 | 7.9 | 39.3 | 8.9 | 14.3 | 3.1 | 14.4 | 2.7 |
| Anhedonic depression subscale scoreb | 86.7 | 9.3 | 85.2 | 9.3 | 43.4 | 7.9 | 46.7 | 9.9 |
| General distress anxiety subscale scoreb | 22.2 | 5.6 | 25.8 | 6.9 | 13.8 | 2.8 | 12.3 | 1.8 |
| Anxious arousal subscale scoreb | 22.1 | 4.2 | 26.3 | 8.7 | 19.4 | 3.1 | 17.9 | 1.3 |
| Snaith-Hamilton Pleasure Scaleb | 32.9 | 4.5 | 32.8 | 6.2 | 21.5 | 6.0 | 22.6 | 6.8 |
| Duration of current major depressive episode (months) | 18.1 | 16.4 | 21.3 | 42.2 | ||||
| Number of past depressive episodes | 3.9 | 3.0 | 4.6 | 3.2 | ||||
| N | % | N | % | N | % | N | % | |
| Female | 21 | 91.3 | 16 | 69.6 | 18 | 78.3 | 15 | 75.0 |
| Caucasian | 10 | 43.5 | 10 | 43.5 | 10 | 43.5 | 5 | 25.0 |
| Current comorbid anxiety disorders | 3 | 13.0 | 2 | 8.7 | ||||
| Past comorbid anxiety disorders | 3 | 13.0 | 2 | 8.7 | ||||
TABLE 1. Demographic and Clinical Characteristics of Participants in a Study of Dopaminergic Enhancement of Striatal Response to Reward in Major Depressiona
Procedure
Participants first completed a clinical evaluation to determine eligibility (based on the SCID interview) and self-report measures of depression and anhedonia (Table 1; see also the Supplementary Methods section in the online data supplement). Eligible participants were invited to take part in the neuroimaging session, and those who participated were randomly assigned to receive amisulpride or placebo under double-blind conditions. Pharmacokinetic data indicate that plasma concentration of amisulpride has two peaks, approximately 1–1.5 hours and 2.5 hours after administration (18, 19). Therefore, the study physician administered either amisulpride or placebo at the beginning of the neuroimaging session, and fMRI scanning of the monetary incentive delay task started 1 hour after amisulpride or placebo administration to coincide with the first plasma concentration peak. The probabilistic selection task was administered after scan completion, approximately 2.5 hours after amisulpride or placebo administration, to coincide with the second plasma concentration peak. Heart rate, blood pressure, and side effects were assessed by the study physician throughout the session (Figure 1).
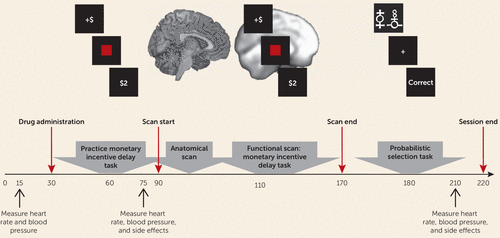
FIGURE 1. Procedure and Timeline for a Study of Dopaminergic Enhancement of Striatal Response to Reward in Major Depressiona
a On arrival to the scanning session, participants completed a pre-MRI safety screening form and provided a urine sample for drug testing and for pregnancy testing in female participants. Participants’ heart rate and blood pressure were then measured by the study physician. Next, the study physician administered a capsule of either amisulpride or placebo (participants were randomly assigned to receive double-blind amisulpride or placebo). Participants then waited for 1 hour in a quiet room to allow amisulpride plasma concentration to reach its first peak. During the waiting period, participants practiced the monetary incentive delay task. Forty-five minutes after drug administration, the study physician measured participants’ heart rate and blood pressure again, and participants completed a questionnaire on drug side effects. Next, participants completed an MRI scan (approximately 1.5 hours) that included structural scans and a functional scan while completing the monetary incentive delay task. After the scan, and approximately 2.5 hours after drug administration, participants completed the probabilistic selection task (administered to coincide with the second peak in amisulpride plasma concentration). After participants completed the probabilistic selection task, the study physician once again assessed their heart rate, blood pressure, and side effects. Participants were then debriefed, paid, and discharged.
fMRI Task
The monetary incentive delay task involves anticipation and receipt of monetary rewards and penalties, which have been shown to elicit robust striatal response in healthy individuals (25). Previous studies using this task have revealed reduced striatal activation and reduced corticostriatal functional connectivity in depressed compared with healthy adults during anticipation and receipt of monetary reward (7, 26), making it well suited for the present study (see the Supplementary Methods section in the data supplement).
Behavioral Task
A probabilistic selection task was used to probe learning from positive and negative feedback (20). In the learning phase, participants repeatedly viewed three pairs of stimuli (AB, CD, and EF) and had to integrate feedback over several trials to learn which stimulus in each pair was rewarded most consistently. In the test phase, the most reliably rewarded (A) and penalized (B) stimuli were presented in conjunction with all other stimuli (e.g., AC, AD, AE, AF); participants’ ability to “choose A” or to “avoid B” were used as measures of reward or penalty learning, respectively (see the Supplementary Methods section in the data supplement).
MRI Acquisition Parameters
The MRI acquisition parameters are described in the Supplementary Methods section of the data supplement.
fMRI Data Analysis
fMRI data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/). Preprocessing included coregistration of functional and anatomical images, segmentation, nonlinear volume-based spatial normalization (using Montreal Neurological Institute [MNI] space), and spatial smoothing with a Gaussian filter (6 mm full width at half maximum).
Hemodynamic responses were modeled using a canonical hemodynamic response function that was convolved with the onset times of task regressors in order to compute a general linear model at the single-subject level. The general linear model included nine task-related regressors: three cues (reward, penalty, no incentive), the target, and five outcomes (win [reward outcome following reward cue], no win [no-change outcome following reward cue], loss [penalty outcome following penalty cue], no loss [no-change outcome following penalty cue], and no change [no-change outcome following no-incentive cue]). The general linear model also included high-pass temporal filtering (0.008 Hz), seven rigid-body movement parameters, nuisance regressors accounting for no-response trials, and outlier time points (see the Supplementary Methods section in the data supplement).
To test a priori hypotheses regarding striatal responses to reward (7), we conducted a region-of-interest analysis in which activations (beta weights) were extracted from anatomical masks of the caudate, the nucleus accumbens, and the putamen for each participant and for each task regressor (relative to baseline). To avoid any biases, masks were defined using a manually segmented MNI-152 brain and implemented as overlays on the SPM12 canonical brain (see Figure S1 in the data supplement; see also reference 27). Activations reported throughout the analyses were quantified by averaging beta weights from all voxels within a mask. Exploratory whole brain analyses were also conducted (see the Supplementary Methods and Results sections of the data supplement).
Psychophysiological interaction analyses were performed to examine the effects of reward and penalty outcomes on striatal functional connectivity. Because hemispheric effects on task activation were nonsignificant, striatal masks were collapsed across hemispheres, yielding three bilateral seeds (caudate, nucleus accumbens, putamen). Analyses retained the subject-level general linear models described above, adding regressors corresponding to the seed time course and the interaction of the seed time course with the task condition of interest (separately for reward and penalty outcome). Single-subject connectivity maps for the interaction between each seed time course and the regressor of interest were entered into second-level whole brain random-effects analysis. Effects were thresholded at a peak p value of <0.001, whole brain family-wise error corrected to p<0.05 at the cluster level.
Statistical Analysis
The methods for statistical analysis are detailed in the Supplementary Methods section of the online data supplement.
Results
Behavioral Results
Accuracy in “choose A” and “avoid B” trials of the probabilistic selection task test phase were used as measures of reward and penalty learning, respectively. A repeated-measures analysis of variance (ANOVA) with learning type (“choose A” and “avoid B” accuracy) as the within-subject variable and diagnosis (depressed versus control group) and drug (amisulpride versus placebo) as between-subject variables revealed no significant main effects or interactions (see Figure S2A in the online data supplement). Because the primary focus of this study was reward processing, we also performed analyses that separately probed group differences in reward learning (which may be driven by a mixture of reward responsiveness and learning ability) and penalty learning (which may be driven by both penalty sensitivity and learning ability). Factorial ANOVAs were conducted separately with either reward or penalty learning (i.e., accuracy in “choose A” and “avoid B” trials, respectively) as the dependent variable and diagnosis (depressed versus control group) and drug (amisulpride versus placebo) as between-subject variables. For reward learning, there was a main effect of diagnosis (F=6.28, df=1, 75, p=0.014), due to reduced reward learning in the depressed compared with the control group. No significant group differences in penalty learning were observed. Thus, depressed participants exhibited impaired reward learning, but not penalty learning, relative to control participants, and this impairment was not affected by drug administration. Nevertheless, the lack of a significant type-by-diagnosis interaction (“choose A” and “avoid B” accuracy; depressed versus control group) in the repeated-measures ANOVA precludes any strong inferences about the specificity of these findings. No other significant effects of diagnosis or drug emerged across behavioral analyses of either experimental task (see the Supplementary Results section of the data supplement).
Striatal Response to Cues
A repeated-measures ANOVA was performed for each striatal region with the following factors: hemisphere (left versus right) and cue (reward, penalty, no-incentive) as within-subject variables and diagnosis (depressed versus control group) and drug (amisulpride versus placebo) as between-subject variables. These analyses revealed a main effect of cue in all three regions (caudate: F=56.55, df=2, 170, p<0.001; nucleus accumbens: F=61.33, df=2, 170, p<0.001; putamen: F=40.31, df=2, 170, p<0.001). Consistent with previous studies (7), post hoc analyses indicated that this effect was driven by increased striatal responses to reward cues, followed by penalty cues, followed by no-incentive cues (see Figure S3 in the data supplement). Relevant to the study hypotheses, a diagnosis-by-drug interaction also emerged for all regions (caudate: F=9.65, df=1, 85, p=0.003; putamen: F=5.84, df=1, 85, p=0.018; the interaction fell short of statistical significance for the nucleus accumbens: F=3.35, df=1, 85, p=0.071). These effects were driven by increased striatal response to cues (regardless of cue type) in depressed participants receiving amisulpride relative to depressed participants receiving placebo (caudate: p=0.022; nucleus accumbens: p=0.036; putamen: p=0.049) and relative to control participants receiving amisulpride (caudate: p=0.017; the interaction fell short of statistical significance for the nucleus accumbens: p=0.063). Together, these results indicate that amisulpride enhanced striatal responses to cues, regardless of cue valance, in depressed but not healthy participants (Figure 2A).
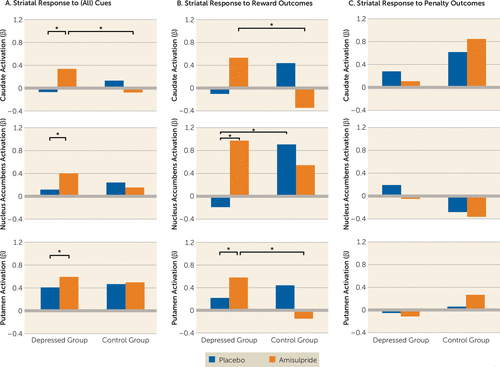
FIGURE 2. Striatal Response to Cues and Outcomes in Depressed Individuals and Healthy Control Subjects With and Without Dopaminergic Enhancementa
a In panel A, striatal response to cues, across all types, was greater in depressed participants receiving amisulpride compared with depressed participants receiving placebo (caudate: p=0.022; nucleus accumbens: p=0.036; putamen: p=0.049), as well as compared with healthy control participants receiving amisulpride (caudate: p=0.017; the group difference in nucleus accumbens fell short of statistical significance: p=0.063). In panel B, striatal response to reward outcomes was greater in depressed participants receiving amisulpride compared with depressed participants receiving placebo (nucleus accumbens, p=0.007; putamen, p=0.050), as well as compared with control participants receiving amisulpride (caudate, p=0.044; putamen, p=0.003); nucleus accumbens activation in response to reward outcome was also higher in control participants receiving placebo relative to depressed participants receiving placebo (p=0.026). In panel C, striatal response to penalty outcomes did not differ significantly across groups and there was no consistent pattern across regions in the penalty condition.
*p<0.05.
Striatal Response to Outcomes
A repeated-measures ANOVA was performed for each striatal region with the following factors: hemisphere (left versus right) and outcome (reward outcome versus penalty outcome) as within-subject variables and diagnosis (depressed versus control group) and drug (amisulpride versus placebo) as between-subject variables. These analyses revealed a main effect of outcome in the nucleus accumbens (F=11.30, df=1, 85, p=0.001) related to greater nucleus accumbens activation to rewards than to penalties across participants. Critically, all three striatal regions showed an outcome-by-diagnosis-by-drug interaction (caudate: F=4.64, df=1, 85, p=0.034; putamen: F=6.73, df=1, 85, p=0.011; the interaction fell short of statistical significance for the nucleus accumbens: F=3.17, df=1, 85, p=0.078). As shown in Figure 2B, amisulpride administration in depressed participants enhanced striatal response to reward outcomes relative to placebo administration (nucleus accumbens: p=0.007; putamen: p=0.050) and relative to amisulpride administration in control participants (caudate: p=0.044; putamen: p=0.003). Nucleus accumbens response to reward outcome was also greater in control participants receiving placebo than in depressed participants receiving placebo (p=0.026). In contrast, no significant group differences emerged in striatal response to penalty outcome (Figure 2C). In sum, amisulpride selectively enhanced striatal response to reward outcomes, but not penalty outcomes, in depressed (but not healthy) participants.
Striatal Connectivity in Response to Outcomes
Whole-brain psychophysiological interaction analyses were conducted to separately investigate the effects of reward and penalty outcomes on striatal functional connectivity. A whole brain diagnosis-by-drug ANOVA (depressed versus control group; amisulpride versus placebo) revealed no significant group differences for striatal connectivity in response to reward or penalty outcomes at peak p<0.001, whole brain family-wise error corrected p<0.05. Next, striatal connectivity at the whole brain level was investigated across the entire sample (N=89). These analyses revealed that in response to reward but not penalty outcomes, participants exhibited increased functional connectivity bilaterally between the caudate and a region (k=22 voxels) of the dorsal anterior cingulate cortex, as well as bilaterally between the nucleus accumbens and a region (k=13 voxels) of the midcingulate cortex (Figure 3A; see also Table S1 in the data supplement). Post hoc analyses were conducted to investigate whether depression or amisulpride moderated these reward-related corticostriatal connectivity patterns. To this end, caudate–dorsal anterior cingulate cortex and nucleus accumbens–midcingulate cortex connectivity values were extracted and used as the dependent variables in mixed-effect ANOVAs with diagnosis (depressed versus control group) and drug (amisulpride versus placebo) as between-subject variables. For both analyses investigating caudate–dorsal anterior cingulate cortex as well as nucleus accumbens–midcingulate cortex connectivity, significant diagnosis-by-drug interactions emerged (F=4.26, df=1, 85, p=0.043, and F=6.25, df=1, 85, p=0.015, respectively). Post hoc analyses revealed that control participants receiving placebo exhibited stronger reward-related caudate–dorsal anterior cingulate cortex functional connectivity relative to all three other groups (all p values, <0.033) (Figure 3B). With regard to nucleus accumbens–midcingulate cortex functional connectivity, both control participants receiving placebo and depressed participants receiving amisulpride showed stronger connectivity than depressed participants receiving placebo (p=0.037 and p=0.022, respectively) (Figure 3C).
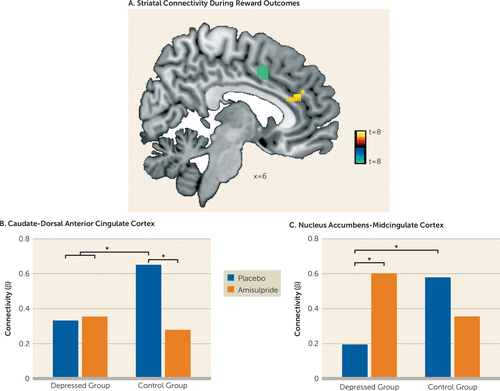
FIGURE 3. Striatal Connectivity in Response to Reward Outcomes in Depressed Individuals and Healthy Control Subjects With and Without Dopaminergic Enhancementa
a In panel A, whole-brain psychophysiological interaction analyses revealed increased functional connectivity bilaterally between the caudate and regions of the dorsal anterior cingulate cortex (yellow) and bilaterally between the nucleus accumbens and regions of the midcingulate cortex (green) in response to reward outcomes across the entire sample at peak p<0.001, family-wise error corrected p<0.05. No changes in striatal connectivity were found in response to penalty outcomes. In panel B, caudate–dorsal anterior cingulate cortex functional connectivity was significantly higher in control participants receiving placebo relative to all other groups (all p values <0.033). In panel C, nucleus accumbens–midcingulate cortex functional connectivity was significantly higher in both control participants receiving placebo and depressed participants receiving amisulpride than in depressed participants receiving placebo (p=0.037 and p=0.022, respectively).
Striatal Connectivity During Reward Outcomes and Reward Learning
Given the observed effects of amisulpride on nucleus accumbens–midcingulate cortex functional connectivity in depressed participants, multiple regression analyses were conducted to investigate the relationship between reward-related nucleus accumbens–midcingulate cortex connectivity and reward learning. Specifically, diagnosis (depressed group coded as +1, control group as −1), drug (amisulpride coded as +1, placebo as −1), reward learning (“choose A” accuracy from the probabilistic selection task), and their interactions were regressed on reward-related nucleus accumbens–midcingulate cortex functional connectivity. The results revealed a significant diagnosis-by-drug-by-reward learning interaction (F=5.76, df=1, 67, p=0.019). Post hoc simple regression analyses within each group revealed positive relationships between reward learning and reward-related nucleus accumbens–midcingulate cortex functional connectivity in depressed participants receiving amisulpride (r=0.65, p=0.003) and in control participants receiving placebo (r=0.54, p=0.029), but not in depressed participants receiving placebo (r=−0.24, p=0.35) or control participants receiving amisulpride (r=0.08, p=0.74) (Figure 4). These results indicate that amisulpride administration enhanced nucleus accumbens–midcingulate cortex functional connectivity in response to reward outcome in depressed individuals to a level comparable to that exhibited by healthy subjects receiving placebo. Furthermore, the magnitude of nucleus accumbens–midcingulate cortex functional connectivity during reward outcome for both depressed individuals receiving amisulpride and control participants receiving placebo was positively associated with reward learning in the behavioral probabilistic selection task.
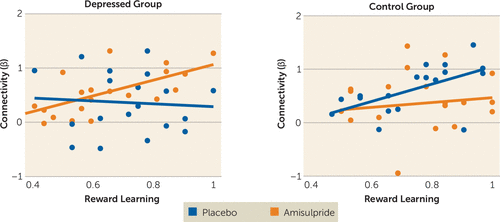
FIGURE 4. Reward Learning and Nucleus Accumbens–Midcingulate Cortex Functional Connectivity in Depressed Individuals and Healthy Control Subjects With and Without Dopaminergic Enhancementa
a Regression analyses revealed positive relationships between reward learning and reward-related nucleus accumbens–midcingulate cortex functional connectivity in depressed participants receiving amisulpride (r=0.65, p=0.003) and healthy control participants receiving placebo (r=0.54, p=0.029), but not in depressed participants receiving placebo (r=−0.24, p=0.354) or control participants receiving amisulpride (r=0.08, p=0.740). Reward learning refers to “choose A” accuracy from the probabilistic selection task.
Discussion
Major depression is a debilitating psychiatric disorder characterized by high rates of relapse and recurrence. Discovering treatment tools that target putative mechanisms of illness in depression—such as blunted response to reward—is therefore a key clinical priority. Findings from this proof-of-mechanism study suggest that an acute pharmacological challenge transiently increased striatal response to reward among adults with major depressive disorder, putatively via enhancement of dopaminergic transmission owing to autoreceptor blockade. Specifically, depressed participants receiving amisulpride exhibited increased striatal activity in response to cues, and increased striatal activity and corticostriatal functional connectivity in response to reward outcomes. Furthermore, stronger corticostriatal functional connectivity between the nucleus accumbens and the midcingulate cortex in depressed participants who received amisulpride was associated with better reward learning performance, a pattern similar to that observed in healthy control subjects receiving placebo. Together, these results provide converging evidence for abnormalities in neural reward systems in depression and highlight the potential of targeted pharmacological treatments to normalize reward processing in depression.
Extensive preclinical research has emphasized the key role of striatal dopamine signaling in mediating reward-related behaviors (2–4) and has postulated links between reduced striatal dopamine function and blunted reward processing and reinforcement learning in depression (9, 10). Interestingly, previous research indicates that dopamine differentially mediates anticipatory and consummatory phases of reward processing (28) and thus may uniquely affect their putative dysfunction in anhedonia and depression (29). In support of this idea, we observed that acute administration of amisulpride enhanced striatal response to cues regardless of valence (e.g., signaling potential rewards, penalties, or null outcomes), yet in response to outcomes, striatal enhancement was selective to reward.
In addition to increasing striatal activity in response to rewards, enhancement of dopamine signaling in depressed individuals was also associated with amplified functional connectivity between the striatum and areas of the midcingulate cortex. This finding is consistent with a model in which abnormal coordinated activity among large-scale brain circuits, including corticostriatal pathways, is central to the pathophysiology of depression (30, 31). Critically, those depressed individuals who exhibited the strongest nucleus accumbens–midcingulate cortex connectivity in response to rewards after amisulpride administration also exhibited better reward learning in an independent behavioral task, and this pattern was not found among depressed individuals who received placebo. Of relevance to the present findings, increased functional connectivity has been observed between midcingulate and striatal regions (and the insula) during learning (32), supporting the importance of this corticostriatal subcircuit in dopamine-mediated functioning. Coordination between dopamine-rich areas of the striatum and midline regions involved in processing behavioral salience may therefore be an important dimension of healthy reinforcement learning, and dopamine enhancement may help to regulate this functional circuit in depression. In fact, given preclinical evidence that amisulpride has a particularly high affinity for mesolimbic pathways (18, 19), one may speculate that amisulpride may enhance striatal function by affecting regulatory mechanisms beyond the striatum, and in particular in regions of the mesocorticolimbic pathway that communicate with the striatum via dopaminergic signaling to enable reward motivation and reinforcement learning (29). Thus, while in the present study we investigated the effects of amisulpride on striatal functioning, other brain systems that have exhibited abnormal activity or functional connectivity in depression (e.g., prefrontal cortex) may be important targets of dopamine manipulation.
Several additional questions remain open for future investigation. First, evidence from preclinical studies linking reinforcement learning and motivation with phasic dopamine signaling in the striatum suggests that amisulpride enhancement of reward processing in depressed individuals most likely occurs via increased phasic dopamine signaling (2–4). Nevertheless, the mechanisms by which amisulpride may act to enhance striatal response to reward are complex and may involve modifications of phasic and tonic levels of dopamine, as well as of additional neurotransmitters (33, 34). Additional research, especially in humans, investigating the effects of amisulpride on tonic and phasic dopamine release is needed. A second area for future investigation is motivated by differences between our findings and the results of previous investigations in which dopaminergic manipulation in healthy individuals resulted in better reward learning and increased striatal activity. The modest amisulpride dose used in the present study (50 mg as opposed to 200 mg and 400 mg in past studies [15, 35]) may have contributed to these discrepancies. We selected a 50-mg dose based on animal work showing that low doses of amisulpride potentiate striatal dopamine release, have strong hedonic effects, and increase the incentive value of environmental cues (18, 19). In humans, a 50-mg dose of amisulpride has been associated with reduced blockade of postsynaptic D2/D3 receptor in comparison to higher doses of 200–400 mg (23), increasing the likelihood of presynaptic effects. Perhaps more importantly, (sustained) 50-mg amisulpride dosing has been shown to have antidepressant and antianhedonic effects in depressive disorders (21, 22), suggesting that the present pharmacological manipulation may preferentially benefit depressed individuals as compared with healthy subjects. Nevertheless, while the pharmacological manipulation enhanced striatal function in depressed individuals, it had no such effect on behavior (i.e., reward learning). One potential reason for this could relate to the fact that we administered only a single dose of the drug. Thus, while the drug may have an immediate effect on neural function, modifying behavior may require longer and more chronic exposure. In support of this idea, antidepressant effects of amisulpride among depressed individuals have been observed after sustained (but not acute) administration (21, 22).
In conclusion, in depressed individuals, but not healthy subjects, acute pharmacological challenge transiently increased striatal activity and corticostriatal functional connectivity in response to rewards, putatively via enhancement of dopaminergic transmission. These findings suggest that an acute pharmacological manipulation believed to increase dopamine transmission may help normalize reward processing in depressed individuals through the enhancement of key corticostriatal mechanisms.
1 : Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-5. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Predictive reward signal of dopamine neurons. J Neurophysiol 1998; 80:1–27Crossref, Medline, Google Scholar
3 : Dopamine, learning, and motivation. Nat Rev Neurosci 2004; 5:483–494Crossref, Medline, Google Scholar
4 : The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007; 191:391–431Crossref, Medline, Google Scholar
5 : Reinforcement learning models and their neural correlates: an activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci 2015; 15:435–459Crossref, Medline, Google Scholar
6 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26Crossref, Medline, Google Scholar
7 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Link, Google Scholar
8 : Reinforcement learning in depression: a review of computational research. Neurosci Biobehav Rev 2015; 55:247–267Crossref, Medline, Google Scholar
9 : The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64:327–337Crossref, Medline, Google Scholar
10 : The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 2006; 59:1151–1159Crossref, Medline, Google Scholar
11 : Dysfunctional reward processing in depression. Curr Opin Psychol 2015; 4:114–118Crossref, Medline, Google Scholar
12 : Corticostriatal pathways contribute to the natural time course of positive mood. Nat Commun 2015; 6:10065Crossref, Medline, Google Scholar
13 : Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA 2009; 106:22445–22450Crossref, Medline, Google Scholar
14 : The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14:609–625Crossref, Medline, Google Scholar
15 : Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J Neurosci 2011; 31:1606–1613Crossref, Medline, Google Scholar
16 : Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 2006; 442:1042–1045Crossref, Medline, Google Scholar
17 : Dopamine restores reward prediction errors in old age. Nat Neurosci 2013; 16:648–653Crossref, Medline, Google Scholar
18 : Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther 1997; 280:83–97Medline, Google Scholar
19 : Amisulpride: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs 1996; 6:237–256Crossref, Google Scholar
20 : By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 2004; 306:1940–1943Crossref, Medline, Google Scholar
21 : Faster response on amisulpride 50 mg versus sertraline 50–100 mg in patients with dysthymia or double depression: a randomized, double-blind, parallel group study. Int Clin Psychopharmacol 2001; 16:317–324Crossref, Medline, Google Scholar
22 : Efficacy and safety of amisulpride 50 mg versus paroxetine 20 mg in major depression: a randomized, double-blind, parallel group study. Int Clin Psychopharmacol 2002; 17:27–32Crossref, Medline, Google Scholar
23 : D2 receptor occupancy during high- and low-dose therapy with the atypical antipsychotic amisulpride: a 123I-iodobenzamide SPECT study. J Nucl Med 2005; 46:1028–1033Medline, Google Scholar
24 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
25 : fMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 2000; 12:20–27Crossref, Medline, Google Scholar
26 : Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med 2015; 45:121–131Crossref, Medline, Google Scholar
27 : Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry 2015; 78:67–76Crossref, Medline, Google Scholar
28 : Parsing reward. Trends Neurosci 2003; 26:507–513Crossref, Medline, Google Scholar
29 : Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35:537–555Crossref, Medline, Google Scholar
30 : The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 2012; 22:229–251Crossref, Medline, Google Scholar
31 : Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72:603–611Crossref, Medline, Google Scholar
32 : Temporal prediction errors modulate cingulate-insular coupling. Neuroimage 2013; 71:147–157Crossref, Medline, Google Scholar
33 : Amisulpride promotes cognitive flexibility in rats: the role of 5-HT7 receptors. Behav Brain Res 2013; 248:136–140Crossref, Medline, Google Scholar
34 : 5-HT7 receptor activation: procognitive and antiamnesic effects. Psychopharmacology (Berl) 2015; 232:595–603Crossref, Medline, Google Scholar
35 : Differential modulation of reinforcement learning by D2 dopamine and NMDA glutamate receptor antagonism. J Neurosci 2014; 34:13151–13162Crossref, Medline, Google Scholar



