Outcomes of Citalopram Dosage Risk Mitigation in a Veteran Population
Abstract
Objective:
A public safety communication issued by the Food and Drug Administration declared that citalopram dosages exceeding 40 mg/day were no longer considered safe because of a newly recognized risk of dosage-dependent QT interval prolongation. The authors compared the incidence of hospitalizations and mortality when higher dosages of citalopram were or were not reduced to ≤40 mg/day.
Method:
National electronic medical records compiled by the Veterans Health Administration were used to conduct a retrospective study of a population filling citalopram prescriptions for more than 40 mg/day when the safety communication was first issued in August 2011. Hospitalizations and mortality after dosages of citalopram were or were not reduced to ≤40 mg/day were compared using multivariable Cox regression.
Results:
The at-risk cohort of 35,848 veterans (mean age, 58 years [SD=11]; 92% male) had citalopram prescriptions for 64 mg/day (SD=8.3), on average. Within 180 days after the safety communication was issued, 60% had filled prescriptions for ≤40 mg/day. All-cause hospitalizations or deaths were found to significantly increase after dosage reductions (adjusted hazard ratio=4.5, 95% CI=4.1–5.0), as were hospitalizations for depression or all-cause death (adjusted hazard ratio=2.2, 95% CI=1.8–2.6). Mortality did not decline (adjusted hazard ratio=1.0, 95% CI=0.8–1.3), and neither did hospitalizations for arrhythmias or all-cause deaths (adjusted hazard ratio=1.3, 95% CI=1.0–1.7).
Conclusions:
Reduction of prescribed citalopram dosages to a new safety limit was associated with a higher rate of hospitalization in a large patient population who had been treated with substantially higher dosages. Stipulating a safety limit for citalopram dosages before the benefits and risks of doing so were firmly established appears to have had unintended clinical consequences.
The Food and Drug Administration (FDA) may issue a safety communication when a marketed medication is associated with a new serious adverse effect (1, 2). Safety warnings that stipulate actions to mitigate risk can create a clinical conundrum (3). Studies of at-risk patient populations are needed to determine the ultimate outcomes of clinical responses to safety warnings (4).
On Aug. 24, 2011, the FDA issued a safety communication that stipulated prescribed daily dosages of the antidepressant citalopram should no longer exceed 40 mg because of the risk of dosage-dependent QT interval prolongation (5). Within a week, the Department of Veterans Affairs (VA) Center for Medication Safety disseminated a similar national safety bulletin to make sure that health care providers were aware of the new dosage limit. Dosages exceeding 40 mg/day had been used in several clinical trials and were frequently prescribed despite a lack of unequivocal evidence of additional therapeutic benefit (6–8). Absent definitive evidence about the incidence of life-threatening QT interval prolongation or the effectiveness of higher dosages in clinical practice, clinicians needed to decide whether the risk of worsening mental health disorders outweighed the risk and liability of continuing to prescribe higher dosages of citalopram (9–12).
The intended outcome of limiting dosages of citalopram to 40 mg/day was to reduce the incidence of fatalities and serious health problems due to QT interval prolongation. However, we observed several cases of suicidal ideation and hospitalization for worsening depression after citalopram dosages were reduced. Given the seemingly low incidence of the targeted adverse effect and the frequent clinical use of higher dosages of citalopram for presumably more difficult-to-treat cases, unintended increases in suicide and hospitalization due to worsening depression could offset the intended benefits of limiting citalopram dosage levels. We compared rates of hospitalizations and mortality in an at-risk veteran patient population whose prescribed dosages of citalopram were >40 mg/day when the safety warning was issued, and whose dosages either were reduced to ≤40 mg/day or were not reduced thereafter.
Method
Study Cohort
A search of the VA’s national electronic medical records database found 265,795 veterans who filled at least one outpatient citalopram prescription in the 3 months before August 2011; of these, 53,468 (20%) had received a prescription exceeding 40 mg/day. Those whose last citalopram prescription was filled before August 2011 and did not exceed 40 mg/day (N=903; 1.7%), and those whose last dispensed supply of citalopram, plus a 30-day grace period for a refill, would not continue into August 2011 if taken as prescribed (N=5,916; 11.1%) were excluded. Employee and other medical records flagged as sensitive by the VA (N=4,603) were also excluded. The remaining cohort included 42,046 patients who were actively filling VA prescriptions for citalopram at dosages >40 mg/day on Aug. 1, 2011, approximately 3 weeks before the safety communications were issued. After the safety communications were disseminated during the last week of August 2011, 35,848 patients still had active citalopram prescriptions exceeding 40 mg/day. The Minneapolis VA Institutional Review Board waived informed consent requirements for this study of available electronic records.
Baseline Variables
Baseline data were extracted for the 12 months prior to August 2011. Health care variables included VA eligibility category, the geographical Veterans Integrated Service Networks, having other health insurance, number of days with outpatient encounters, and number of hospitalizations. Inpatient and outpatient ICD-9 codes were used to indicate the possible presence of depression, posttraumatic stress disorder (PTSD), anxiety, cardiac arrhythmias, other heart diseases, and several other comorbidities based on Charlson or Elixhauser classifications of ICD-9 codes (13).
Prescriptions were categorized into 39 pharmacologic classes, such as tricyclic antidepressants, antipsychotics, and so forth, based on the VA national formulary. Additional variables were created to indicate other prescription medications associated with prolongation of the QT interval, or medications that could inhibit the CYP2C19 enzyme that metabolizes citalopram (14, 15). Baseline citalopram prescriptions were characterized by the total days of supply provided by prescriptions for more than 40 mg/day, the last prescribed dosage, days of supply, and type of prescriber (psychiatrist, other physician, or physician extender such as a nurse practitioner or pharmacist). Outpatient visit records were searched for codes signifying that an electrocardiogram had been done. Procedure codes were searched for any indication that an internal cardiac defibrillator was placed or being monitored.
Study Outcomes
The primary outcome was time to the first all-cause hospitalization or death to capture all hospitalizations or deaths that were directly or indirectly related to QT interval prolongation or citalopram dosage reduction and to avoid reliance on discharge diagnoses. Unrelated hospitalizations and deaths were assumed to be unaffected. The hundreds of principal discharge diagnoses were crudely categorized into 21 major diagnostic categories, such as mental health or cardiovascular system disorders, to determine which types of diagnoses contributed most to any differences in all-cause hospitalizations. Hospitalizations at non-VA facilities that were paid for by the VA, and therefore noted in VA records, were included. Dates of death were determined using the VA vital status file that comprehensively compiles death records from the Social Security Administration, the Centers for Medicare and Medicaid Services, and VA hospital discharge, burial, and compensation and pension records (16). Hospitalization and vital status files were accessed late in 2014 to allow for data lag.
Two more specific composite secondary endpoints were analyzed. One was a hospitalization with a principal diagnosis of depression, any diagnosis of self-injury, or all-cause death. Depression was defined by ICD-9 principal discharge diagnosis codes used by the VA National Registry for Depression (293.83, 296.2x, 296.3x, 296.90, 296.99, 298.0, 300.4, 301.12, 309.0, 309.1, 309.28, and 311). Self-inflicted injury was indicated by codes E950–E958 (17–19). The other more specific composite was all-cause death or a hospitalization with a principal diagnosis of paroxysmal ventricular tachycardia (427.1), ventricular fibrillation or flutter (427.4x), cardiac arrest (427.5), long QT syndrome (426.82), unspecified arrhythmias (427.89, 427.9), syncope and collapse (780.2), or sudden death (798, 798.1, 798.2, 798.9) (20–22).
Follow-Up
Citalopram prescriptions filled through August 2012 were extracted to determine the duration of continued use and the first date a citalopram prescription for ≤40 mg/day was filled. Follow-up was censored 30 days after a subject’s previous fill presumably ran out if taken as prescribed. A sensitivity analysis allowed a 90-day gap in citalopram supply before censoring follow-up. Follow-up of those who continued to regularly fill citalopram prescriptions continued until an endpoint hospitalization or death, the dosage of citalopram was increased to >40 mg/day after it had been reduced to ≤40 mg/day, or Aug. 31, 2012.
Data Analysis
Continuous variables are described by their means and standard deviations. Proportions are expressed as percentages. The cumulative proportion that had a citalopram dosage reduction to <40 mg/day is depicted as a Kaplan-Meier curve starting on Aug. 1, 2011, 3 weeks before the safety communications were issued.
To calculate incidence rates per 100 person-years, follow-up time was partitioned into periods before or after a subject’s prescribed dosage of citalopram was reduced to ≤40 mg/day. The distributions of 109 baseline characteristics of those whose dosage was reduced to ≤40 mg/day during the 1-year follow-up period were compared with those who continued to receive prescriptions for dosages exceeding 40 mg/day by calculating standardized differences (see Table S1 in the data supplement that accompanies the online edition of this article) (23). A propensity score for dosage reduction was estimated using Cox regression of times to prescription of ≤40 mg/day in relation to the 109 baseline variables.
Multivariable Cox regression models were employed to test for an association (hazard ratio) between citalopram dosage reductions, which were observed at varying times during follow-up, and study outcomes (24, 25). The multivariable models included all 109 baseline variables. Each component of the primary composite outcome was also analyzed separately. When hospitalizations were analyzed separately, death was a competing risk rather than a primary study outcome. In addition, the propensity scores of the group whose citalopram dosage was reduced were matched 1:1 to the nearest neighbor in the group whose dosage was not reduced to ≤40 mg/day (see Table S2 and Figure S1 in online data supplement), and the matched subset was used to estimate adjusted hazard ratios for citalopram dosage reduction.
Only a few less critical baseline variables had missing values, such as race (7.4%) and Hispanic ethnicity (4.9%). The percentages of the groups being compared that had missing values were similar. Therefore, we elected to simply assign the missing values to the most prevalent category of each variable.
All p values and 95% confidence intervals are two-sided. Stata, version 12.1 (StataCorp, College Station, Tex.) was used for all analyses.
Results
As shown in Figure 1, the cumulative proportion of patients receiving citalopram prescriptions for dosages ≤40 mg/day increased rapidly after the safety communications were issued. Substantial numbers of subjects were censored after a 30-day gap in citalopram resupply that may have resulted in part from unrecognized citalopram dosage reductions or discontinuation. Of those who filled citalopram prescriptions for ≤40 mg/day during the year of follow-up (N=18,407), 3.9% (cumulative proportion; N=519) subsequently filled prescriptions for >40 mg/day.
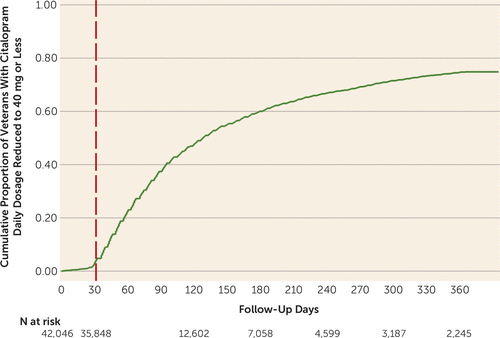
FIGURE 1. Time Taken to Reduce Prescribed Citalopram Dosages to ≤40 mg/day After FDA and VA Safety Communicationsa
a Day 0 is Aug. 1, 2011, approximately 3 weeks before the safety communications were nationally disseminated on days 24 and 31. Follow-up for subsequent citalopram dosage reductions or continuation of prescriptions for >40mg/day, as well as follow-up for study outcomes, began after the vertical dashed line, when there were 35,848 at-risk patients.
Selected characteristics of subjects who continued to have active citalopram prescriptions for >40 mg/day after the FDA and VA safety communications were issued (N=35,848), and who did (N=18,407) or did not (N=17,441) have their dosage reduced thereafter, are described in Table 1. Their last prescribed dosages before the safety communications were issued averaged 64 mg/day (SD=8.6) and 63 mg/day (SD=7.9), respectively. The two groups had a similar number of VA health care encounters and diagnoses, as well as medications related to mental health or cardiac disorders. All 109 baseline variables, including standardized differences, are summarized in the data supplement in Table S1 and, after matching on propensity scores, in Table S2. The majority of standardized differences were negligible (<10%) before matching on propensity scores, and only one slightly exceeded 10% in the matched groups.
| Characteristic | Citalopram Dosage Not Reduced (N=17,441) | Citalopram Dosage Reduced (N=18,407) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 16,004 | 92 | 16,956 | 92 |
| Admitted to hospital | 2,511 | 14 | 2,749 | 15 |
| Mean | SD | Mean | SD | |
| Age (years) | 58 | 12 | 58 | 11 |
| Outpatient visit days | 14 | 14 | 15 | 15 |
| Prescription drug classes | 6.1 | 3.0 | 6.3 | 3.1 |
| Diagnoses recorded in the past year | N | % | N | % |
| Depression | 12,042 | 69 | 12,837 | 70 |
| Posttraumatic stress disorder | 8,971 | 51 | 9,985 | 54 |
| Self-injury | 121 | 0.7 | 141 | 0.8 |
| Anxiety | 4,156 | 24 | 4,792 | 26 |
| Psychosis | 2,320 | 13 | 3,005 | 16 |
| Substance abuse | 3,839 | 22 | 4,103 | 22 |
| Tachycardia | 327 | 1.9 | 337 | 1.8 |
| Atrial fibrillation | 586 | 3.4 | 631 | 3.4 |
| Other arrhythmia | 634 | 3.6 | 674 | 3.7 |
| Long QT syndrome | 5 | 0.03 | 7 | 0.04 |
| Atrioventricular block | 440 | 2.5 | 443 | 2.4 |
| Ischemic heart disease | 3,123 | 18 | 3,471 | 19 |
| Heart failure | 762 | 4.4 | 801 | 4.4 |
| Prescriptions in the past year | ||||
| Tricyclic antidepressant | 1,273 | 7.3 | 1,288 | 7.0 |
| Sedative or hypnotic | 6,970 | 40 | 8,050 | 44 |
| Antipsychotic | 4,254 | 24 | 5,374 | 29 |
| Beta-adrenergic blocker | 5,879 | 34 | 6,126 | 33 |
| Beta-adrenergic stimulant | 60 | 0.3 | 66 | 0.4 |
| Digoxin | 245 | 1.4 | 225 | 1.2 |
| Antiarrhythmic | 142 | 0.8 | 149 | 0.8 |
| Others that prolong QT interval | 3,918 | 22 | 4,395 | 24 |
TABLE 1. Baseline Characteristics of Subjects Whose Citalopram Dosage Was or Was Not Reduced to ≤40 mg/day Following FDA and VA Safety Communications
The unadjusted incidence of all-cause hospitalizations or deaths was more than 2.5 times higher after citalopram dosages were reduced to ≤40 mg/day (Table 2). The unadjusted hazard ratio for the primary all-cause outcome was 4.4 (95% CI=4.0–4.8; p<0.001). The mortality rate continued to be 1.9 per 100 person-years after dosages were reduced, and the adjusted hazard ratio was 1.0 (95% CI=0.8–1.3; p=0.97). Therefore, the higher risk observed for the primary composite outcome was driven by a higher risk of hospitalizations (unadjusted hazard ratio=5.3, 95% CI=4.6–6.0; p<0.001), with deaths analyzed as a competing risk. The only remarkable difference in principal discharge diagnoses was in the mental health disorder category, which accounted for 20.1% (N=401) of the 1,997 all-cause hospitalizations that occurred after the dosage was reduced to ≤40 mg/day, compared with 14.0% (N=132) of the 944 all-cause hospitalizations that occurred when higher dosages were continued. PTSD accounted for nearly half of the difference (6.6% [N=132] and 3.8% [N=36], respectively). There was little difference in the other 20 major diagnostic categories of all-cause hospitalizations, including circulatory system disorders (19.6% compared with 18.8%).
| Citalopram Dosage Not Reduced to ≤40 mg/day | Citalopram Dosage Reduced to ≤40 mg/day | |||||
|---|---|---|---|---|---|---|
| Endpoint | Events / Person-Years | Incident Rate (per 100 person-years) | 95% CI | Events / Person-Years | Incident Rate (per 100 person-years) | 95% CI |
| All-cause deaths or hospitalizations | 944 / 8,906 | 10.6 | 10.0–11.3 | 1,997 / 7,315 | 27.3 | 26.1–28.5 |
| All-cause deaths or depression hospitalizations | 233 / 8,962 | 2.6 | 2.2–2.9 | 329 / 7,833 | 4.2 | 3.8–4.7 |
| All-cause deaths or arrhythmia hospitalizations | 195 / 9,286 | 2.1 | 1.8–2.4 | 196 / 7,840 | 2.5 | 2.2–2.8 |
| All-cause deaths | 178 / 9,368 | 1.9 | 1.7–2.2 | 152 / 8,000 | 1.9 | 1.6–2.2 |
TABLE 2. Hospitalization and Mortality Rates Following Reduction of Citalopram Prescriptions to ≤40 mg/day
The unadjusted incidence rates for the secondary composite outcome of hospitalizations for depression, diagnoses of self-injury, or death were also higher after citalopram dosages were reduced (Table 2). The unadjusted hazard ratio was 9.8 (95% CI=6.2–15.5; p<0.001) when deaths were analyzed as a competing risk rather than as an outcome event. The composite unadjusted incidence of hospitalizations for cardiac arrhythmias or all-cause deaths (Table 2) was not lower after dosages of citalopram were reduced, and neither was the unadjusted hazard ratio (hazard ratio=1.3, 95% CI=1.0–1.6; p=0.02). The unadjusted hazard ratio of these more specific arrhythmia-related hospitalizations was 7.1 (95% CI=2.9–17.1; p<0.001) when deaths were analyzed as a competing risk rather than as an outcome. The increased risk of this composite outcome after citalopram dosages were reduced came from more hospitalizations with a principal discharge diagnosis of syncope or collapse. Excluding these less specific diagnoses, the unadjusted incidence of hospitalizations for cardiac arrhythmias or all-cause mortality was approximately 2.1 per 100 person-years, regardless of whether high citalopram dosages were reduced or continued.
The multivariable regression outcome model and the propensity matching are presented in Table 3. Neither had a substantial effect on the hazard ratio estimates for the primary or secondary composite outcomes. Allowing a 90-day gap, rather than a 30-day gap, for receipt of another citalopram supply allowed us to include many more subjects and more follow-up time in the analysis, but the adjusted time frame did not substantially alter the estimated adjusted hazard ratios (Table 3).
| Unadjusted Analysis (N=35,848) | Multivariable Analysis (N=35,848)a | Matched Analysis (N=29,524)b | 90-Day Gap in VA Citalopram Supply (N=42,162)c | |||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| All-cause deaths or hospitalizations | 4.4*** | 4.0–4.8 | 4.5*** | 4.1–5.0 | 4.1*** | 3.7–4.5 | 5.4*** | 5.0–5.8 |
| All-cause deaths or depression hospitalizations | 2.3*** | 1.9–2.7 | 2.2*** | 1.8–2.6 | 2.0*** | 1.6–2.4 | 2.2*** | 1.9–2.6 |
| All-cause deaths or arrhythmia hospitalizations | 1.3* | 1.0–1.6 | 1.3* | 1.0–1.7 | 1.2 | 1.0–1.6 | 1.3** | 1.1–1.6 |
TABLE 3. Unadjusted and Adjusted Hazard Ratios for Outcomes of Citalopram Dosage Reduction in a Veteran Population
Discussion
Reductions of prescribed dosages of citalopram to a new safety limit of 40 mg/day were associated with a substantial increase in all-cause and depression-related hospitalizations. Rapidly reducing dosages from >60 mg/day to ≤40 mg/day may have precipitated worsening symptoms of depression, PTSD, and other mental health disorders. On the other hand, there was no noticeable reduction in hospitalizations for cardiac arrhythmias or all-cause mortality. Although citalopram can prolong the QT interval in a dose-dependent manner, the mortality risk may have been too low to detect even in this large cohort of patients who had survived high dosages of citalopram (26, 27). Previous research has found no increased risk of ventricular arrhythmia or death when citalopram dosages >40 mg/day were compared with lower dosages (28). In the present study, deaths that were directly or possibly indirectly related to worsening mental health (e.g., poor adherence to cardiovascular medications and self-care) may have offset deaths from cardiac arrhythmias incurred by a continuance of higher prescription dosages. The safety warning may have prompted electrocardiograms that led to dosage reductions that prevented some hospitalizations for cardiac arrhythmias and deaths. However, the net effect of the large number of citalopram dosage reductions that occurred shortly after the safety communications were issued appeared to manifest as an increase in hospitalizations for mental health disorders. The outcomes may differ if high citalopram dosages are more selectively reduced for patients at greatest risk of clinically significant QT interval prolongation, although the FDA dosage limit was applicable to all patients treated with citalopram.
Citalopram prescriptions for ≤40 mg/day increased impressively shortly after the safety warnings were issued by the FDA and disseminated to all VA health care systems by the VA Center for Medication Safety. There were relatively few dosage reductions during the weeks before the new dosage limit was issued, and these dosage reductions were excluded from the outcome analyses. Baseline clinical characteristics were similar in the groups that did or did not have their prescribed citalopram dosages reduced. Therefore, most of the observed citalopram dosage reductions were presumably prompted by the new safety limit. If worsening mental health prompted outpatient visits when citalopram dosages were reduced, it seems more likely that these dosage reductions would be prompted by the dosage limit rather than the patients’ worsening mental health, although clinicians and patients may have thought the high dosages were no longer effective. If so, worsening depression prior to dosage reductions may have led to some hospitalizations and deaths following dosage reduction.
Outcome events could be misclassified into the group that continued on higher dosages if the citalopram dosages were reduced or discontinued but not reflected in the medical records. To limit the effects of this type of misclassification, follow-up was censored as soon as there was a 30-day gap in the dispensed citalopram supply. The results were essentially the same when a 90-day gap was allowed before follow-up was censored. Furthermore, this type of misclassification would place a bias against finding a higher incidence of hospitalizations or death following citalopram dosage reductions.
We used multivariable Cox regression models of the composite outcome and the propensity to reduce the dosage of citalopram to try to reduce bias from differences in measured variables. However, we could not exclude the possibility of residual confounding based on differences in unmeasured confounders (24). Bias arising from differences in the unmeasured severity of depression or PTSD may not have been controlled via its relationship to the prescribed dosage of citalopram, use of other prescription medications, use of VA health care, and so forth. The risk of QT interval prolongation may not have been controlled by incorporating into the regression models other cardiac dysrhythmias and conditions, other medications that can alter the QT interval, and the amount of previous exposure to high dosages of citalopram. If some of the variables in the propensity model were related to the likelihood of citalopram dosage reductions but not to the study outcome, their inclusion could have increased bias in the estimated hazard ratios (25). Nevertheless, we did not find substantial differences in the large number of measured confounders, and accordingly neither regression method we used to try to reduce bias substantially altered the estimated hazard ratios. Any residual bias due to differences in unmeasured confounders would need to be much larger to affect the estimated hazard ratios. Thus, residual confounding bias most likely does not entirely explain the observed association between the citalopram dosage reductions and the adverse patient outcomes.
Providers' Perspective
Shortly after the release of the FDA and VA national safety bulletins stipulating that dosages of citalopram should no longer exceed 40 mg/day, psychiatrists at the Minneapolis VA Medical Center identified all patients whose daily dosage of citalopram exceeded 40 mg and held staff meetings to discuss patient risk of QT prolongation and torsade de pointes. Lacking published evidence, we had difficulty assessing the risk and were concerned about lowering dosages of citalopram that had been clinically titrated. However, we were also reluctant not to comply with the FDA dosage limit because of concerns about malpractice, should a fatal arrhythmia occur in a patient being treated with more than 40 mg/day. Most clinicians decided to discuss the FDA’s warnings with patients and to recommend that their dosage be reduced.
Over the next several months, we noted some cases of worsening depressive symptoms and hospitalizations associated with reductions in citalopram dosages. For example, a middle-aged patient, who after 1 year of treatment had been stabilized on 80 mg of citalopram, had the dosage reduced and experienced a subsequent increase in depressive symptoms. The patient was switched to sertraline, which did not reduce the symptoms. The process of reducing the dosage of citalopram had a significant effect on the patient's functioning, including loss of work and relationship strain. Eventually, the patient requested to be placed back on citalopram, was titrated to a dosage of 80 mg/day, and felt better. Another elderly veteran patient required hospitalization after a reduction of citalopram dosage from 60 mg/day to 20 mg/day, as was recommended in the safety bulletins. The patient felt suicidal, and during hospitalization the patient was titrated back to 60 mg/day and reported feeling better thereafter.
Our analysis is also limited by lack of data for some non-VA hospitalizations. We cannot exclude the possibility that the observed higher relative risk of VA hospitalizations was because of less utilization of non-VA hospital care after citalopram dosages were reduced, compared with when high VA dosages of citalopram continued to be prescribed. We did not pursue Medicare data to try to explore this issue because the majority of the at-risk population was younger than age 65, and many subjects had private health care insurance.
Presumably, any diagnostic misclassification in the VA data would not be related to whether clinicians decided to prescribe lower dosages of citalopram, and it certainly would not affect the primary composite outcome of all-cause hospitalizations or death. Counting hospitalizations that were not directly or indirectly associated with the dosage reductions as outcomes would be expected to reduce the estimated hazard ratio, assuming unrelated hospitalizations were equally likely when citalopram dosages were or were not reduced as they appeared to be when major discharge diagnosis categories were examined.
A national VA medication safety communication helped ensure that VA health care providers were aware of the new FDA dosage limit for citalopram. Indeed, the majority of veterans who continued to receive citalopram prescriptions had lower dosages prescribed soon after the warning was disseminated to all VA health care systems. Many VA health care providers may have felt obligated to prescribe dosages that did not exceed 40 mg/day.
In conclusion, reduction of prescribed citalopram dosages to a new safety limit was associated with a higher rate of hospitalization in a large patient population who had been treated with substantially higher dosages. We hope this research will encourage more empirical studies of patient outcomes after risk mitigation initiatives and thereby help to improve medication safety warnings and clinical risk management.
1 : “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol 2006; 117:34–39Crossref, Medline, Google Scholar
2 : Guidance for Industry: Drug Safety Information: FDA’s Communication to the Public, Silver Spring, Md, 2012 (http://www.fda.gov/downloads/Drugs/guidanceComplianceRegulatoryInformation/Guidances/UCM295217.pdf)Google Scholar
3 : Drug warnings that can cause fits: communicating risks in a data-poor environment. N Engl J Med 2008; 359:991–994Crossref, Medline, Google Scholar
4 : The public health implications of the 1995 ‘pill scare’. Hum Reprod Update 1999; 5:621–626Crossref, Medline, Google Scholar
5 FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). US Food and Drug Administration, Silver Spring, Md, 2012 (http://www.fda.gov/Drugs/DrugSafety/ucm269086.htm)Google Scholar
6 : Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med 2012; 125:859–868Crossref, Medline, Google Scholar
7 : Citalopram therapy for depression: a review of 10 years of European experience and data from U.S. clinical trials. J Clin Psychiatry 2000; 61:896–908Crossref, Medline, Google Scholar
8 : Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? a systematic review. Eur Arch Psychiatry Clin Neurosci 2005; 255:387–400Crossref, Medline, Google Scholar
9 : The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55:580–592Crossref, Medline, Google Scholar
10 : Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry 2006; 63:1358–1367Crossref, Medline, Google Scholar
11 : Economic consequence of switching to citalopram after its generic entry for adult patients with major depressive disorder (MDD) treated with escitalopram: a 6-month retrospective study. J Med Econ 2010; 13:599–609Crossref, Medline, Google Scholar
12 : Associations between depression and all-cause and cause-specific risk of death: a retrospective cohort study in the Veterans Health Administration. J Psychosom Res 2015; 78:324–331Crossref, Medline, Google Scholar
13 : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139Crossref, Medline, Google Scholar
14 Woosley RL: Drugs that prolong the Qt interval and/or induce Torsades de Pointes. (www.azcert.org/medical-pros/drug-lists/printable-drug-list.cfm)Google Scholar
15 : Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013; 138:103–141Crossref, Medline, Google Scholar
16 : Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4:2Crossref, Medline, Google Scholar
17 : Variation in the risk of suicide attempts and completed suicides by antidepressant agent in adults: a propensity score-adjusted analysis of 9 years’ data. Arch Gen Psychiatry 2010; 67:497–506Crossref, Medline, Google Scholar
18 : Suicide-related behaviors in older patients with new anti-epileptic drug use: data from the VA hospital system. BMC Med 2010; 8:4Crossref, Medline, Google Scholar
19 : Antidepressant dose, age, and the risk of deliberate self-harm. JAMA Intern Med 2014; 174:899–909Crossref, Medline, Google Scholar
20 : Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf 2010; 19:555–562Crossref, Medline, Google Scholar
21 : Effect of macrolide and fluoroquinolone antibacterials on the risk of ventricular arrhythmia and cardiac arrest: an observational study in Italy using case-control, case-crossover and case-time-control designs. Drug Saf 2009; 32:159–167Crossref, Medline, Google Scholar
22 : QTc-prolonging drugs and hospitalizations for cardiac arrhythmias. Am J Cardiol 2003; 91:59–62Crossref, Medline, Google Scholar
23 : Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–3107Crossref, Medline, Google Scholar
24 : Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol 2005; 161:891–898Crossref, Medline, Google Scholar
25 : A review of covariate selection for non-experimental comparative effectiveness research. Pharmacoepidemiol Drug Saf 2013; 22:1139–1145Crossref, Medline, Google Scholar
26 : Reconciling the risk of QT interval prolongation in antidepressants. Pharmacoepidemiol Drug Saf 2012; 21:329–330Crossref, Medline, Google Scholar
27 : QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ 2013; 346:f288Crossref, Medline, Google Scholar
28 : Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry 2013; 170:642–650Link, Google Scholar



